Published online Feb 28, 2016. doi: 10.4329/wjr.v8.i2.210
Peer-review started: July 30, 2015
First decision: October 30, 2015
Revised: December 4, 2015
Accepted: December 18, 2015
Article in press: December 21, 2015
Published online: February 28, 2016
Processing time: 213 Days and 23.3 Hours
AIM: To report the results of functional magnetic resonance imaging (fMRI) studies pertaining internet addiction disorder (IAD) in young adults.
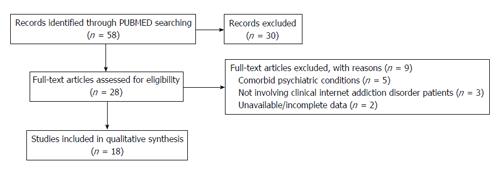
METHODS: We conducted a systematic review on PubMed, focusing our attention on fMRI studies involving adult IAD patients, free from any comorbid psychiatric condition. The following search words were used, both alone and in combination: fMRI, internet addiction, internet dependence, functional neuroimaging. The search was conducted on April 20th, 2015 and yielded 58 records. Inclusion criteria were the following: Articles written in English, patients’ age ≥ 18 years, patients affected by IAD, studies providing fMRI results during resting state or cognitive/emotional paradigms. Structural MRI studies, functional imaging techniques other than fMRI, studies involving adolescents, patients with comorbid psychiatric, neurological or medical conditions were excluded. By reading titles and abstracts, we excluded 30 records. By reading the full texts of the 28 remaining articles, we identified 18 papers meeting our inclusion criteria and therefore included in the qualitative synthesis.
RESULTS: We found 18 studies fulfilling our inclusion criteria, 17 of them conducted in Asia, and including a total number of 666 tested subjects. The included studies reported data acquired during resting state or different paradigms, such as cue-reactivity, guessing or cognitive control tasks. The enrolled patients were usually males (95.4%) and very young (21-25 years). The most represented IAD subtype, reported in more than 85% of patients, was the internet gaming disorder, or videogame addiction. In the resting state studies, the more relevant abnormalities were localized in the superior temporal gyrus, limbic, medial frontal and parietal regions. When analyzing the task related fmri studies, we found that less than half of the papers reported behavioral differences between patients and normal controls, but all of them found significant differences in cortical and subcortical brain regions involved in cognitive control and reward processing: Orbitofrontal cortex, insula, anterior and posterior cingulate cortex, temporal and parietal regions, brain stem and caudate nucleus.
CONCLUSION: IAD may seriously affect young adults’ brain functions. It needs to be studied more in depth to provide a clear diagnosis and an adequate treatment.
Core tip: We systematically reviewed the functional magnetic resonance imaging studies on adults affected by internet addiction disorder (IAD), without any other psychiatric condition. We found 18 studies, mostly conducted in East Asia and enrolling young males with internet gaming disorder. Internet addicts showed functional alterations in regions involved in cognitive control and reward/punishment sensitivity (orbitofrontal cortex, anterior and posterior cingulate, insula, dorsolateral prefrontal cortex, temporoparietal regions, brain stem and caudate nucleus) that are similar to those observed in substance use disorder. IAD is a disabling condition needing careful consideration due to its severe impact on young people’s brain functioning.
- Citation: Sepede G, Tavino M, Santacroce R, Fiori F, Salerno RM, Di Giannantonio M. Functional magnetic resonance imaging of internet addiction in young adults. World J Radiol 2016; 8(2): 210-225
- URL: https://www.wjgnet.com/1949-8470/full/v8/i2/210.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i2.210
Internet addiction disorder (IAD), also called pathologic/problematic internet use (PIU), may be defined as an impulse control disorder characterized by an uncontrolled Internet use, associated with a significant functional impairment or clinical distress[1]. IAD is not classified as a mental disorder in the Diagnostic and Statistical Manual of Mental Disorders-fifth edition, but a subtype of IAD, the internet gaming disorder (IGD) (also called videogame addiction), is included in the section 3 as a topic deserving future studies[2]. A recent meta-analysis on IAD[3] involving more than 89000 participants from 31 nations reported a global prevalence estimate of 6%, with the higher prevalence in the Middle East (10.9%) and the lowest prevalence in Northern and Western Europe (2.6%). A higher prevalence of IAD was significantly associated with lower subjective and environmental conditions. A recent study conducted on Indian college students[4] reported 8% of moderate IAD and identified the following variables as risk factors: Male gender, continuous availability online, using the internet more for making new friendships/relationships and less for coursework/assignment. Due to their high computer skill and easy Internet access, young adults are at augmented risk for IAD[5].
Some of the clinical characteristics of IAD are similar to those observed in behavioral or substance misuse disorders (loss of control, craving, withdrawal symptoms), Obsessive Compulsive Disorder, or Bipolar Disorder so the nature of IAD (primary psychiatric disorder or “online variant” of other psychiatric conditions) is still debated[6-9].
Functional imaging techniques increase the possibility to investigate the neural basis of IAD, enhancing the sensitivity and the statistical power of clinical data. Functional magnetic resonance imaging (fMRI), in particular, is a worldwide used non-invasive technique to study the neural underpinnings of psychiatric disorders[10-12]. By means of fMRI, brain signal changes may be analyzed in terms of functional fluctuations with respect to a given “baseline” (activations/deactivations analysis) or in terms of functional connectivity among different brain regions (network analysis). Metabolic activity changes in the brain can be monitored during the execution of paradigms (task related fMRI) or during the spontaneous cerebral activity (resting state fMRI)[13-16].
Aim of the present study was to systematically review the resting state and task related fMRI studies conducted on adult subjects with IAD, looking for reliable biomarkers of this challenging mental condition.
We searched PubMed to identify fMRI studies investigating IAD in adult subjects. The following search words were used, both alone and in combination: fMRI, Internet addiction, Internet dependence, functional neuroimaging. The search was conducted on April 20th, 2015 and yielded 58 records.
Inclusion criteria were the following: Articles written in English, patients’ age ≥ 18 years, patients affected by IAD, studies providing fMRI results during resting state or cognitive/emotional paradigms. Structural MRI studies, functional imaging techniques other than fMRI, studies involving adolescents, patients with comorbid psychiatric, neurological or medical conditions were excluded.
By reading titles and abstracts, we excluded 30 records. By reading the full texts of the 28 remaining articles, we identified 18 papers meeting our inclusion criteria and therefore included in the qualitative synthesis (Figure 1).
Statistics were performed by Dr. Gianna Sepede, who has a certificated experience in Biomedical Statistics, Systematic Reviews and Meta-analysis. In the present paper, PRISMA 2009 checklist (http://www.prisma-statement.org/) was used to describe eligibility criteria, conduct the search, select the studies and report the qualitative synthesis results. Statistical methods were therefore adequately described, correct and conducted on homogeneous data. Number of subjects and dropouts were given. When appropriate, confidence limits and significant P values were calculated and reported.
We found 18 papers fulfilling our inclusion criteria, all published from 2009 to 2015[17-35]. The studies were all conducted in the Asian Continent (China, South Korea, Taiwan), with the only exception of the paper published by Lorenz et al[23], which was conducted in Germany.
In total, 666 subjects were tested by the 18 studies included in the qualitative synthesis: 347 patients with IAD (IADp), 304 normal comparisons (NC) and 15 subjects with Alcohol Use Disorder (AUDp) The large majority of IADp were male (n = 331, 95.4%) and very young (mean age ranged from 21 to 25 year). The number of patients involved in each study ranged from 8 to 74. For what regards the subtypes of IAD, 15 out of 18 studies focused on IGD[19-24,26-34], so more than 85% of all the IADp (n = 297) were IGD patients (IGDp). Different diagnostic criteria were used to assess IAD, such as Beard’s Diagnostic criteria for Internet addiction[35], Ko’s diagnostic criteria of Internet addiction for college students[36], Chinese Internet addiction test (C-IAT)[37] and Grüsser and Thalemann’s computer game addiction criteria[38].
The most used questionnaire to assess the severity of IAD was the Young’s IAT[1], with different cut-off (usually > 80, in a few studies > 50). To diagnose IGD, online gaming was also required to be the principal Internet activity (more than 80% of the time spent online or more than 30 h/wk).
In order to exclude subjects with comorbid psychiatric conditions or substance use disorders, structured interviews and psychometric scales to address depression, anxiety, impulsivity, substance addiction were usually provided.
MRI data were acquired with a 3 T scanner in 17 studies, and with a 1.5 T scanner in one study[19]. In 4 articles, only resting state fMRI was recorded, whereas 13 articles reported task related fMRI data, and one paper acquired both resting state and task related functional activations[31]. Seventeen studies were transversal observational reports, whereas the paper by Han et al[19] was a 6-wk longitudinal study.
The participants in the 18 selected studies were all free of any psychopharmacological treatment at the moment of the scanning (and at study enter for the above mentioned longitudinal study).
A total number of five studies were selected[18,21,31,32,34]. The characteristic of the groups and the results of the studies are reported in Table 1. Right-handedness was an inclusion criterion in 4 studies[18,21,31,34], as well as male gender[21,31,32,34]. A total number of 298 subjects (Males n = 280, 94%), all medication free, were involved: 159 IADp (140 IGDp), 124 NC and 15 AUDp. Patients were usually very young (mean age ranging from 21 to 24 years).
| Ref. | Design and aims | Participants | Diagnostic criteria and evaluation scales | fMRI methods | fMRI results |
| Liu et al[18] | Resting state fMRI study | n = 38, age range 18-25 yr | IAD: Beard's DQIA "5 + | Scanner: 3 T | Between group significant effects: |
| Aim: To analyze encephalic | Medication free 100% | 1 criteria" plus any one | FMRI Scan duration: | ReHo | |
| functional characteristic of | Right-handed 100% | of: ≥ 6 h/d for 3 mo | 9 min | IAD > NC in: Cerebellum, | |
| IAD under resting state | Normal neurological examination 100% | Decline in academic performance | Software used: SPM2 | brainstem, R CG, bilateral PH, R FL, L SFG, L precuneus, R PoCG, | |
| No comorbid psychiatric disorders | Unable to maintain normal school learning | ReHo measured by means of KCC | R MOG, R ITG, L STG, MTG | ||
| Groups: IGA n = 19 | Signal analyzed: | ||||
| Mean age: 21.0 ± 1.3 yr | BOLD | ||||
| Males n = 11 (57.9%) | Both whole brain and | ||||
| NC n = 19 (50%) | ROI based analysis | ||||
| Mean age: 20.0 ± 1.8 yr | |||||
| Males n = 11 (57.9%) | |||||
| Dong et al[21] | Resting state fMRI study | n = 29, age 24.2 ± 3.8 yr | IGD: YIAT ≥ 80 | Scanner: 3 T | Between group significant effects: |
| Aim: To investigate the | Males 100% | > 80% of the online time | FMRI Scan duration: | ReHo | |
| effects of long-time online | Medication free 100% | was spent playing | 9 min | IGA > NC in: Bilateral brainstem, | |
| game playing on visual and | Right-handed 100% | videogames | Software used: | bilateral IPL, L posterior | |
| auditory brain regions | No nicotine, cocaine or marijuana use | BDI < 5; MINI: No Axis I | DPARSF; ReHo measured by | cerebellum, L MiFG; IGA < NC in: L STG, L ITG, L OL, | |
| Groups: IGD n = 15; Age 24.2 ± 3.5 yr | psychiatric disorders | means of KCC; Signal analyzed: | L PL | ||
| NC n = 14; Age 24.6 ± 3.8 yr | BOLD; Both whole brain and | ||||
| ROI based analysis | |||||
| Seed based connectivity analysis | |||||
| 1Dong et al[31] | Resting-state and task | n = 71 | IGD: Young's IAT ≥ 50 | Scanner: 3 T | Between group significant effects: |
| related fMRI | Age 22.35 | > 80% of the online time | Rs fMRI Scan | RsFc | |
| Aim: To examine the Fc of | Males 100% | was spent playing | duration: 7 min | IGD < NC in: Total ECN and L | |
| ECN during both resting | Medication free 100% | videogames | Software used: REST, | ECN | |
| state and Stroop task | Right-handed 100% | BDI < 5 | DPARSF, SPM8, FSL | ||
| performing | No DSM 5 psychiatric disorders | MINI: No Axis I psychiatric disorders | Signal analyzed: BOLD | ||
| Groups: IGD n = 35 | Whole brain analysis | ||||
| Age 22.2 ± 3.8 yr | |||||
| NC n = 36 | |||||
| Age 22.8 ± 2.4 yr | |||||
| Kim et al[32] | Resting state fMRI study | n = 45 | For all participants: | Scanner: 3 T | Between group significant effects: |
| Aim: To compare the brain | Males 100% | WAIS III ≥ 80 | Rs fMRI Scan | ReHo | |
| functioning of IGD, AUD, | Medication free 100% | For IGD: YIAT ≥ 70 | duration: 8 min | (1) IGD vs NC | |
| and NC during resting state | Groups: IGD n = 16 | > 4.5 h/d were spent | Software used: | IGD > NC in L PCC | |
| Age 21.6 ± 5.9 yr | playing online | DPARSF, SPM8, | IGD < NC in R STG | ||
| AUD n = 14 | For AUD: SCID criteria | REST | (2) IGD vs AUD | ||
| Age 28.6 ± 5.9 yr | AUDIT-K | ReHo measured by | IGD < NC in R STG | ||
| NC n = 15 | < 2 h/d were spent | means of KCC | (3) AUD vs NC | ||
| Age 25.4 ± 5.9 yr | online | Signal analyzed: | AUD > NC in R PCC, R insula, L | ||
| IGD were significantly | Other scales | BOLD | MTG | ||
| younger than AUD | administered to all | Whole brain analysis | AUD < NC in R ACC | ||
| (P < 0.01) | subjects: | ||||
| BDI: IGD and AUD > NC (P < 0.01) | |||||
| BAI: AUD > NC (P < 0.01) | |||||
| BIS-11: IGD and AUD > NC (P < 0.01) | |||||
| Zhang et al[34] | Resting state fMRI study | n = 115 | IGD: CIAS ≥ 67 | Scanner: 3 T | Between group significant effects: |
| fMRI in young adults with Internet gaming disorder | Males 100%; Medication free 100% | Internet gaming > 14 h/wk for 1 yr | Rs fMRI Scan duration: 7 min | RsFC; L anterior insula | |
| using rsFC | Right-handed 100%; Groups: IGD n = 74 | Playing as the principal online activity | Software used: DPABI, REST, SPM8 | IGD > NC in R putamen, R angular gyrus, IFG | |
| Aim: To study resting-state | age 22.3 ± 2 yr; n = 57 alcohol drinkers | NC: CIAS < 60 | Signal analyzed: BOLD | R anterior insula; IGD > NC in ACC, middle CG, | |
| functional connectivity of | n = 8 cigarette smokers | internet gaming < | Seed based | L angular gyrus, L precuneus, | |
| the insula in IGD | NC n = 41; | 2 h/wk | connectivity analysis | Bilateral SFG and STG | |
| age 23.0 ± 2.1 yr | Other scales: FTND | L posterior insula | |||
| n = 29 alcohol drinkers | BDI: IGD > NC | IGD > NC in bilateral PoCG, L | |||
| Cigarette use and frequency of alcohol use were higher in IGD with respect to NC (P < 0.05 and P < 0.01) | (P < 0.001); BAI: IGD > NC (P < 0.01) | precentral gyrus, R SMA, STG; R posterior insula; IGD > NC in bilateral STG | |||
| No comorbid psychiatric disorders |
In all the five selected studies, fMRI images were acquired using a 3 T scanner and scan duration ranged from 7 to 9 min. Resting state functional connectivity (RsFc) and/or Regional Homogeneity (ReHo) were calculated to assess between group differences. As a result, all the selected studies identified significant differences between patients and controls.
Liu et al[18], in their research on 19 IAD patients, reported an increased synchronization among frontal areas, cingulate gyrus, temporal and occipital regions, cerebellum and brain stem, with respect to matched normal comparisons. So the authors suggested an altered functional connectivity in regions belonging to the reward system of the brain. All the four papers focused on IGD patients[21,31,32,34] reported significant between group effects. Dong et al[21] observed that, when compared to controls, IGD patients showed an enhanced ReHo in sensorimotor coordination areas (brainstem, cerebellum, bilateral inferior parietal lobule, and left middle frontal gyrus), and a reduced ReHO in left-sided visual and auditory cortex. In a larger sample of IGD patients, Dong and colleagues[31] observed a reduced functional connectivity in areas belonging to the Executive Control Network, especially in the left hemisphere: Ventromedial prefrontal cortex, dorsolateral prefrontal cortex and parietal cortex.
In a recent study, Kim et al[32] compared the resting state brain functioning of IGD patients not only with healthy subjects, but also with a group of AUD patients, looking for similarities and differences between these two “addictive conditions”. As a result, they found that both IGD and AUD shared an augmented ReHo in posterior cingulate cortex with respect to healthy controls, whereas a reduced ReHo in the right superior temporal gyrus was observed in the IGD patients only. The authors also reported a negative correlation between the left inferior temporal cortex and the level of impulsivity.
To assess the role of the insular cortex in IGD, Zhang et al[34] conducted a seed-based resting state connectivity study in 74 patients with IGD and compared them with 41 normal controls. IGD patients exhibited enhanced rsFC between the anterior insula and anterior cingulate cortex, precuneus, angular gyrus and basal ganglia (all areas involved in cognitive control, salience, attention and craving). When analyzing the posterior part of the insula, they found an augmented rsFC in areas playing a key role in sensory-motor integration, such as post central and precentral gyrus, supplementary motor area and superior temporal gyrus. Moreover, they observed a positive correlation between the insula-superior temporal gyrus connectivity and the level of IGD severity.
Summarizing the rsfMRI studies, the more relevant abnormalities observed in IGD were localized in the superior temporal gyrus. Other important alterations were detected in limbic areas, medial frontal regions (anterior cingulate cortex, supplementary motor area) and parietal regions. Results in not gaming IAD were limited due to the small number of patients involved (n = 19) and reported altered functioning in reward-related brain regions (frontal, parietal, temporal regions, cingulated gyrus, brain stem and cerebellum).
We found 14 studies reporting task-related neural correlates of IAD[17,19,20,22-31,33]. The characteristic of the groups and the results of the studies are reported in Table 2. Right-handedness was an inclusion criterion in all but two studies[19,23]. Only male participants were included in 13 studies, whereas a mixed gender sample was enrolled by Liu et al[33] (2015).
| Ref. | Design and aims | Participants | Diagnostic criteria and evaluation scales | Task and behavioral results | fMRI methods | fMRI results |
| Ko et al[17] | Task related fMRI study | n = 20; Males 100% | DCIA-C | Task used: Cue-reactivity paradigm. | Scanner: 3 T | Between group significant effects: IGD > NC in: R OFC, R basal ganglia (caudatum and accumbens), bilateral ACC, bilateral MFG, R DLPFC |
| Aim: To identify the neural substrates of IGD by means of a cue-reactivity paradigm | Medication free 100% | MINI | Task design: Videogame viewing | fMRI scan duration: 4.8 min | ||
| Right-handed 100% | CIAS | Behavioral results: Gaming craving: IGD > NC | Acquisition method: Block design | |||
| Normal neurological examination 100% | Software used: SPM2 | |||||
| No comorbid psychiatric disorders or substance use | AUDIT | Signal analyzed: BOLD | ||||
| Groups: | FTND | Whole brain and ROI based analysis | ||||
| IGD n = 10 | Gaming craving scale | |||||
| Mean age: 22 ± 1.5 yr | For IGD: Addiction to World of Warcraft | |||||
| NC n = 10 | Playing > 30 h/wk | |||||
| Mean age: 22.7 ± 1.3 yr | ||||||
| Han et al[19] | Six-week open label pharmacological study with task related fMRI acquisition | n = 19; Males 100% | SCID | Task used: Cue-reactivity paradigm. | Scanner: 1.5 T | Between group significant effects: |
| Aim: To evaluate the efficacy of bupropion SR in reducing game craving and influencing brain activity in IGD | Medication free (at study enter) 100% | BDI < 17 | Task design: Videogame viewing | fMRI scan duration: 7.5 min | At baseline: | |
| Normal neurological examination 100% | 7 point Gaming Craving VAS | Behavioral results: Gaming craving: IGD > NC | Acquisition method: Block design | (1) IGD > NC in: L occipital lobe, cuneus, L DLPFC, L PH | ||
| No comorbid psychiatric disorders or substance use disorders | For IGD: YIAT > 50 | Bupropion effects in the IGD group: Significant decreases of: Craving (23.6%, P = 0.04) | Software used: Brain voyager | After 6 wk of Bupropion treatment on IGD: | ||
| Groups: IGD n = 11 | Playing > 4 h/d and 30 h/wk | Playing game time (35.4%, P = 0.01) | Signal analyzed: BOLD | (2) Significant decreased activation in L DLPFC | ||
| Mean age: 21.5 ± 5.6 yr | Addiction to star craft | YIAT scores (15.4%, P = 0.01) | Acquisition time: | |||
| Study treatment: Bupropion SR for 6 wk | (1) At study enter (baseline); | |||||
| NC n = 8 | (2) After 6 wk of Bupropion treatment | |||||
| Mean age: 20.3 ± 4.1 yr | Whole brain analysis | |||||
| Dong et al[20] | Task related fMRI study | n = 27; Males 100% | MINI | Task used: Guessing task | Scanner: 3 T | Between group significant effects: |
| Aim: To investigate reward and punishment processing in IGD during a guessing task | Medication free 100% | For IGD: YIAT > 80 | Task design: Two-choices gain or loss guessing task | fMRI scan duration: 16.3 min | In WIN condition: IGD > NC in L OFC (BA 11) | |
| Right-handed 100% | C-IAT criteria | Behavioral results: No between group significant differences in accuracy and reaction times | Acquisition method: Block design | In LOSS condition: NC > IGD in ACC | ||
| Normal neurological examination 100% | Spending most of their time playing online Internet games | Software used: SPM5 | ||||
| No comorbid psychiatric disorders or substance use disorders | For NC: YIAT < 20 | Signal analyzed: BOLD | ||||
| Groups: IGD n = 14 | Whole brain analysis | |||||
| Mean age: 23.4 ± 3.3 yr | ||||||
| NC n = 13 | ||||||
| Mean age: 24.1 ± 3.2 yr | ||||||
| Dong et al[22] | Task related fMRI study | n = 24; Males 100% | For all participants: BDI < 13 | Task used: Cognitive control task | Scanner: 3 T | Between group significant effects: |
| Aim: To identify the neural correlates of response inhibition in individuals with and without IGD | Medication free 100% | For IGD: YIAT > 80 | Task design: Three-choices color-word Stroop task | fMRI scan duration: 12 min | During Stroop effect: IGD > HC in: ACC, PCC, L insula, MiFG, MFG, L thalamus, R IFG, R SFG | |
| Right-handed 100% | C-IAT criteria | Behavioral results: No between group significant differences | Acquisition method: Event-related design | |||
| No comorbid psychiatric disorders or substance use disorders | Spending most of their time playing online Internet games | Software used: SPM5 | ||||
| Non smokers 100% | YIAT < 20 | Signal analyzed: BOLD | ||||
| Groups: IGD n = 12 | Whole brain analysis | |||||
| Mean age: 23.6 ± 3.5 yr | ||||||
| NC n = 12 | ||||||
| Mean age: 24.2 ± 3.1 yr | ||||||
| Lorenz et al[23] | Task related fMRI study | n = 17; Males 100% | World of warcraft addiction inventory | Task used: Attentional bias/cue reactivity task | Scanner: 3 T | Between group significant effects |
| Aim: To assess neural correlates of attentional bias and cue reactivity in IGD | Groups: IGD n = 8 | CSVK | Task design: Two-choice dot probe paradigm during SP and LP trials | fMRI scan duration: 30 min | During SP trials | |
| Mean age: 25 ± 7.4 yr | Vocabulary test (WST-IQ) | Stimulus class: | Acquisition method: Block design | IGD > NC in bilateral ACC, R MPFC, L OFC, L PH, MTG, precuneus, cerebellum, R amygdala | ||
| NC n = 9 | Test of attention | (1) IAPS based emotional images (neutral and positive valences) | Software used: SPM8b | During LP trials IGD > NC in: R IFG, R Hippocampus, bilateral lingual gyrus and R calcarine gyrus | ||
| Mean age: 24.8 ± 6.9 yr | Social interaction anxiety scale | (2) Computer generated stimuli (neutral images and World of Warcraft based images) | Signal analyzed: BOLD | PPI results: IGD > NC in connectivity between R IFG and: | ||
| STAI | Behavioral results: | Whole brain analysis | IFG, orbital gyrus, MFG, MTG, MOG, STG, ITG, Angular gyrus, precuneus, basal ganglia | |||
| BDI | In SP trials: IGD: RT congruent < RT incongruent | Connectivity analysis: Post hoc PPI, using R IFG as seed region | ||||
| BIS 11 | ||||||
| Iowa Gambling test | ||||||
| For IGD: ≥ 3 Grüsser and Thalemann’s criteria for computer game addiction | ||||||
| Dong et al[24] | Task related fMRI study | n = 30; Males 100% | MINI | Task used: Cognitive control task | Scanner: 3 T | Between group significant effects |
| Aim: To investigate error-monitoring ability in IGD | Medication free 100% | For IGD | Task design: Three-choices color-word Stroop task | fMRI scan duration: 12 min | During correct responses: | |
| Right-handed 100% | YIAT > 80 | Focus: Error monitoring | Acquisition method: Event-related design | IGD < NC in OFC and ACC | ||
| Non smokers 100% | Spending > 80% of their time online playing games | Behavioral results: No significant between | Software used: SPM8 | During incorrect responses: IGD > NC in ACC | ||
| No comorbid psychiatric disorders or substance use disorders | For NC: YIAT < 30 | group effects | Signal analyzed: BOLD | |||
| Groups: IGD n = 15 | Whole brain analysis | |||||
| Mean age: 23.8 ± 3.7 yr | ||||||
| NC n = 15 | ||||||
| Mean age: 24.1 ± 3.3 yr | ||||||
| Dong et al[25] | Task related fMRI study | n = 31; Males 100% | MINI | Task used: Guessing task | Scanner: 3 T | Between group significant effects |
| Aim: To investigate brain correlates of decision-making in IAD | Medication free 100% | BDI < 5 | Task design: Two-choices gain or loss guessing task | fMRI scan duration: 21 min | In WIN condition: IAD > NC in: ACC, insula and IFG | |
| Right-handed 100% | For IAD: YIAT > 80 | Behavioral results: | Acquisition method: Block design | IAD < NC in: PCC and caudatum | ||
| No comorbid psychiatric disorders or substance use disorders | For NC: YIAT < 30 | In LOSS condition: RT | Software used: SPM5 | In LOSS condition: IAD > NC in: Inferior CG | ||
| Groups: IAD n = 16 | IAD > NC | Signal analyzed: BOLD | IAD < NC in: PCC | |||
| Mean age 21.4 ± 3.1 yr | Whole brain analysis | |||||
| NC n = 15 | ||||||
| Mean age: 22.1 ± 3.6 yr | ||||||
| Dong et al[26] | Task related fMRI study | n = 31; Males 100% | MINI | Task used: Guessing task | Scanner: 3 T | Between group significant effects |
| Aim: To investigate reward/punishment sensitivities in IGD during a guessing task | Medication free 100% | BDI < 5 | Task design: Two-choices gain or loss guessing task | fMRI scan duration: 21 min | In WIN condition: IGD > NC in L SFG | |
| Right-handed 100% | For IGD: YIAT > 80 | No behavioral response was required | Acquisition method: Block design | In LOSS condition: IGD > NC in L SFG | ||
| No comorbid psychiatric disorders or substance use disorders | Spending > 80% of their time online playing games | Post scanning self-report questionnaire | Software used: SPM5 | IGD < NC in bilateral PCC | ||
| Groups: IGD n = 16 | For NC: YIAT < 30 | (1) On subjective experiences | Signal analyzed: BOLD | In WIN-LOSS contrast condition | ||
| Mean age 21.4 ± 3.1 yr | During LOSS condition: | Whole brain analysis | IGD > NC in L SFG | |||
| NC n = 15 | IGD < NC in reporting negative emotions | |||||
| Mean age: 22.1 ± 3.6 yr | (2) On craving for win: | |||||
| IGD > NC in both WIN and LOSS conditions | ||||||
| Dong et al[27] | Task related fMRI study | n = 30; Males 100% | MINI | Task used: Cognitive control task | Scanner: 3 T | Between group significant effects |
| Aim: To explore cognitive flexibility in IGD during a color-word Stroop task | Medication free 100% | BDI < 5 | Task design: Three-choices color-word Stroop task | fMRI scan duration: 16 min | Task switching | |
| Right-handed 100% | For IGD: YIAT > 80 | Focus: Cognitive flexibility during task switching (from easy to difficult condition and viceversa) | Acquisition method: Event-related design | (1) From difficult to easy condition | ||
| No comorbid psychiatric disorders or substance use disorders | Spending > 80% of their time online playing games | Behavioral results: No significant between group differences | Software used: SPM5 | IGD > NC in: Bilateral insula, R STG | ||
| Groups: IGD n = 15 | For NC: YIAT < 30 | Signal analyzed: BOLD | (2) From easy to difficult condition: IGD > NC in: Bilateral precuneus, L STG, L angular gyrus | |||
| Mean age 21.2 ± 3.2 yr | Whole brain analysis | |||||
| NC n = 15 | ||||||
| Mean age: 22.1 ± 3.6 yr | ||||||
| Ko et al[28] | Task related fMRI study | n = 49; Males 100% | MINI | Task used: Cognitive control task | Scanner: 3 T | Between group significant effects |
| Aim: To evaluate impulsivity and brain correlates of response inhibition and error processing in IGD | Medication free 100% | CIAS | Task design: Go/No-go Task | fMRI scan duration: 15.5 min | During response inhibition | |
| Right-handed 100% | BIS-11 | Behavioral results: No significant between group differences | Acquisition method: event-related design | IGD > NC in bilateral caudate and L OFG (BA 47) | ||
| No comorbid psychiatric disorders or substance use disorders | Dickman’s impulsivity scale | Software used: SPM5 | During error processing | |||
| Groups: IGD n = 26 | For IGD: Fulfilling DCIA criteria | Signal analyzed: BOLD | IGD < NC in R insula | |||
| Mean age 24.6 ± 3.2 | Addiction to online gaming | Whole brain and ROI based analysis | ||||
| NC n = 23 | ||||||
| Mean age: 24.4 ± 2.1 yr | ||||||
| Liu et al[29] | Task related fMRI study | n = 22; Males 100% | DCIA-C | Task used: Cognitive control task | Scanner: 3 T | Between group significant effects |
| Aim: To investigate brain correlates of response inhibition under gaming cue distraction in IGD | Medication free 100% | MINI | Task design: Modified Go/no-go Task with gaming cue distracters | fMRI scan duration: 8.5 min | During no gaming distracting condition | |
| Right-handed 100% | CIAS | Behavioral results: During gaming distracting condition | Acquisition method: Block design | IGD > NC in R SPL | ||
| No comorbid psychiatric disorders or substance use disorders | FTND < 5 | Commission errors IGD > NC | Software used: SPM5 | During gaming distracting condition | ||
| Groups: IGD n = 11 | Signal analyzed: BOLD | NC > IGD in R DLPFC, R SPL and cerebellum | ||||
| Mean age 23.4 ± 2.3 yr | Whole brain and ROI based analysis | ROI based analysis results | ||||
| NC n = 11 | In IGD | |||||
| Mean age: 22.4 ± 1.7 yr | R DLPFC and R SPL activations were positively associated to commission errors during gaming distracting condition | |||||
| Chen et al[30] | Task related fMRI study | n = 30; Males 100% | MINI | Task used: Cognitive control task | Scanner: 3 T | Between group significant effects |
| Aim: To evaluate neural correlates of response inhibition among subjects with IGD | Medication free 100% | CIAS | Task design: Go/no-go Task | fMRI scan duration: 6 min | During response inhibition | |
| Right-handed 100% | Behavioral results: No significant between group differences | Acquisition method: Block design | NC > IGD in R SMA/pre-SMA | |||
| No comorbid psychiatric disorders or substance use disorders | BIS-11 | Software used: SPM5 | ||||
| Groups: IGD n = 15 | For IGD: Fulfilling DCIA criteria | Signal analyzed: BOLD | ||||
| Mean age 24.7 ± 3.1 yr | Addiction to World of Warcraft | ROI based analysis | ||||
| NC n = 15 | ||||||
| Mean age: 24.5 ± 2.8 yr | ||||||
| 1Dong et al[31] | Resting-state and task related fMRI | All participants: n = 71 age 22.35 | IGD: YIAT ≥ 50 | Task used: Cognitive control task | Scanner: 3 T | Between group significant effects |
| Aim: To examine the Fc of ECN during both resting state and Stroop task performing | Participants who performed the fMRI Stroop task: n = 35 | > 80% of the online time was spent playing videogames | Task design: Three-choices color-word Stroop task | fMRI scan duration: 15 min | During incongruent trials: IGD > NC in bilateral SFG | |
| Males 100% | BDI < 5 | Behavioral results: No significant between group differences | Acquisition method: Event-related design | IGD < NC in L | ||
| Medication free 100% | MINI: No Axis I psychiatric disorders | Software used: SPM8 | DLPFC, ACC and left OFC | |||
| Right-handed 100% | IAD | Signal analyzed: BOLD | ||||
| No DSM 5 psychiatric disorders | Whole brain and ROI based analysis | |||||
| Groups performing fMRI Stroop task: | ||||||
| IGD n = 16 | ||||||
| NC n = 15 | ||||||
| Liu et al[33] | Task related fMRI study | n = 38; Males 58% | HAM-A | Task used: Cue-reactivity paradigm. | Scanner: 3 T | Between group significant effects |
| Aim: To investigate brain function in IGD individuals during a cue-reactivity paradigm | Medication free 100% | BDI | Task design: Videogame viewing | fMRI scan duration: 7.5 min | IGD > NC in: R SPL, R precuneus, R insula, R CG, R STG, L brain stem | |
| Right-handed 100% | For IGD: Beard's DQIA "5 + 1 criteria" plus any one of: ≥ 6 h/d for 3 mo; Decline in academic performance; Unable to maintain normal school learning | No behavioral response was required | Acquisition method: Block design | |||
| No comorbid psychiatric disorders or substance use disorders | Software used: Brain Voyager | |||||
| Groups: IGD n = 19 | Signal analyzed: BOLD | |||||
| Males n = 11 (58%) | Whole brain analysis | |||||
| Mean age 21.4 ± 1.0 yr | ||||||
| NC n = 19 | ||||||
| Mean age: 20.1 ± 1.1 yr | ||||||
| Males n = 11 (58%) |
A total number of 368 subjects (males n = 352, 95.6%: Mean age ranging from 21 to 25 years) were involved: 188 IADs (IGDs n = 157) and 180 NC. Participants were all medication free at the moment of the scanning and at study enter for the longitudinal study by Han et al[19]. FMRI images were acquired using a 3 T scanner and scan duration ranged from 5 to 30 min.
The paradigms administered to the participants were: cue-reactivity tasks (three studies)[17,19,33], guessing tasks (three studies)[20,25,26] or cognitive control tasks of different kinds (eight studies)[22-24,27-31]. In more than half of the studies[20,22,24,27,28,30,31,33] no behavioral differences were found between cases and controls, but all of them reported significant group effects in functional activation of several brain regions, especially orbitofrontal gyrus, anterior cingulate cortex, insula, dorsolateral prefrontal cortex, precuneus, posterior cingulate cortex and superior temporal gyrus.
In cue-reactivity paradigms, addicted subjects are exposed to stimuli designed to elicit a craving for substance or behavior: In case of IAD, i.e., viewing images or videos related to videogames or Internet scenarios[17,39,40].
In probabilistic guessing tasks, participants are required to bet on different outcomes (i.e., on cards, dices, colors) and their brain response to win or loss conditions can be analyzed, to evaluate reward and punishment neural systems[41].
In cognitive control tasks, participants have to choice between different conflicting responses. Stimuli can be manipulated to increase difficulty and to measure particular cognitive abilities, such as sustained attention, response inhibition, impulsivity, task switching ability and error processing. Frequently used cognitive control tasks are the Stroop tasks: Participants are required to detect only a salient characteristic of the stimuli, ignoring the others (i.e., color words printed in different colored ink and participants have to ignore the word and name its color)[42]. When the different features of the stimuli are incongruent, the task difficulty increases and affects the performance (Stroop effect)[43]. Another important category of control tasks is the “go no-go paradigm”: Stimuli (i.e., digits, letters, shapes) are presented in a continuous stream and participants perform a binary decision on each stimulus. One of the outcomes requires participants to make a motor response (go), whereas the other requires participants to withhold a response (no-go)[44].
When the study is focused on the influence of emotion or salience on selective attention, dot prob paradigms are frequently used: Participants view neutral or salient stimuli appearing randomly on either side of the screen, then a dot is presented in the location of one former stimulus and participants have to indicate the correct location of the dot, so an attentional bias toward salient stimuli can be detected[45,46].
In their study on 10 IGDp addicted to the videogame World of Warcraft (WOW) Ko et al[17] found that IGDp reported a higher gaming urge when passive viewing WOW images with respect to NC. Moreover, a significant higher activation was observed in right orbitofrontal cortex, right basal ganglia (caudatum and accumbens), bilateral anterior cingulate cortex, bilateral medial prefrontal cortex, right dorsolateral prefrontal cortex.
Han et al[19] conducted a six-week open label pharmacological study aiming to evaluate bupropion efficacy in reducing game craving and modulate brain activation in 11 IGDp addicted to the videogame Starcraft. At baseline, all participants were medication free and the authors observed an higher game urging and an augmented activation of left dorsolateral prefrontal cortex, L parahippocampus, left occipital lobe and cuneus in IGDp, with respect to NC during Starcraft cue presentation. After bupropion treatment, a significant decreased activation of left dorsolateral prefrontal cortex was observed in IGDp. Bupropion, being an antidepressant agent modulating dopamine and norepinephrine reuptake, was reported to be efficacious in patients with substance use disorder, with or without comorbid mood disorders[47,48] and in pathological gambling[49]. So the authors hypothesized that bupropion reduced craving in IGD by modulating dorsolateral prefrontal cortex functional activity.
In a recent study using videogame stimuli, Liu et al[33] (2015) enrolled a mixed-gender sample of 19 IGDp (males 58%) and reported a significant dysfunction of the frontal cortex, with increased activation in right-sided temporo-parietal and limbic regions: Superior parietal lobe, insula, cingulate gyrus and superior temporal gyrus.
Guessing task fMRI studies in IAD
To evaluate reward and punishment sensitivity in IGDp, Dong et al[20] simulated a gain/loss situation: Participants had to choice between two covered playing cards and at the end of the fMRI scan session they received a money sum based on their wins and losses. fMRI data analysis revealed that during win condition IGDs showed an higher activation of left orbitofrontal cortex (BA11) with respect to NC, whereas in loss condition the opposite was true for anterior cingulate cortex activation. So the authors concluded that a reduced sensitivity to negative experiences (monetary loss) and an augmented sensitivity to positive events (monetary gain) throughout an altered functioning of orbitofrontal cortex and anterior cingulate cortex could explain why IADp persisted in their habit despite the negative consequences on their everyday life.
Using a similar guessing task, Dong et al[25] found that IGDp were significantly slower than NC when exposed to continuous losses, whereas no behavioral group effects were observed after continuous wins. In terms of brain activations, IGDs showed a reduced activation of posterior cingulate cortex and an increased activation of inferior frontal gyrus during both win and loss conditions, whereas an augmented activation of anterior cingulate cortex and insula was observed during win condition only. These results suggested that decision-making ability was impaired in IGDp, due to a functional inefficiency in the inferior frontal gyrus (higher activation but lower behavioral performance) and a reduced involvement of posterior cingulate cortex and caudate. In the same study sample, with a modified guessing paradigm (a different control condition was added to wins and losses) Dong et al[26] asked the participants to describe their subjective experience after the scan section: IGDp reported higher craving for win in both continuous win and loss conditions and reduced negative emotions during loss conditions. In terms of functional activations, the results were similar, but not identical to those previously reported[25] (probably due to the different control condition): IGDp hyperactivated the left superior frontal gyrus in both wins and losses (but the level of activation was higher during wins) and hypoactivated the posterior cingulate cortex during losses. The authors concluded that superior frontal gyrus in IGDp was insensitive to negative situations and posterior cingulate cortex failed to exert its cognitive control on environmental changes.
In the eight cognitive controls fMRI studies we selected, Stroop tasks were used in four studies[22,24,27,31], go/no-go paradigms in three studies[28-30] and a dot/prob paradigm in one study[23].
Dong et al[22] enrolled 12 male, drug free and no-smokers IGDp and compared them with healthy peers during a three-choices color-word Stroop task. The groups did not differ in terms of behavioral performance, but during Stroop effect (incongruent - congruent stimuli contrast) IGDp showed a significant hyperactivation in anterior cingulate cortex, posterior cingulate cortex, left insula, middle frontal gyrus, medial frontal gyrus, left thalamus, right inferior frontal gyrus, right superior frontal gyrus.
The authors speculated that a greater activation of posterior cingulate cortex in IGD group could indicate a failure to optimize task related attentional resources due to an incomplete disengagement of Default Mode Network. Furthermore, the hyperactivation of the anterior cingulate cortex, insula and prefrontal regions might reflect a cognitive inefficiency of fronto-limbic regions playing a key role in conflict monitoring and “top down” inhibitory control.
In a larger sample, Dong et al[24] administered the same Stroop paradigm with an event-related design and separately analyzed the functional correlates of correct and error responses to stimuli. IGDp and NC performed similarly, but differences emerged in activation patterns: during correct responses IGDp failed to activate anterior cingulate cortex and orbitofrontal cortex, whereas an abnormal activation of anterior cingulate cortex was observed during errors, thus suggesting an impaired error monitoring ability.
More recently, Dong et al[27] analyzed the cognitive flexibility of a group of IGD during a modified version of the Stroop task, adding a monetary reward for correct responses and creating easy and difficult task conditions. The two group did not significantly differ behaviorally. On the other hand, when the task switched from difficult to easy condition IGDp activated the bilateral insula and right superior temporal gyrus more than NC; when the task switched from easy to difficult condition, they hyperactivated the bilateral precuneus, left superior temporal gyrus and left angular gyrus. The authors hypothesized that an higher (and therefore less efficient) activation of limbic and temporoparietal regions playing a key role in inhibitory control and cognitive flexibility was a biomarker of IGD.
The same inhibitory control impairment was found in another study by Dong et al[31]. As a part of a larger resting state connectivity study, a subsample of IGDs performed a Stroop task during an event related fMRI scanning. The authors observed that during incongruent trials, IGDs showed an augmented activation of bilateral superior frontal gyrus and a reduced activation of left dorsolateral prefrontal cortex, left orbitofrontal cortex and anterior cingulate cortex, all regions implicated in executive control.
Ko et al[28] used a go/no-go paradigm with digit stimuli to assess response inhibition and error processing in 26 male IGDp. The authors did not found significant behavioral deficits in IGDp, with respect to NC. On the contrary, when analyzing fMRI data, they reported significant group effects: During successful response inhibition, IGDp activated the bilateral caudate and left orbitofrontal gyrus more than NC; during error committion they failed to activate the right insula. Orbitofrontal gyrus and insula are key regions in modulating inhibitory control and error processing, so the authors suggested that IGDp needed to hyperactivate the orbitofrontal gyrus to successfully perform the task and compensate for the insular hypofunction.
In a recent article, Chen et al[30] used a block design to analyze the functional correlates of cognitive control in IGDp by means of a short go/no-go task. Even though behaviorally intact, IGDp showed a reduced activation of supplementary motor area/pre supplementary motor area, a key region in selecting the appropriate behavior, withholding wrong responses.
Liu et al[29] enrolled a mixed gender sample of IGDp and used a modified go/no-go paradigm, entering gaming picture as background distracters. They observed similar group performance in the original paradigm, but more commission errors during the cue distraction condition in the IGD group. Moreover, during the original task, IGDp hyperactivated the right superior parietal lobe, whereas during the gaming distracting condition they hypoactivated right dorsolateral prefrontal cortex, right superior parietal lobe and cerebellum. A Region of Interest based analysis revealed that in IGDp the rate of commission errors was positively associated with the right dorsolateral prefrontal cortex and right superior parietal lobe activation. The authors therefore suggested that gaming cues significantly affected inhibitory control in IGDp, throughout a failure of dorsolateral prefrontal cortex and superior parietal lobe function.
A cognitive task with emotional and cue distracters was also used by Lorenz et al[23] in a small group of IGDp: They administered a two-choice dot probe paradigm during short (SP) and long presentation (LP) trials in order to elicit attentional bias and cue reactivity, respectively. Stimuli were International Affective Picture System based emotional images (with neutral or positive valence) and computer generated images (neutral pictures or images based on World of Warcraft videogame). IGDp showed a significant attentional bias vs both game related and affective pictures with positive valence. Compared to NC, IGDp showed an abnormal activation of medial prefrontal cortex, anterior cingulate cortex, left orbitofrontal cortex and amygdala during SP trials and of occipital regions, right inferior frontal gyrus and right hippocampus during LP trials. In authors’ opinion, IGDp patients showed a behavioral and neural response similar to that observed in patients with substance use disorder, giving more attention to positive stimuli.
In this paper we systematically reviewed the resting state and task related fMRI studies on adult patients with IAD. All but one of the papers included in our qualitative synthesis were conducted in the Asian continent, confirming the great attention given to this potential harmful condition by Eastern governments[50].
The majority of the studies were conducted on young male IGDp (mean age ≤ 25 years), with only a few females and subjects with non-gaming Internet addiction. To avoid any confounding effects of other conditions, we included only studies conducted in subjects free of any comorbid psychiatric or substance use disorder.
Summarizing the literature findings, we highlighted that IGDp differed from healthy comparisons in the functioning of several brain regions involved in reward and executive control/attention processing, even when they were behaviorally intact.
In particular, the most reported cortical dysfunctions were located in orbitofrontal gyrus, anterior cingulate cortex, insula, dorsolateral prefrontal cortex, superior temporal gyrus, inferior frontal gyrus, precuneus and posterior cingulate cortex, whereas for subcortical regions, functional alterations were often found in brainstem and caudate.
Orbitofrontal cortex is involved in decision-making, value-guided behaviors and reward/punishment sensitivity[51,52]: Through its multiple connections with prefrontal, limbic and sensorial regions, it estimates the potential reward of a given stimulus and the appropriate behavior in order to achieve a positive outcome. In patients with substance addiction, an altered functioning of orbitofrontal cortex has been linked to craving and impaired decision-making[53]. Anterior cingulate cortex and insular cortex are both relevant in sustained attention, conflict monitoring, error signaling[54] and processing of unpleasant stimuli[55]. They provide a hub between different cerebral systems, binding emotion to cognition[56,57]. Altered functioning of anterior cingulate cortex and insula have been found in alcohol and drug addiction[58,59].
Dorsolateral prefrontal cortex is a region involved in different cognitive tasks, such as working memory[60] and motor skill learning[61]. An abnormal activation of dorsolateral prefrontal cortex was found in heavy alcohol drinkers with respect to light drinkers during a go/no go task[62] and in pathological gamblers during a cue-reactivity task[63].
Superior temporal gyrus was found activated in the processing of audiovisual stimuli with an emotional content[64] and during task shifting[65]. A reduced activation of superior temporal gyrus was reported in cocaine addicts during a Stroop task[66].
Inferior frontal gyrus has a role in cognitive inhibition[67], target detection[68], decision making[69] and emotional processing[70]. In response to decision-making involving uncertainty and during aversive interoceptive processing, young adults with problematic use of cocaine and amphetamine exhibited a reduced inferior frontal gyrus activation with respect to both former stimulant users and healthy controls[71]. The precuneus has a pivotal role in self-consciousness, visuo-spatial imagery, episodic memory retrieval[72] and target detection during high difficulty tasks[73]. In their work on internet addicts with comorbid nicotine dependence, Ko et al[74] reported an increased activation of precuneus during game cue exposure in acutely ill IGDp, but not in remitted IGD.
Posterior cingulate cortex is considered part of the default mode of the brain[75] and its deactivation during high demanding cognitive tasks is seen as an expression of a reallocation of processing resources[76]. An altered function of posterior cingulate cortex and other components of Default Mode Network was reported in cocaine addicts, especially in those with chronic use[77].
The importance of brainstem in providing ascending and descending pathways between brain and body is well documented[78]. In particular, prefrontal regions and anterior cingulate cortex are deeply connected to the brainstem, so a dysfunction of this subcortical structure leads to attentional and executive impairment[79].
Caudate nucleus is involved in posture, motor control and modulation of approach/attachment behavior[80]. In response to alcohol cues, heavy alcohol users showed higher caudate activation with respect to moderate users[81].
Radiological imaging is a useful research strategy in psychiatric and neurological fields, and may be considered as a form of “molecular pathological epidemiology”[82,83], an interdisciplinary research area aiming to investigate the complex relationships among genes, environment, molecular alterations and long term outcome of clinical disorders[84].
Taken together, the results of our systematic review suggest that young adult with IGD, without any other psychiatric disorder, showed a pattern of functional brain alterations similar to those observed in substance addiction.
Altered functioning of anterior and posterior cingulate cortex, prefrontal and parietal regions, limbic areas and subcortical structures results in impaired response inhibition and abnormal sensitivity to reward and punishment. As observed in substance use disorders, patients with IAD show a reduced cognitive flexibility, more stereotyped responses and inappropriate behavior, with negative consequences on social and working life[85-87].
The majority of patients enrolled in the reviewed studies were males IGDp, so the conclusions can’t be extended to other subtypes of IAD or to female patients. Focusing our review on adult subjects, we excluded fMRI studies conducted on pediatric and adolescent populations.
Internet addiction disorder (IAD) is an impulse control disorder characterized by an uncontrolled Internet use, associated with a significant functional impairment or clinical distress. Even if it is not classified as a mental disorder in the current edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), it is a highly debated condition, due to its relevant prevalence among adolescents and young adults.
Some of the clinical characteristics of IAD, such as loss of control, craving and withdrawal symptoms when patients are not allowed to use the Internet are similar to those observed in behavioral or substance use disorders. Therefore, in the last years several neuroimaging studies have been conducted aiming to investigate the relation between the clinical presentation of IAD and the functioning of cortical and subcortical regions involved in reward processing and cognitive control.
Neuroimaging research is nowadays a promising approach to fill the gap between the molecular basis of psychiatric disorders and their clinical manifestations. The scientific literature on debated diagnosis such as IAD is rapidly growing, so providing an updated review of the last published data may be of interest for the readers. Focusing the authors’ systematic review on homogeneous study samples (only adult patients, no psychiatric comorbid conditions allowed) results of different researches can be easily compared to find similarities and discordances.
In clinical settings, patients with the same psychiatric condition often differ from one another in terms of clinical symptoms, response to pharmacological treatments and long-term outcome. Studying their brains and behaviors in details could help to provide more accurate diagnosis and treatments.
IAD: An impulse control disorder characterized by an uncontrolled Internet use, associated with a significant functional impairment or clinical distress; IGD: A subtype of IAD, also called videogame addiction, characterized by excessive online gaming as the principal Internet activity.
This is a very interesting article.
P- Reviewer: Gumustas OG, Matsumoto S S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Young KS. Internet addiction: the emergence of a new clinical disorder. Cyberpsychol Behav. 1998;1:237-244. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2427] [Cited by in RCA: 1955] [Article Influence: 72.4] [Reference Citation Analysis (1)] |
| 2. | American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders (5th ed). Washington, DC, 2013. Available from: http://www.psychiatry.org/. |
| 3. | Cheng C, Li AY. Internet addiction prevalence and quality of (real) life: a meta-analysis of 31 nations across seven world regions. Cyberpsychol Behav Soc Netw. 2014;17:755-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 354] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 4. | Krishnamurthy S, Chetlapalli SK. Internet addiction: Prevalence and risk factors: A cross-sectional study among college students in Bengaluru, the Silicon Valley of India. Indian J Public Health. 2015;59:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Rumpf HJ, Vermulst AA, Bischof A, Kastirke N, Gürtler D, Bischof G, Meerkerk GJ, John U, Meyer C. Occurence of internet addiction in a general population sample: a latent class analysis. Eur Addict Res. 2014;20:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Choi SW, Kim HS, Kim GY, Jeon Y, Park SM, Lee JY, Jung HY, Sohn BK, Choi JS, Kim DJ. Similarities and differences among Internet gaming disorder, gambling disorder and alcohol use disorder: a focus on impulsivity and compulsivity. J Behav Addict. 2014;3:246-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Bipeta R, Yerramilli SS, Karredla AR, Gopinath S. Diagnostic Stability of Internet Addiction in Obsessive-compulsive Disorder: Data from a Naturalistic One-year Treatment Study. Innov Clin Neurosci. 2015;12:14-23. [PubMed] |
| 8. | Wölfling K, Beutel ME, Dreier M, Müller KW. Bipolar spectrum disorders in a clinical sample of patients with Internet addiction: hidden comorbidity or differential diagnosis? J Behav Addict. 2015;4:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Tonioni F, Mazza M, Autullo G, Cappelluti R, Catalano V, Marano G, Fiumana V, Moschetti C, Alimonti F, Luciani M. Is Internet addiction a psychopathological condition distinct from pathological gambling? Addict Behav. 2014;39:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Walton E, Turner JA, Ehrlich S. Neuroimaging as a potential biomarker to optimize psychiatric research and treatment. Int Rev Psychiatry. 2013;25:619-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Bullmore E. The future of functional MRI in clinical medicine. Neuroimage. 2012;62:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Mitterschiffthaler MT, Ettinger U, Mehta MA, Mataix-Cols D, Williams SC. Applications of functional magnetic resonance imaging in psychiatry. J Magn Reson Imaging. 2006;23:851-861. [PubMed] |
| 13. | van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2151] [Cited by in RCA: 2148] [Article Influence: 143.2] [Reference Citation Analysis (0)] |
| 14. | Sava S, Yurgelun-Todd DA. Functional magnetic resonance in psychiatry. Top Magn Reson Imaging. 2008;19:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 858] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 16. | Honey GD, Fletcher PC, Bullmore ET. Functional brain mapping of psychopathology. J Neurol Neurosurg Psychiatry. 2002;72:432-439. [PubMed] |
| 17. | Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, Yen CF, Chen CS. Brain activities associated with gaming urge of online gaming addiction. J Psychiatr Res. 2009;43:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 357] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 18. | Liu J, Gao XP, Osunde I, Li X, Zhou SK, Zheng HR, Li LJ. Increased regional homogeneity in internet addiction disorder: a resting state functional magnetic resonance imaging study. Chin Med J (Engl). 2010;123:1904-1908. [PubMed] |
| 19. | Han DH, Hwang JW, Renshaw PF. Bupropion sustained release treatment decreases craving for video games and cue-induced brain activity in patients with Internet video game addiction. Exp Clin Psychopharmacol. 2010;18:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Dong G, Huang J, Du X. Enhanced reward sensitivity and decreased loss sensitivity in Internet addicts: an fMRI study during a guessing task. J Psychiatr Res. 2011;45:1525-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Dong G, Huang J, Du X. Alterations in regional homogeneity of resting-state brain activity in internet gaming addicts. Behav Brain Funct. 2012;8:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Dong G, Devito EE, Du X, Cui Z. Impaired inhibitory control in ‘internet addiction disorder’: a functional magnetic resonance imaging study. Psychiatry Res. 2012;203:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Lorenz RC, Krüger JK, Neumann B, Schott BH, Kaufmann C, Heinz A, Wüstenberg T. Cue reactivity and its inhibition in pathological computer game players. Addict Biol. 2013;18:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Dong G, Shen Y, Huang J, Du X. Impaired error-monitoring function in people with Internet addiction disorder: an event-related fMRI study. Eur Addict Res. 2013;19:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Dong G, Hu Y, Lin X, Lu Q. What makes Internet addicts continue playing online even when faced by severe negative consequences? Possible explanations from an fMRI study. Biol Psychol. 2013;94:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Dong G, Hu Y, Lin X. Reward/punishment sensitivities among internet addicts: Implications for their addictive behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Dong G, Lin X, Zhou H, Lu Q. Cognitive flexibility in internet addicts: fMRI evidence from difficult-to-easy and easy-to-difficult switching situations. Addict Behav. 2014;39:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Ko CH, Hsieh TJ, Chen CY, Yen CF, Chen CS, Yen JY, Wang PW, Liu GC. Altered brain activation during response inhibition and error processing in subjects with Internet gaming disorder: a functional magnetic imaging study. Eur Arch Psychiatry Clin Neurosci. 2014;264:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Liu GC, Yen JY, Chen CY, Yen CF, Chen CS, Lin WC, Ko CH. Brain activation for response inhibition under gaming cue distraction in internet gaming disorder. Kaohsiung J Med Sci. 2014;30:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Chen CY, Huang MF, Yen JY, Chen CS, Liu GC, Yen CF, Ko CH. Brain correlates of response inhibition in Internet gaming disorder. Psychiatry Clin Neurosci. 2015;69:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Dong G, Lin X, Potenza MN. Decreased functional connectivity in an executive control network is related to impaired executive function in Internet gaming disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 32. | Kim H, Kim YK, Gwak AR, Lim JA, Lee JY, Jung HY, Sohn BK, Choi SW, Kim DJ, Choi JS. Resting-state regional homogeneity as a biological marker for patients with Internet gaming disorder: A comparison with patients with alcohol use disorder and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2015;60:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Liu J, Li W, Zhou S, Zhang L, Wang Z, Zhang Y, Jiang Y, Li L. Functional characteristics of the brain in college students with internet gaming disorder. Brain Imaging Behav. 2015;Epub ahead of print. [PubMed] |
| 34. | Zhang JT, Yao YW, Li CS, Zang YF, Shen ZJ, Liu L, Wang LJ, Liu B, Fang XY. Altered resting-state functional connectivity of the insula in young adults with Internet gaming disorder. Addict Biol. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Beard KW, Wolf EM. Modification in the proposed diagnostic criteria for Internet addiction. Cyberpsychol Behav. 2001;4:377-383. [PubMed] |
| 36. | Ko CH, Yen JY, Chen SH, Yang MJ, Lin HC, Yen CF. Proposed diagnostic criteria and the screening and diagnosing tool of Internet addiction in college students. Compr Psychiatry. 2009;50:378-384. [PubMed] |
| 37. | Wang WZ, Tao R, Niu YJ, Chen Q, Jia J, Wang XL, Kong QM, Tian CH. Preliminarily proposed diagnostic criteria of pathological Internet use. Chinese Ment Health J. 2009;23:890e4. |
| 38. | Grüsser SM, Thalemann CN. Verhaltenssucht: Diagnostik, Therapie, Forschung. Bern: Huber 2006; . |
| 39. | Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563-579. [PubMed] |
| 40. | Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211-214. [PubMed] |
| 41. | Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147-148. [PubMed] |
| 42. | MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163-203. [PubMed] |
| 43. | Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643-662. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10260] [Cited by in RCA: 10406] [Article Influence: 115.6] [Reference Citation Analysis (0)] |
| 44. | Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex. 2004;14:986-994. [PubMed] |
| 45. | Bradley B, Field M, Mogg K, De Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: component processes of biases in visual orienting. Behav Pharmacol. 2004;15:29-36. [PubMed] |
| 46. | MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol. 1986;95:15-20. [PubMed] |
| 47. | Castells X, Casas M, Pérez-Mañá C, Roncero C, Vidal X, Capellà D. Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev. 2010;CD007380. [PubMed] |
| 48. | Sepede G, Di lorio G, Lupi M, Sarchione F, Acciavatti T, Fiori F, Santacroce R, Martinotti G, Gambi F, Di Giannantonio M. Bupropion as an add-on therapy in depressed bipolar disorder type I patients with comorbid cocaine dependence. Clin Neuropharmacol. 2014;37:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Dannon PN, Lowengrub K, Musin E, Gonopolski Y, Kotler M. Sustained-release bupropion versus naltrexone in the treatment of pathological gambling: a preliminary blind-rater study. J Clin Psychopharmacol. 2005;25:593-596. [PubMed] |
| 50. | Ahn DH. Korean policy on treatment and rehabilitation for adolescents’ Internet addiction in 2007 International Symposium on the Counseling and Treatment of Youth Internet Addiction. 2007;49. |
| 51. | Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545-556. [PubMed] |
| 52. | Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86:216-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10:334-342. [PubMed] |
| 54. | Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215-222. [PubMed] |
| 55. | Petrovic P, Pleger B, Seymour B, Klöppel S, De Martino B, Critchley H, Dolan RJ. Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. J Neurosci. 2008;28:10509-10516. [PubMed] |
| 56. | Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318-1326. [PubMed] |
| 57. | Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 973] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 58. | Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642-1652. [PubMed] |
| 59. | Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18:121-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 395] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 60. | Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133:44-54. [PubMed] |
| 61. | Seidler RD, Bo J, Anguera JA. Neurocognitive contributions to motor skill learning: the role of working memory. J Mot Behav. 2012;44:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 62. | Ames SL, Wong SW, Bechara A, Cappelli C, Dust M, Grenard JL, Stacy AW. Neural correlates of a Go/NoGo task with alcohol stimuli in light and heavy young drinkers. Behav Brain Res. 2014;274:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Crockford DN, Goodyear B, Edwards J, Quickfall J, el-Guebaly N. Cue-induced brain activity in pathological gamblers. Biol Psychiatry. 2005;58:787-795. [PubMed] |
| 64. | Robins DL, Hunyadi E, Schultz RT. Superior temporal activation in response to dynamic audio-visual emotional cues. Brain Cogn. 2009;69:269-278. [PubMed] |
| 65. | Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35-45. [PubMed] |
| 66. | Barrós-Loscertales A, Bustamante JC, Ventura-Campos N, Llopis JJ, Parcet MA, Avila C. Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Res. 2011;194:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170-177. [PubMed] |
| 68. | Mantini D, Corbetta M, Perrucci MG, Romani GL, Del Gratta C. Large-scale brain networks account for sustained and transient activity during target detection. Neuroimage. 2009;44:265-274. [PubMed] |
| 69. | Reckless GE, Ousdal OT, Server A, Walter H, Andreassen OA, Jensen J. The left inferior frontal gyrus is involved in adjusting response bias during a perceptual decision-making task. Brain Behav. 2014;4:398-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Frühholz S, Grandjean D. Processing of emotional vocalizations in bilateral inferior frontal cortex. Neurosci Biobehav Rev. 2013;37:2847-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 71. | Stewart JL, Parnass JM, May AC, Davenport PW, Paulus MP. Altered frontocingulate activation during aversive interoceptive processing in young adults transitioning to problem stimulant use. Front Syst Neurosci. 2013;7:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564-583. [PubMed] |
| 73. | Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689-4699. [PubMed] |
| 74. | Ko CH, Liu GC, Yen JY, Chen CY, Yen CF, Chen CS. Brain correlates of craving for online gaming under cue exposure in subjects with Internet gaming addiction and in remitted subjects. Addict Biol. 2013;18:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 75. | Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178-1184. [PubMed] |
| 76. | McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394-408. [PubMed] |
| 77. | Konova AB, Moeller SJ, Tomasi D, Goldstein RZ. Effects of chronic and acute stimulants on brain functional connectivity hubs. Brain Res. 2015;1628:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 78. | Angeles Fernández-Gil M, Palacios-Bote R, Leo-Barahona M, Mora-Encinas JP. Anatomy of the brainstem: a gaze into the stem of life. Semin Ultrasound CT MR. 2010;31:196-219. [PubMed] |
| 79. | Hurley RA, Flashman LA, Chow TW, Taber KH. The brainstem: anatomy, assessment, and clinical syndromes. J Neuropsychiatry Clin Neurosci. 2010;22:iv, 1-7. [PubMed] |
| 80. | Villablanca JR. Why do we have a caudate nucleus? Acta Neurobiol Exp (Wars). 2010;70:95-105. [PubMed] |
| 81. | Dager AD, Anderson BM, Rosen R, Khadka S, Sawyer B, Jiantonio-Kelly RE, Austad CS, Raskin SA, Tennen H, Wood RM. Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction. 2014;109:585-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 452] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 83. | Nishi A, Kawachi I, Koenen KC, Wu K, Nishihara R, Ogino S. Lifecourse epidemiology and molecular pathological epidemiology. Am J Prev Med. 2015;48:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Ogino S, Lochhead P, Chan AT, Nishihara R, Cho E, Wolpin BM, Meyerhardt JA, Meissner A, Schernhammer ES, Fuchs CS. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol. 2013;26:465-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 85. | Lundqvist T. Imaging cognitive deficits in drug abuse. Curr Top Behav Neurosci. 2010;3:247-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Luijten M, Machielsen MW, Veltman DJ, Hester R, de Haan L, Franken IH. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci. 2014;39:149-169. [PubMed] |
| 87. | Zhu Y, Zhang H, Tian M. Molecular and functional imaging of internet addiction. Biomed Res Int. 2015;2015:378675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |