Published online Apr 28, 2016. doi: 10.4329/wjr.v8.i4.403
Peer-review started: December 22, 2015
First decision: January 18, 2016
Revised: January 27, 2016
Accepted: February 16, 2016
Article in press: February 17, 2016
Published online: April 28, 2016
Processing time: 123 Days and 1.1 Hours
AIM: To identify computed tomography (CT) findings that are associated with the presence of bacteremia in patients with acute pyelonephritis (APN) due to Escherichia coli (E. coli).
METHODS: The clinical data and contrast-enhanced CT findings of 128 patients who were diagnosed with APN due to E. coli and showed renal abnormality on contrast-enhanced CT between January 2003 and November 2013 were retrospectively reviewed. The patients were divided into two groups according to the presence of bacteremia: The bacteremia group and the non-bacteremia group. The abnormality on contrast-enhanced CT were categorized into 5 renal and 4 extrarenal CT findings and compared between the two groups using the χ2 test and multivariate logistic regression.
RESULTS: Among the 128 patients, 34 patients (26.6%) were classified into the bacteremia group and 94 patients (73.4%) into the non-bacteremia group. There was no statistically significant difference in gender between the two groups (P = 0.09), but the age of the patients in the bacteremia group was higher than that of the patients in the non-bacteremia group (P < 0.01). Compared to the non-bacteremia group, 1 renal CT finding such as urothelial thickening and 3 extrarenal CT findings such as diffuse peritoneal thickening, cystitis and pulmonary congestion were more frequently observed in the bacteremia group with statistical significance. The logistic regression analysis revealed that CT findings, including urothelial thickening, diffuse peritoneal thickening, cystitis and pulmonary congestion were suggested as the predictive CT findings of bacteremic APN.
CONCLUSION: On CT, urothelial thickening, diffuse peritoneal thickening, cystitis, and pulmonary congestion are more frequently observed in patients with bacteremic APN due to E. coli.
Core tip: This is a retrospective study to evaluate the different computed tomography (CT) findings between the bacteremic and non-bacteremic acute pyelonephritis (APN) due to Escherichia coli (E. coli). Current study includes 128 subjects diagnosed with APN due to E. coli and performed contrast-enhanced CT. The usefulness of blood culture that was routinely performed in the patients with APN have been doubted; therefore, several studies which aimed to determine the predictive model of bacteremia by using some clinical features have been published. Here, we analyzed the CT findings of APN according to the presence of bacteremia and suggested the significant CT features predicting bacteremia which were urothelial thickening, diffuse peritoneal thickening, cystitis, and pulmonary congestion.
- Citation: Oh SJ, Je BK, Lee SH, Choi WS, Hong D, Kim SB. Comparison of computed tomography findings between bacteremic and non-bacteremic acute pyelonephritis due to Escherichia coli. World J Radiol 2016; 8(4): 403-409
- URL: https://www.wjgnet.com/1949-8470/full/v8/i4/403.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i4.403
Acute pyelonephritis (APN) is an inflammation of the upper urinary tract, including renal parenchyma, calyces, and pelvis, which is considered as an ascending infection from the lower urinary tract[1-3]. The clinical spectrum of APN is diverse ranging from dysuria to systemic illness complicated by sepsis or death. The urine and blood cultures are performed as routine workups to identify the true pathogen and to obtain the antibiotic susceptibility test[4-7]. Although several studies have suggested that blood cultures provide no useful information toward the clinical management of APN, it is still essential to differentiate the patients with bacteremia from those without bacteremia, considering that the mortality from urosepsis reaches 7.5%-30%, especially at the initial presentation[8-11]. There have been several studies that aimed to determine the predictive model of bacteremia in the patients with APN by using the clinical features[11-14]; however, there has been no study that evaluates the radiologic features that are associated with bacteremic APN. Therefore, the purpose of this study was to identify computed tomographic (CT) findings associated with the presence of bacteremia in patients with APN due to Escherichia coli (E. coli).
This retrospective study was approved by the institutional review board and was compliant with HIPAA. The requirement for informed consent was waived.
This study included all consecutive subjects who underwent contrast-enhanced CT scan of the kidneys with a clinical diagnosis of APN between January 2003 and November 2013. A total of 491 patients met these two criteria. Then, we selected the following patients: (1) the patients with renal abnormality on contrast-enhanced CT that was performed at their first visit; and (2) the patients in whom both blood and urine cultures were performed at their first visit. One hundred and eighty-three patients were selected, and we excluded the following patients in the next step: (1) the patients in whom a microorganism other than E. coli was isolated from urine culture; and (2) the patients with the history of comorbidity other than APN which may affect the result of blood or urine culture. Finally, 128 patients (109 females, 19 males, median age 43 ± 25 years) comprised our study population. From this cohort, the patients were divided into the two groups according to the results of blood culture: The bacteremia group in which E. coli was isolated from blood culture and the non-bacteremia group with no bacterial growth in blood.
Contrast-enhanced CT studies were performed by using two multidetector CT scanners (Brilliance 64, Philips Medical System, Cleveland, OH, United States; Aquilion 64, Toshiba Medical Systems, Tochigi, Japan) with intravenous contrast media (Ultravist 300 or 370, Bayer-Schering, Berlin, Germany; Omnipaque 300, GE Healthcare) administered at a rate of 3-4 mL/s, using power injectors (Medrad, Pittsburgh, PA, United States). The volume of contrast media administered varied depending on the body weight of each patient (2 mL/kg of body weight).
CT scanning parameters were 100-120 kVp, 130-700 mAs, and 3-5 mm slice thickness with no gap. CT protocols applied to the patients were diverse due to retrospective enrollment of the patients. Forty-three patients underwent 3-phase CT scan which was obtained during pre-contrast, portal venous, and excretory phases; 27 patients underwent 2-phase CT scan which was obtained during pre-contrast and portal venous phases; 21 patients underwent 4-phase CT scan which was obtained during pre-contrast, arterial, portal venous, and excretory phases; 13 patients underwent single phase CT scan which was obtained during portal venous phase; 9 patients underwent 2-phase CT scan which was obtained during arterial and excretory phases; 5 patients underwent 2-phase CT scan which was obtained during portal venous and excretory phases; 5 patients underwent 3-phase CT scan which was obtained during arterial, portal venous, and excretory phases; 3 patients underwent 3-phase CT scan which was obtained during pre-contrast, arterial and excretory phases; and 2 patients underwent 2-phase CT scan which was obtained during pre-contrast and excretory phases. After acquisition of the pre-contrast images, arterial phase scanning was performed 10-15 s after the trigger attenuation threshold (100 HU) was reached at the level of the supra-celiac abdominal aorta. The time delays were 70-80 s for portal venous phase and 180 s for excretory phase.
Contrast-enhanced CT images of 128 patients were reviewed on the Picture Archiving and Communication System workstation (G3; Infinitt Co., Seoul, South Korea) by two radiologists in consensus (the first author with 15 years of experience in radiology and the second author with 4 years of experience in radiology). The readers were aware that E. coli was isolated from urine culture, but were blinded to the results of blood culture.
Contrast-enhanced CT findings were categorized into the renal and extrarenal findings.
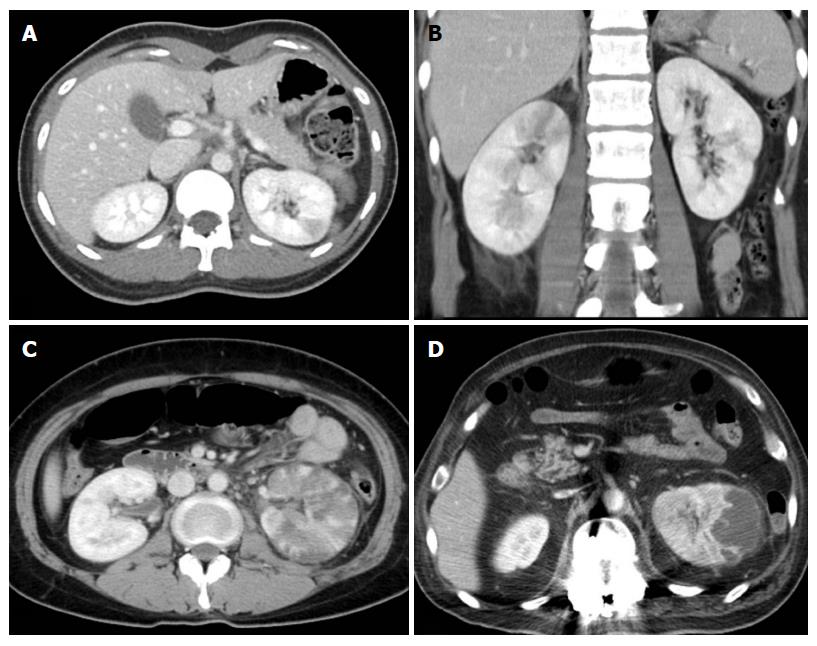
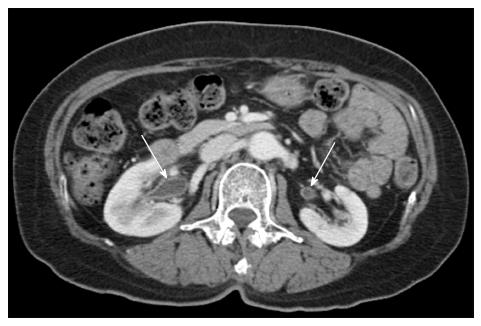
The renal findings were recorded as follows: (1) APN grade on a scale of 4; (2) the presence of urothelial thickening; (3) bilateral involvement; and (4) perirenal infiltration; (5) rupture of renal abscess. APN grades were defined as follows (Figure 1): Grade 1 was simple APN presenting with a focal, wedge-shaped hypodensity radiating from the papilla in the medulla to the cortical space of the kidney; grade 2 was severe APN presenting as multifocal or diffusely scattered hypodensities in the kidney; grade 3 was a microabscess which was less than 2 cm in size; grade 4 was a macroabscess which was more than 2 cm in longest diameter. The urothelial thickening is defined as thickening of renal pelvis, calyces, and ureter (Figure 2). In cases with bilateral APN of different grades in each kidney, the higher grade was recorded. Perirenal infiltration was defined as the obliteration of perirenal fat or thickening of Gerota’s fascia.
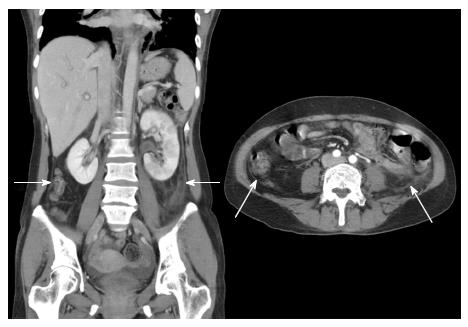
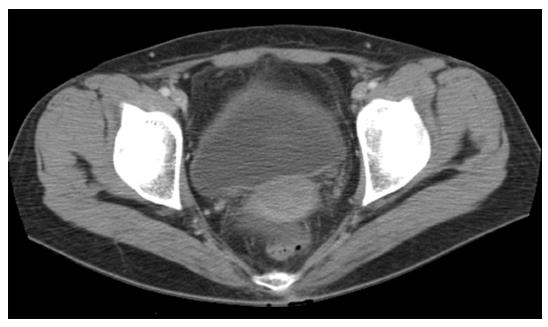
The extrarenal findings were recorded as follows: (1) diffuse peritoneal thickening; (2) cystitis; (3) pulmonary congestion; and (4) pleural effusion (Figure 3). Cystitis was defined as the presence of a thickened and enhanced wall of the urinary bladder accompanied by infiltration into the perivesical fat (Figure 4).
The frequency of each CT finding was compared statistically between the two groups by χ2 test using SPSS® software (Version 20.0 for Windows, SPSS Institute, Chicago, IL, United States). All variables were entered into a logistic regression model to assess the predictive CT findings of bacteremia. The multivariate logistic regression was performed to calculate the odds ratios with 95%CI and P values. In both methods of analyses, a P value of < 0.05 was considered to be statistically significant.
A total of 128 CT scans of 128 patients were included: 34 patients (32 females, 2 males, median age 63 ± 18 years) in the bacteremia group and 94 patients (77 females, 17 males, median age 39 ± 25 years) in the non-bacteremia group. There was no statistically significant difference in gender between the two groups (P = 0.09), but the age of the patients in the bacteremia group was higher than that of the patients in the non-bacteremia group in the result of t test (P < 0.01).
The frequencies of renal and extrarenal CT findings in the two groups and the results of χ2 test are shown in Table 1. Compared to the non-bacteremia group, 4 CT findings, including 1 renal finding such as urothelial thickening and 3 extrarenal findings such as diffuse peritoneal thickening, cystitis and pulmonary congestion, were more frequently observed in the bacteremia group with statistical significance (Figure 2).
| CT findings | Patients with bacteremia (n = 34) | Patients without bacteremia (n = 94) | P value |
| Renal findings | |||
| APN grade | 0.88 | ||
| 1 | 2 (5.8) | 7 (7.0) | |
| 2 | 21 (61.8) | 50 (53.2) | |
| 3 | 8 (23.5) | 24 (25.5) | |
| 4 | 3 (8.8) | 13 (13.8) | |
| Urothelial thickening | 29 (85.3) | 61 (64.9) | 0.03 |
| Bilateral involvement | 10 (29.4) | 31 (33.0) | 0.70 |
| Perirenal infiltration | 27 (79.4) | 69 (73.4) | 0.49 |
| Rupture of abscess | 0 | 4 (4.3) | 0.22 |
| Extrarenal findings | |||
| Diffuse peritoneal thickening | 9 (26.5) | 4 (4.3) | < 0.01 |
| Cystitis | 15 (44.1) | 19 (20.2) | < 0.01 |
| Pulmonary congestion | 16 (47.1) | 20 (21.3) | < 0.01 |
| Pleural effusion | 8 (23.5) | 14 (14.9) | 0.25 |
To identify a predictive CT finding of bacteremia, we performed multivariate logistic regression analysis (Table 2). The logistic regression analysis revealed that urothelial thickening, diffuse peritoneal thickening, cystitis, and pulmonary congestion were significantly associated with bacteremia.
| CT findings | OR (98%CI) | P value |
| Renal findings | ||
| Urothelial thickening | 3.16 (1.20-8.28) | 0.02 |
| Bilateral involvement | 1.05 (0.49-2.29) | 0.90 |
| Perirenal infiltration | 1.50 (0.61-3.67) | 0.38 |
| Rupture of abscess | 0.56 (0.06-5.19) | 0.61 |
| Renal findings | ||
| Diffuse peritoneal thickening | 7.50 (2.18-25.81) | < 0.01 |
| Cystitis | 4.15 (1.82-9.45) | < 0.01 |
| Pulmonary congestion | 4.73 (2.15-10.42) | < 0.01 |
| Pleural effusion | 1.61 (0.63-4.08) | 0.32 |
Urinary tract infection (UTI) usually starts from the bladder and can spread up to the kidneys, which is called APN[6]. When the infection spreads into the bloodstream, it results in a systemic illness called bacteremia. The identification of bacteremia is important, because it may lead to severe urosepsis and mortality reaching 7.5%-30%[4-7,13,15-18]. Blood culture is a simple test which can isolate the microorganism with high specificity, although the general yields are less than 10%[12,13]. Among the patients with APN, blood cultures have been reported to be positive in 18%-32% of APN cases[11,13,15-17,19,20]. Some studies have doubted the usefulness of routine blood cultures in the patients with uncomplicated APN, based on the limited information from blood culture and little effect of antibiotic therapy[8-10,15,18]. However, blood cultures are still performed frequently, due to particular concern about higher mortality and complication rate related with bacteremia and increasing prevalence of resistant strains[21,22]. Considering the prediction of bacteremia before or without obtaining the result of blood culture, several studies aimed to determine the predictive model of bacteremia in the patients with APN by using the clinical features[11-14]; age, systolic blood pressure of < 90 mmHg, body temperature of > 39 °C, and procalcitonin level of > 0.5 ng/dL in the study by Lee et al[11], and indwelling urinary catheters before UTI and high C-reactive protein in the study by Shigemura et al[14]. However, there has been no study which evaluates the radiologic features that are associated with bacteremic APN.
CT has a role in defining the extent of APN and in identifying the presence of complications[23]. Review of the literatures identified two studies which introduced the grading system of APN, using CT images[24,25]. Lim et al[24] graded APN into 3 groups according to the progression of disease, such as simple, severe and abscess formation. In addition, Paick et al[25] graded APN into 4 groups according to the extent of renal involvement; no renal parenchymal involvement as grade 1; less than 25% involvement as grade 2; 25% to 50% involvement as grade 3; and greater than 50% as grade 4. Since we enrolled the patients whose CT findings showed renal abnormality on contrast-enhanced CT, we modified the Lim’s APN grading system on a scale of 4. In this study, the grades of APN showed no statistical difference between the patients with and without bacteremia, as the proportion of the patients in each APN grade were similar in both groups.
Among the CT findings, urothelial thickening, diffuse peritoneal thickening, cystitis and pulmonary congestion were more frequently observed in the patients with bacteremia with statistical significance; moreover, these findings were also significantly associated with bacteremia on multivariate analysis. All renal CT findings other than urothelial thickening were not associated with the presence of bacteremia, while 3 of the 4 extrarenal CT findings were statistically significantly associated with the presence of bacteremia. Based on these results, we presumed that there were 3 concerns with respect to bacteremia associated with APN. First, we could assume that the 4 significant CT findings indicated the entrance of the pathogen from the urinary tract into the blood-stream, which might occur through the peritoneal cavity, and not through the kidney. The microorganism in the urinary bladder, ureter, and renal pelvis might have directly spread through the peritoneal cavity, which could explain the 4 significant CT findings in our results. In contrast, perirenal infiltration in APN or the rupture of a renal abscess showed no statistically significant difference between the two groups, which might suggest that the inflammation in the perirenal space did not lead to bacteremia. Second, we could assume that the 4 significant CT findings were secondary to sepsis in the patients with bacteremia. Earlier, Zissin et al[26] described the extrarenal CT findings of APN in 21 cases, such as gallbladder wall thickening, periportal tracking, peritoneal fluid, congested inferior vena cava, pleural fluid, and interlobular septal thickening in the basal lungs, and suggested that the cause of the extrarenal CT findings was probably APN-related sepsis. Third, we could assume that pulmonary congestion was due to the increased permeability of capillaries caused by sepsis or direct pleural irritation caused by spreading peritonitis.
Besides the CT findings, we compared age and gender between the two groups. The age of the patients in the bacteremia group was higher than that of the patients in the non-bacteremia group, which was in agreement with the result of a recent study by Lee et al[11]. They concluded that old age was one of the predictive factors associated with bacteremia, in conjunction with low systolic blood pressure, high body temperature, and increased level of serum procalcitonin[11]. Even though there was a female predominance in the enrolled patients of our study, patients’ gender was not statistically different between the two groups (P = 0.09).
This study has several limitations. First, we categorized the patients into the bacteremia or non-bacteremia group based on the clinical data from our institute. Data on previous treatment with antibiotics or previous hospitalization before visiting our institute were limited, which was inevitable due to the retrospective study design. The administration of antibiotics prior to the CT scan or blood and urine culture could impact on the number of the patients in both groups. Second, considering the number of patients, we limited the patients to those in whom E. coli was isolated, as E. coli was the most predominant bacteria isolated from urine cultures. Among the 183 patients, there were other bacterial strains isolated in urine cultures, such as Klebsiella pneumoniae (6%), Enterococcus species (5%), Staphylococcus species (3%), Streptococcus species (2%), Proteus mirabilis (1%), and Pseudomonas species (1%). Since we aimed to perform the statistical analysis between the bacteremia group and the non-bacteremia group, we enrolled the most frequently affected microorganism. In the future, other species but E. coli could be enrolled with more data. Third, there was a possibility that a patient whose renal function was severely impaired did not undergo contrast-enhanced CT scan, and hence he/she was excluded from this study.
In conclusion, renal CT findings, such as urothelial thickening, and extrarenal CT findings, such as diffuse peritoneal thickening, cystitis, and pulmonary congestion, were more commonly observed in the patients with bacteremia with statistical significance, as compared with the patients without bacteremia. Therefore, these CT findings may help physicians to identify bacteremia when managing the patients and initiating the proper treatment to decrease the mortality from bacteremia in APN.
Urinary tract infection (UTI) usually starts from the bladder and can spread up to the kidneys, which is called Acute pyelonephritis (APN). APN is an inflammation of the upper urinary tract, including renal parenchyma, calyces, and pelvis, which is considered as an ascending infection from the lower urinary tract. The clinical spectrum of APN is diverse. When the infection spreads into the bloodstream, it results in a systemic illness called bacteremia that may lead to severe urosepsis and mortality reaching 7.5%-30%. Therefore, the urine and blood cultures are usually performed as routine workups to identify the true pathogen and to obtain the antibiotic susceptibility test. Although several studies have suggested that blood cultures provide the limited information and little effect of antibiotic therapy toward the clinical management of APN, it is still essential to differentiate the patients with bacteremia from those without bacteremia, considering that the mortality from urosepsis reaches 7.5%-30%, especially at the initial presentation. There have been several studies that aimed to determine the predictive model of bacteremia in the patients with APN by using the clinical features; age, systolic blood pressure of < 90 mmHg, body temperature of > 39 °C, and procalcitonin level of > 0.5 ng/dL, indwelling urinary catheters before UTI, and high C-reactive protein.
Computed tomography (CT) has a role in defining the extent of APN and in identifying the presence of complications. However, there has been no study that evaluates the radiologic features that are associated with bacteremic APN. The result of this study contributes to identifying CT findings associated with the presence of bacteremia in patients with APN due to Escherichia coli (E. coli).
In this study, 1 renal CT finding such as urothelial thickening and 3 extrarenal CT findings such as diffuse peritoneal thickening, cystitis and pulmonary congestion were more frequently observed in the bacteremia group with statistical significance, compared to the non-bacteremia group.
This study suggests that the CT findings, including urothelial thickening, diffuse peritoneal thickening, cystitis and pulmonary congestion can be the predictive CT findings of bacteremic APN due to E. coli.
The study describes a retrospective CT finding analysis of E. coli pyelonephritis cases to predict bacteremic patients. The authors have presented the data well, and it is well written.
P- Reviewer: Schwan WR, Yuksel S S- Editor: Gong ZM L- Editor: A E- Editor: Jiao XK
| 1. | Roberts JA. Management of pyelonephritis and upper urinary tract infections. Urol Clin North Am. 1999;26:753-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Bauer RJ, Zhang L, Foxman B, Siitonen A, Jantunen ME, Saxen H, Marrs CF. Molecular epidemiology of 3 putative virulence genes for Escherichia coli urinary tract infection-usp, iha, and iroN(E. coli). J Infect Dis. 2002;185:1521-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Czaja CA, Scholes D, Hooton TM, Stamm WE. Population-based epidemiologic analysis of acute pyelonephritis. Clin Infect Dis. 2007;45:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Bryan CS, Reynolds KL. Hospital-acquired bacteremic urinary tract infection: epidemiology and outcome. J Urol. 1984;132:494-498. [PubMed] |
| 5. | Bryan CS, Reynolds KL. Community-acquired bacteremic urinary tract infection: epidemiology and outcome. J Urol. 1984;132:490-493. [PubMed] |
| 6. | Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113 Suppl 1A:5S-13S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1148] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 7. | Brown P, Ki M, Foxman B. Acute pyelonephritis among adults: cost of illness and considerations for the economic evaluation of therapy. Pharmacoeconomics. 2005;23:1123-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | McMurray BR, Wrenn KD, Wright SW. Usefulness of blood cultures in pyelonephritis. Am J Emerg Med. 1997;15:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Thanassi M. Utility of urine and blood cultures in pyelonephritis. Acad Emerg Med. 1997;4:797-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Velasco M, Martínez JA, Moreno-Martínez A, Horcajada JP, Ruiz J, Barranco M, Almela M, Vila J, Mensa J. Blood cultures for women with uncomplicated acute pyelonephritis: are they necessary? Clin Infect Dis. 2003;37:1127-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Lee H, Lee YS, Jeong R, Kim YJ, Ahn S. Predictive factors of bacteremia in patients with febrile urinary tract infection: an experience at a tertiary care center. Infection. 2014;42:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Bates DW, Cook EF, Goldman L, Lee TH. Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med. 1990;113:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 193] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Kim KS, Kim K, Jo YH, Kim TY, Lee JH, Lee SJ, Rhee JE, Suh GJ. A simple model to predict bacteremia in women with acute pyelonephritis. J Infect. 2011;63:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Shigemura K, Tanaka K, Osawa K, Arakawa S, Miyake H, Fujisawa M. Clinical factors associated with shock in bacteremic UTI. Int Urol Nephrol. 2013;45:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Chen Y, Nitzan O, Saliba W, Chazan B, Colodner R, Raz R. Are blood cultures necessary in the management of women with complicated pyelonephritis? J Infect. 2006;53:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Litke A, Bossart R, Regez K, Schild U, Guglielmetti M, Conca A, Schäfer P, Reutlinger B, Mueller B, Albrich WC. The potential impact of biomarker-guided triage decisions for patients with urinary tract infections. Infection. 2013;41:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | van Nieuwkoop C, Hoppe BP, Bonten TN, Van’t Wout JW, Aarts NJ, Mertens BJ, Leyten EM, Koster T, Wattel-Louis GH, Delfos NM. Predicting the need for radiologic imaging in adults with febrile urinary tract infection. Clin Infect Dis. 2010;51:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Stalenhoef JE, van Dissel JT, van Nieuwkoop C. Febrile urinary tract infection in the emergency room. Curr Opin Infect Dis. 2015;28:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | MacMillan MC, Grimes DA. The limited usefulness of urine and blood cultures in treating pyelonephritis in pregnancy. Obstet Gynecol. 1991;78:745-748. [PubMed] |
| 20. | Grover SA, Komaroff AL, Weisberg M, Cook EF, Goldman L. The characteristics and hospital course of patients admitted for presumed acute pyelonephritis. J Gen Intern Med. 1987;2:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Talan DA, Krishnadasan A, Abrahamian FM, Stamm WE, Moran GJ. Prevalence and risk factor analysis of trimethoprim-sulfamethoxazole- and fluoroquinolone-resistant Escherichia coli infection among emergency department patients with pyelonephritis. Clin Infect Dis. 2008;47:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Wagenlehner FM, Weidner W, Naber KG. An update on uncomplicated urinary tract infections in women. Curr Opin Urol. 2009;19:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Kawashima A, Sandler CM, Goldman SM, Raval BK, Fishman EK. CT of renal inflammatory disease. Radiographics. 1997;17:851-866; discussion 867-868. [PubMed] |
| 24. | Lim SK, Ng FC. Acute pyelonephritis and renal abscesses in adults--correlating clinical parameters with radiological (computer tomography) severity. Ann Acad Med Singapore. 2011;40:407-413. [PubMed] |
| 25. | Paick SH, Choo GY, Baek M, Bae SR, Kim HG, Lho YS, Jung SI, Park HK. Clinical value of acute pyelonephritis grade based on computed tomography in predicting severity and course of acute pyelonephritis. J Comput Assist Tomogr. 2013;37:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Zissin R, Osadchy A, Gayer G, Kitay-Cohen Y. Extrarenal manifestations of severe acute pyelonephritis: CT findings in 21 cases. Emerg Radiol. 2006;13:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |