Published online Mar 28, 2016. doi: 10.4329/wjr.v8.i3.316
Peer-review started: August 22, 2015
First decision: November 6, 2015
Revised: November 15, 2015
Accepted: January 5, 2016
Article in press: January 7, 2016
Published online: March 28, 2016
Processing time: 213 Days and 22.3 Hours
AIM: To describe the multidetector computed tomography features of pancreatic metastasis from leiomyosarcoma (LMS).
METHODS: Between January 1995 and December 2012, 13 consecutive patients (11 women, 2 men; mean age of 57 years; range, 38-78 years) with pancreatic metastases from LMS were included in our study. Imaging features including location, number, largest dimension, tumor attenuation and enhancement characteristics, presence of necrosis, pancreatic ductal dilatation, common bile duct (CBD) dilatation, presence of pancreatitis, and atrophy were documented.
RESULTS: The most common site of origin of the pancreatic metastases from LMS was uterus (38.5%), followed by retroperitoneum (30.8%) and extremity (23.1%). None of the patients in our study had pancreas as the first site of metastasis. All patients developed pancreatic metastases at a median interval of 24 mo. Pancreatic metastases from LMS were solitary in 8/13 patients and multiple in 5/13 patients, had no predilection for any part of the pancreas, were hypovascular on arterial phase in 10/13 patients and associated with pancreatic duct dilatation in 3/13 patients. None had CBD dilatation. None of the pancreatic metastases in LMS cohort caused pancreatitis, and atrophy. Median duration of follow-up was 19 mo for LMS cohort during which two patients underwent resection of metastasis (median survival 45 mo) while the remaining underwent systemic therapy (median survival 13 mo).
CONCLUSION: Pancreatic metastases from LMS are often solitary and hypovascular masses and less commonly associated with pancreatic ductal dilatation, CBD dilatation, pancreatitis or pancreatic atrophy. Surgical resection of solitary LMS pancreatic metastasis can be considered due to the long survival of these patients.
Core tip: Pancreatic metastases from leiomyosarcoma (LMS) commonly arise in the uterus and are characterized by a long latency period after the diagnosis of primary tumor. Although the imaging features of the pancreatic metastases from LMS are nonspecific, pancreatic metastases from LMS should be considered in the differential diagnosis of solitary or multiple hypovascular masses without pancreatic ductal dilatation, common bile duct dilatation, pancreatitis, atrophy in the pancreas in patients with history of LMS. Surgical resection of solitary LMS pancreatic metastasis can be considered due to the long survival of these patients after detection of pancreatic metastasis.
- Citation: Suh CH, Keraliya A, Shinagare AB, Kim KW, Ramaiya NH, Tirumani SH. Multidetector computed tomography features of pancreatic metastases from leiomyosarcoma: Experience at a tertiary cancer center. World J Radiol 2016; 8(3): 316-321
- URL: https://www.wjgnet.com/1949-8470/full/v8/i3/316.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i3.316
Pancreatic metastases are rare accounting for approximately 2%-5% of all malignant pancreatic neoplasms[1-3]. Metastatic spread to pancreas can occur from a variety of primary malignancies with the most common primaries being renal cell carcinoma (RCC), lung cancer, breast cancer, colorectal cancer, and malignant melanoma[2,4]. The overall incidence of pancreatic metastasis in patients with diffuse metastatic disease varies between 3% and 12% in various autopsy series. Leiomyosarcoma (LMS) is a rare and aggressive malignant tumor composed of cells of smooth muscle origin. The most common primary sites of LMS include genitourinary tract, usually the uterus, retroperitoneum and gastrointestinal tract[5]. The common sites of distant metastases from LMS are lungs, kidney, and liver[6,7]. Pancreatic metastasis from LMS is extremely rare[8-10].
Pancreatic metastases are often asymptomatic and can come to clinical attention on imaging either at the time of initial work or post-operative surveillance of non-pancreatic primary neoplasm. Less commonly, they can present with symptoms due to biliary obstruction. Characterization of pancreatic metastasis on imaging studies can be challenging as well as crucial because they can mimic primary pancreatic malignancy and determine the prognosis and management[11]. Most of the radiology literature has been focused on the imaging features of pancreatic metastasis from RCC. Being a rare disease entity, the imaging features of pancreatic metastasis from LMS have not been reported so far. The available literature focuses on management[12,13] and are small series of case reports[8-10]. Accordingly, the purpose of this article is to describe the multidetector computed tomography (MDCT) features of pancreatic metastasis from LMS.
This study was a Health Insurance Portability and Accountability Act (HIPAA) compliant, institutional review board-approved retrospective study with waiver for informed consent. Between January 2000 and December 2012, 323 patients with LMS arising in uterus (n = 116), extremities (n = 55), retroperitoneum (n = 36), skin (n = 25), inferior vena cava (n = 23), mesentery (n = 15), bowel (n = 1) and other sites (n = 42) were either primarily treated or referred to our institute. The radiology reports of all these 323 patients were searched electronically to identify patients with pancreatic metastases which returned 13 patients (11 women, 2 men; mean age of 57 years; range, 38-78 years). These 13 consecutive patients with pancreatic metastases from LMS on MDCT scans were included in our study and the remaining 310 patients were excluded. The histopathology of the primary tumor was confirmed as LMS in all the patients as part of the routine oncology care.
Pretreatment contrast-enhanced CT scan and follow-up imaging were available in all 13 patients. In total we reviewed 148 CT examinations. The CT scans of were performed at our institute on multidetector scanners [four-slice (GE Healthcare, Barrington, IL, United States), 16-row (Siemens Medical Solutions, Forchheim, Germany), and 64-row (Toshiba America Medical Systems, Tustin, CA, United States) MDCT systems with 0.5 mm collimation, 120 kVp, 500 mA (max), gantry rotation time 0.5 s, table speed of 26.5 mm/rotation. The anatomic coverage extended from the clavicles to the pubic symphysis according to the standard department protocol. After intravenous contrast administration, the images of the chest and upper abdomen were acquired after a delay of 25-30 s followed by images of the abdomen and pelvis after 60-70 s. Oral contrast was administered in all the patients.
The imaging was reviewed in consensus by two oncoradiology fellowship trained radiologists (SHT and NHR) with 9 and 16 years of experience each. Imaging features of pancreatic metastases from LMS including location, number, largest dimension, tumor attenuation and enhancement characteristics on CT with respect to the paraspinal muscles (less than, similar to or greater than skeletal muscles) in the portal venous phase, presence of necrosis, pancreatic ductal dilatation, common bile duct (CBD) dilatation, presence of pancreatitis, atrophy, peripancreatic soft tissue infiltration, peripancreatic lymphadenopathy, and vessel involvement were recorded. For determining hypervascularity, the arterial phase images of chest CT which included the upper sections of the abdomen were used. On follow-up imaging, the number of metastasis and size of metastasis were recorded. The metastatic disease was confirmed either by histopathology or by serial imaging demonstrating interval change in the index lesions.
The clinical information for all the 13 patients including demographic data, site of primary tumor, first site of metastases, the date of pancreatic metastasis, duration of follow-up and outcome were documented from the electronic medical record. The time from diagnosis of metastasis to diagnosis of pancreatic metastasis and overall survival were calculated from the clinical data.
Statistical analysis was performed with SPSS software (SPSS, version 21; IBM, Armonk, NY) by one of the authors (CHS). P < 0.05 was considered to indicate a significant difference.
The distribution of the primary tumor in our study is shown in Table 1. In 11 (84.6%) patients, first site of metastases was lung. Other sites of first metastasis were right paracolic gutter, subcutaneous tissue, spine, and bones. None of the patients had pancreas as the first site of metastases. All patients developed pancreatic metastases at a median interval of 24 mo (range, 1-77 mo).
| Site | n (%) |
| Uterus | 5 (38.5) |
| Retroperitoneum | 4 (30.8) |
| Extremity | 3 (23.1) |
| Inferior vena cava | 1 (7.7) |
The mean size of pancreatic metastasis in our study was 2.0 cm (range, 1.0-3.5 cm). Table 2 summarizes the imaging characteristic of pancreatic metastasis from LMS. The location of pancreatic metastasis from LMS was the head in six patients (46.2%), body in two patients, tail in one patient, head and body in three patients, and body and tail in one patient. Pancreatic metastases from LMS were solitary in eight patients (61.5%) and multiple in five patients (38.5%). The pancreatic metastasis were hypovascular on the arterial phase in 10/13 (76.9%) patients and hypervascular in the remaining patients. On the venous phase, the enhancement was homogeneous in seven patients (53.8%), and heterogeneous in 6 patients (46.2%). CT evidence of necrosis was present in six tumors. Pancreatic ductal dilatation (mean, 4.7 mm; range, 3-8 mm) was noted in 3 patients (23.1%). None of the patients had CBD dilatation, CT evidence of pancreatitis, atrophy, peripancreatic lymphadenopathy, or vascular invasion.
| Imaging characteristic | Pancreatic metastasis from LMS (n = 13) |
| Age (yr) | 57 |
| Sex (male: female) | 2:11 |
| Size of largest (cm) | 2.0 ± 0.8 |
| Location | |
| Head | 6 (46.2) |
| Body | 2 (15.4) |
| Tail | 1 (7.7) |
| Head and body | 3 (23.1) |
| Body and tail | 1 (7.7) |
| Head and tail | 0 |
| Head, body, and tail | 0 |
| Solitary | 8 (61.5) |
| Multiple | 5 (38.5) |
| Tumor attenuation on arterial phase | |
| Hypovascular | 10 (76.9) |
| Hypervascular | 3 (23.1) |
| Homogeneity | |
| Homogeneous enhancement | 7 (54.8) |
| Heterogeneous enhancement | 6 (46.2) |
| Necrosis | 6 (46.2) |
| Pancreatic ductal dilatation | 3 (23.1) |
| Common bile duct dilatation | 0 (0) |
| Pancreatitis | 0 (0) |
| Atrophy | 0 (0) |
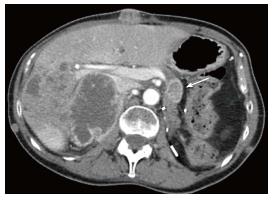
Of the 13 patients in our study, two patients with solitary pancreatic metastasis underwent Whipple’s resection. The remaining eleven patients received systemic treatment. In addition, prophylactic CBD stenting was performed in three patients with metastasis in the head/uncinate process. The median duration of follow-up after detection of pancreatic metastasis in our study was 19 mo (range, 1-48 mo). During follow-up, the two patients who underwent resection of the pancreatic metastasis remained free of pancreatic metastasis on the last follow-up scan. The remaining patients showed increase in mean tumor size from 2.0 cm to 3.9 cm (range, 1.0-7.3 cm). The number of pancreatic metastasis increased in four patients during follow-up (Figure 1).
In patients with pancreatic metastasis from LMS at the time of last follow-up, 12 out of the 13 patients have died at a median interval of 48 mo (interquartile range, 30-61 mo) after the diagnosis of metastases. The median survival after the diagnosis of pancreatic metastasis in these 13 patients was 19 mo (interquartile range, 8-34 mo). In two patients who underwent surgical resection, while one patient died 45 mo after the diagnosis of pancreatic metastasis, another patient was alive at the time of last follow-up. In 11 patients who underwent systemic chemotherapy, the median survival was 13 mo (interquartile range, 8-25 mo).
In our study, the most common site of origin of LMS in patients with pancreatic metastases was uterus (38.5%), followed by retroperitoneum (30.8%) and extremity (23.1%). There was no specific predilection of the metastasis for any part of the pancreas in our study. We found that metastasis to pancreas occurs later in the disease course in LMS at a median interval of 24 mo after the diagnosis of primary and was always preceded by metastasis to other sites like lungs. In a literature review of 333 pancreatic metastases, Minni et al[14] demonstrated that 77.6% of patients developed pancreatic metastasis metachronously at an interval of 9.2 years after the diagnosis of primary tumor. Awareness of late occurrence of pancreatic metastasis in LMS may justify long-term follow-up of these patients and necessitate caution while interpreting images. Pancreas as the first site of metastasis was not seen in any of the patients in our cohort and identification of a pancreatic mass in a patient with LMS who has no other sites of metastasis should raise the suspicion of alternate pathology like primary pancreatic malignancy.
Metastasis to pancreas can be solitary or multiple/diffuse. Pancreatic metastases from LMS in our study were more often solitary at the time of detection. This is in agreement with previous reports[14,15]. Solitary pancreatic metastasis can be difficult to differentiate from primary pancreatic malignancy. However in contrast to primary pancreatic cancer, pancreatic ductal dilatation, pancreatic atrophy, vascular invasion and peripancreatic adenopathy were uncommon in pancreatic metastasis from LMS which can help differentiate the two entities.
LMS can cause widespread hematogenous metastases, particularly to the lungs, liver, soft tissues and bones. Hepatic metastases from LMS are frequently hypervascular[16]. However, in contrast to hepatic metastasis, pancreatic metastases in 10 of 13 (76.9%) patients in our study were hypovascular on arterial phase. This may be explained by relative hypervascularity of the normal pancreatic parenchyma. Pancreatic metastases from RCC, which is the most common primary tumor to metastasize to the pancreas[2,4], are usually hypervasuclar[17].
Surgical resection of pancreatic metastasis is a relatively safe and useful procedure. Pancreatic resections can be performed with low mortality and morbidity rates[4,18,19]. Minni et al[14] in their study reviewed all cases of pancreatic metastasis reported in literature including 5 cases of LMS metastasis and found that 150 of 234 pancreatic metastases underwent pancreatic resection and concluded that surgical resection should be considered in patients with pancreatic metastasis as they tend to have prolonged survival. In our study, two patients with single metastasis underwent Whipple’s operation for pancreatic metastasis and the median survival after the diagnosis of pancreatic metastasis was 45 mo compared to the median survival was 13 mo in the 11 patients who underwent systemic chemotherapy. Therefore, surgical resection of solitary pancreas metastasis could be considered as a treatment option in the patients with pancreatic metastases from LMS.
Our study had several limitations. First, the number of study patients was small and this was retrospective study. Second, limitations of this study include a referral bias of the study populations that may confound our imaging findings. However, these limitations appear unavoidable when dealing with a disease as rare as pancreatic metastasis from LMS at a large referral center. Given the rare nature of this disease process and the lack of other large studies, we believe that our study adds to the existing knowledge about pancreatic metastasis from LMS.
To conclude, we present the MDCT features of pancreatic metastases from LMS. Pancreatic metastases from LMS commonly arise in the uterus and are characterized by a long latency period after the diagnosis of primary tumor. Although the imaging features of the pancreatic metastases from LMS are nonspecific, pancreatic metastases from LMS should be considered in the differential diagnosis of solitary or multiple hypovascular masses without pancreatic ductal dilatation, CBD dilatation, pancreatitis, atrophy in the pancreas in patients with history of LMS. Surgical resection of solitary LMS pancreatic metastasis can be considered due to the long survival of these patients after detection of pancreatic metastasis.
Pancreatic metastases are rare accounting for approximately 2%-5% of all malignant pancreatic neoplasms. The overall incidence of pancreatic metastasis in patients with diffuse metastatic disease varies between 3% and 12% in various autopsy series. The most common primary sites of leiomyosarcoma (LMS) include genitourinary tract, usually the uterus, retroperitoneum and gastrointestinal tract. The common sites of distant metastases from LMS are lungs, kidney, and liver. Pancreatic metastasis from LMS is extremely rare. Pancreatic metastases are often asymptomatic and can come to clinical attention on imaging either at the time of initial work or post-operative surveillance of non-pancreatic primary neoplasm. Less commonly, they can present with symptoms due to biliary obstruction. Characterization of pancreatic metastasis on imaging studies can be challenging as well as crucial because they can mimic primary pancreatic malignancy and determine the prognosis and management. Accordingly, the purpose of this article is to describe the multidetector computed tomography features of pancreatic metastasis from LMS.
Most of the radiology literature has been focused on the imaging features of pancreatic metastasis from renal cell carcinoma. Being a rare disease entity, the imaging features of pancreatic metastasis from LMS have not been reported so far. The available literature focuses on management and are small series of case reports.
In this study, the most common site of origin of LMS in patients with pancreatic metastases was uterus (38.5%), followed by retroperitoneum (30.8%) and extremity (23.1%). There was no specific predilection of the metastasis for any part of the pancreas in this study. The authors found that metastasis to pancreas occurs later in the disease course in LMS at a median interval of 24 mo after the diagnosis of primary and was always preceded by metastasis to other sites like lungs. In a literature review of 333 pancreatic metastases, Minni et al demonstrated that 77.6% of patients developed pancreatic metastasis metachronously at an interval of 9.2 years after the diagnosis of primary tumor. Awareness of late occurrence of pancreatic metastasis in LMS may justify long-term follow-up of these patients and necessitate caution while interpreting images. Pancreas as the first site of metastasis was not seen in any of the patients in this cohort and identification of a pancreatic mass in a patient with LMS who has no other sites of metastasis should raise the suspicion of alternate pathology like primary pancreatic malignancy.
Pancreatic metastases from LMS commonly arise in the uterus and are characterized by a long latency period after the diagnosis of primary tumor. Although the imaging features of the pancreatic metastases from LMS are nonspecific, pancreatic metastases from LMS should be considered in the differential diagnosis of solitary or multiple hypovascular masses without pancreatic ductal dilatation, common bile duct (CBD) dilatation, pancreatitis, atrophy in the pancreas in patients with history of LMS. Surgical resection of solitary LMS pancreatic metastasis can be considered due to the long survival of these patients after detection of pancreatic metastasis.
LMS is a rare and aggressive malignant tumor composed of cells of smooth muscle origin.
The author of this paper evaluated the MDCT features of pancreatic metastases from LMS. Pancreatic metastases from LMS should be considered in the differential diagnosis of solitary or multiple hypovascular masses without pancreatic ductal dilatation, CBD dilatation, pancreatitis, atrophy in the pancreas in patients with history of LMS.
P- Reviewer: Takebayashi S S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Ascenti G, Visalli C, Genitori A, Certo A, Pitrone A, Mazziotti S. Multiple hypervascular pancreatic metastases from renal cell carcinoma: dynamic MR and spiral CT in three cases. Clin Imaging. 2004;28:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Crippa S, Angelini C, Mussi C, Bonardi C, Romano F, Sartori P, Uggeri F, Bovo G. Surgical treatment of metastatic tumors to the pancreas: a single center experience and review of the literature. World J Surg. 2006;30:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Kassabian A, Stein J, Jabbour N, Parsa K, Skinner D, Parekh D, Cosenza C, Selby R. Renal cell carcinoma metastatic to the pancreas: a single-institution series and review of the literature. Urology. 2000;56:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Sohn TA, Yeo CJ, Cameron JL, Nakeeb A, Lillemoe KD. Renal cell carcinoma metastatic to the pancreas: results of surgical management. J Gastrointest Surg. 2001;5:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Rossi CR, Casali P, Kusamura S, Baratti D, Deraco M. The consensus statement on the locoregional treatment of abdominal sarcomatosis. J Surg Oncol. 2008;98:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Rose PG, Piver MS, Tsukada Y, Lau T. Patterns of metastasis in uterine sarcoma. An autopsy study. Cancer. 1989;63:935-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Taylor HB, Norris HJ. Mesenchymal tumors of the uterus. IV. Diagnosis and prognosis of leiomyosarcomas. Arch Pathol. 1966;82:40-44. [PubMed] |
| 8. | Alonso Gómez J, Arjona Sánchez Á, Martínez Cecilia D, Díaz Nieto R, Roldán de la Rúa J, Valverde Martínez A, Lizárraga Febres E, Padillo Ruiz J, Rufián Peña S. Uterine leiomyosarcoma metastasis to the pancreas: report of a case and review of the literature. J Gastrointest Cancer. 2012;43:361-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Iwamoto I, Fujino T, Higashi Y, Tsuji T, Nakamura N, Komokata T, Douchi T. Metastasis of uterine leiomyosarcoma to the pancreas. J Obstet Gynaecol Res. 2005;31:531-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Ozturk S, Unver M, Ozturk BK, Bozbıyık O, Erol V, Kebabcı E, Olmez M, Zalluhoglu N, Bayol U. Isolated metastasis of uterine leiomyosarcoma to the pancreas: Report of a case and review of the literature. Int J Surg Case Rep. 2014;5:350-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Wente MN, Kleeff J, Esposito I, Hartel M, Müller MW, Fröhlich BE, Büchler MW, Friess H. Renal cancer cell metastasis into the pancreas: a single-center experience and overview of the literature. Pancreas. 2005;30:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Hernández S, Martín-Fernández J, Lasa I, Busteros I, García-Moreno F. Pancreaticoduodenectomy for metastasis of uterine leiomyosarcoma to the pancreas. Clin Transl Oncol. 2010;12:643-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Tan CH, Tamm EP, Marcal L, Balachandran A, Charnsangavej C, Vikram R, Bhosale P. Imaging features of hematogenous metastases to the pancreas: pictorial essay. Cancer Imaging. 2011;11:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Minni F, Casadei R, Perenze B, Greco VM, Marrano N, Margiotta A, Marrano D. Pancreatic metastases: observations of three cases and review of the literature. Pancreatology. 2004;4:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Tsitouridis I, Diamantopoulou A, Michaelides M, Arvanity M, Papaioannou S. Pancreatic metastases: CT and MRI findings. Diagn Interv Radiol. 2010;16:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Shah SH, Jagannathan JP, Krajewski K, O’Regan KN, George S, Ramaiya NH. Uterine sarcomas: then and now. AJR Am J Roentgenol. 2012;199:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Palmowski M, Hacke N, Satzl S, Klauss M, Wente MN, Neukamm M, Kleeff J, Hallscheidt P. Metastasis to the pancreas: characterization by morphology and contrast enhancement features on CT and MRI. Pancreatology. 2008;8:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z’graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310-1314; discussion 1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 451] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 19. | Reddy S, Edil BH, Cameron JL, Pawlik TM, Herman JM, Gilson MM, Campbell KA, Schulick RD, Ahuja N, Wolfgang CL. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann Surg Oncol. 2008;15:3199-3206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |