Published online Feb 28, 2016. doi: 10.4329/wjr.v8.i2.192
Peer-review started: August 1, 2015
First decision: September 28, 2015
Revised: October 24, 2015
Accepted: December 18, 2015
Article in press: December 18, 2015
Published online: February 28, 2016
Processing time: 209 Days and 17.2 Hours
AIM: To determine swallowing outcomes and hyolaryngeal mechanics associated with post radiation therapy head and neck cancer (rtHNC) patients using videofluoroscopic swallow studies.
METHODS: In this retrospective cohort study, videofluoroscopic images of rtHNC patients (n = 21) were compared with age and gender matched controls (n = 21). Penetration-aspiration of the bolus and bolus residue were measured as swallowing outcome variables. Timing and displacement measurements of the anterior and posterior muscular slings elevating the hyolaryngeal complex were acquired. Coordinate data of anatomical landmarks mapping the action of the anterior muscles (suprahyoid muscles) and posterior muscles (long pharyngeal muscles) were used to calculate the distance measurements, and slice numbers were used to calculate time intervals. Canonical variate analysis with post-hoc discriminant function analysis was performed on coordinate data to determine multivariate mechanics of swallowing associated with treatment. Pharyngeal constriction ratio (PCR) was also measured to determine if weak pharyngeal constriction is associated with post radiation therapy.
RESULTS: The rtHNC group was characterized by poor swallowing outcomes compared to the control group in regards to: Penetration-aspiration scale (P < 0.0001), normalized residue ratio scale (NRRS) for the valleculae (P = 0.002) and NRRS for the piriform sinuses (P = 0.003). Timing and distance measurements of the anterior muscular sling were not significantly different in the two groups, whereas for the PMS time of displacement was abbreviated (P = 0.002) and distance of excursion was reduced (P = 0.02) in the rtHNC group. A canonical variate analysis shows a significant reduction in pharyngeal mechanics in the rtHNC group (P < 0.0001). The PCR was significantly higher in the test group than the control group (P = 0.0001) indicating reduced efficiency in pharyngeal clearance.
CONCLUSION: Using videofluoroscopy, this study shows rtHNC patients have worse swallowing outcomes associated with reduced hyolaryngeal mechanics and pharyngeal constriction compared with controls.
Core tip: Quality videofluoroscopic imaging of barium swallows is useful to determine swallowing outcomes for safe and efficient swallowing, conventional kinematics, and underlying functional anatomy associated with outcomes. This retrospective study of radiation therapy head and neck cancer (rtHNC) patients compared with age and gender matched controls and found that swallowing outcomes were significantly worse in rtHNC patients. Conventional kinematics indicated a reduction in laryngeal elevation. Computational analysis of swallowing mechanics using coordinate data of anatomical landmarks is here used to visualize impaired functional anatomy associated with poor outcomes in order to suggest particular targets for rehabilitation.
- Citation: Pearson Jr WG, Davidoff AA, Smith ZM, Adams DE, Langmore SE. Impaired swallowing mechanics of post radiation therapy head and neck cancer patients: A retrospective videofluoroscopic study. World J Radiol 2016; 8(2): 192-199
- URL: https://www.wjgnet.com/1949-8470/full/v8/i2/192.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i2.192
The oropharyngeal phase of swallowing is a complex physiological process involving the conversion of a respiratory conduit into an alimentary canal in less than one second. Videofluoroscopic swallowing studies, also known as modified barium swallows (MBS), are the standard instrumental tool for assessing oropharyngeal swallowing safety and efficiency. Swallowing safety is threatened when a bolus penetrates or is aspirated into the airway. The retention of a bolus in the valleculae or piriform sinuses is an indication of swallowing inefficiency. Reduced hyolaryngeal elevation is thought to underlie incomplete clearance of the bolus from the pharynx[1]. Residual bolus retained in pharyngeal spaces such as the piriform sinuses (residue) is predictive of aspiration[2]. Both outcomes are observed among post radiation therapy head and neck cancer (rtHNC) patients[3]. However, the underlying mechanics associated with these outcomes is poorly understood.
Recent anatomical and magnetic resonance imaging (MRI) studies have shown that two muscular slings elevate the hyolaryngeal complex to stretch open the upper esophageal sphincter in young healthy adults[4-6]. It is not known if altered function of one or both of these slings is associated with poor swallowing outcomes (penetration, aspiration, or residue). To determine if poor swallowing outcomes are associated with altered function of the two-sling mechanism, we compared kinematic and morphological data collected from MBS imaging of rtHNC patients with age and gender matched controls.
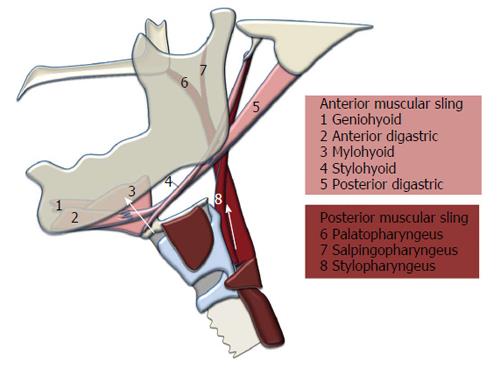
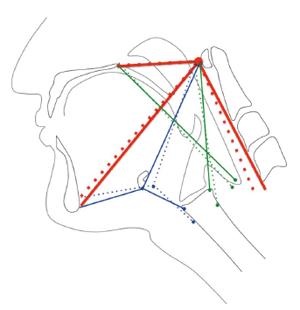
The hyolaryngeal complex is comprised of the hyoid bone, laryngeal cartilages, and associated structures including the cricopharyngeus muscle, which forms the upper esophageal sphincter. Two muscular slings elevate the hyolaryngeal complex in swallowing[5] (Figure 1). The suprahyoid muscles (mylohyoid, geniohyoid, stylohyoid, and digastric) form an anterior sling with proximal attachments to the mandible and cranial base and distal attachments to the body of the hyoid, which translates force to the larynx via the thyrohyoid membrane and likely assisted by the thyrohyoid muscle[4]. A posterior sling comprised of the long pharyngeal muscles (stylopharyngeus, palatopharyngeus and salpingopharyngeus) has superior attachments to the styloid process, auditory tube, and structures associated with the palate, and inferior attachments inserting primarily on the lateral pharyngeal wall and thyroid cartilage[7,8]. These muscular slings function to elevate the hyolaryngeal complex and stretch open the upper esophageal sphincter, but it is unknown whether pathology changes the function of the two-sling mechanism.
High quality MBS imaging that is well collimated provides data useful for analyzing outcomes as well as the underlying mechanisms of swallowing[9]. In this study, outcome variables measuring penetration-aspiration and residue were used to verify differences between test and control groups[10], whereas kinematic measurements[11,12] and multivariate morphometric analysis[6] were used to determine which of the two slings described above is impaired. Additionally, the pharyngeal constriction ratio (PCR), a reliable surrogate for strength of pharyngeal constriction, was measured for each group to document the effect of treatment on the pharyngeal constrictor muscles and the long pharyngeal muscles[13]. We hypothesized that swallowing outcome variables, kinematic measurements, multivariate morphometric analysis, and PCR of the rtHNC group will indicate impairment when compared to the control group.
Under a research protocol approved by the Boston University Medical Campus Institutional Review Board, a review of patient records was used to establish a test and control group. MBS imaging studies were recorded under routine radiographic protocols. An attempt was made to attach a radiopaque marker to each subject as an external scalar. Images were produced by a GE Precision Fluoroscopic unit and recorded digitally by a computer workstation at 30 frames/s. QuickTime™ software was used to trim each imaging study to include one episode of cued lateral view 5 mL swallows of thin liquid barium solution [Varibar Thin Liquid (40% wt/vol)].
Ninety-three patients with MBS studies were identified. Dysphagic patients related to rtHNC were placed in the test group (n = 28), while patients complaining of swallowing difficulty who showed no instrumental evidence of dysphagia were placed in the control group (n = 45). Of the 28 subjects in the rtHNC group, two lacked scalars and five could not be age or gender matched with the “normal” group. This left a final cohort of 21 subjects in each group composed of 14 males and 7 females with a mean age of 64 ± 13 years (test group) and 63 ± 11 years (control group).
Swallowing outcomes were collected along with spatial and temporal data from video files using ImageJ image analysis software equipped with QuickTime™ plug-ins (http://rsbweb.nih.gov/ij). Raters blinded to group assignment analyzed video files. Reliability was tested for all measurements by using a second judge to re-measure variables in 50% of MBS studies. Inter-rater reliability is reported in Table 1. In swallowing episodes requiring more than one swallow to clear the bolus, measurements were taken from the first swallow.
| Intraclass correlation coefficient | Lower 95%CI limit | Upper 95%CI limit | |
| Penetration-aspiration scale | 0.87 | 0.75 | 0.93 |
| Normalized residue ratio scale (valleculae) | 0.93 | 0.85 | 0.97 |
| Normalized residue ratio scale (piriform recess) | 0.91 | 0.82 | 0.96 |
| Anterior sling distance measurement | 0.87 | 0.75 | 0.93 |
| Posterior sling distance measurement | 0.88 | 0.77 | 0.94 |
| Anterior sling time measurement | 0.88 | 0.74 | 0.95 |
| Posterior sling time measurement | 0.90 | 0.77 | 0.96 |
| Pharyngeal constriction ratio | 0.81 | 0.66 | 0.90 |
To measure penetration and aspiration we used a 1-8 ordinal scale called the penetration-aspiration scale (PAS)[14]. In the PAS scoring system 1-2 is considered functionally normal, 3-5 indicates bolus penetration into the laryngeal vestibule, and 6-8 indicates aspiration of the bolus into the airway. To quantify residue we used the normalized residue ratio scale (NRRS) for the valleculae and piriform sinuses[15]. The NRRS is a continuous measurement that incorporates the ratio of residue relative to pharyngeal space (valleculae and piriform sinuses) and the amount of residue scaled by an internal anatomical scalar (C2-C4 measurement described above). Differences in PAS in the two groups were evaluated using Mann-Whitney U tests, and differences in NRRS for the valleculae and piriform sinuses were compared using two-tailed t-tests with Bonferroni correction (α = 0.05, P < 0.025).
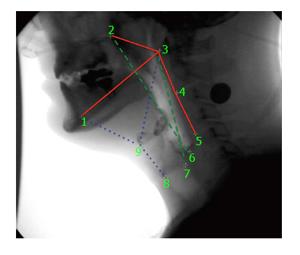
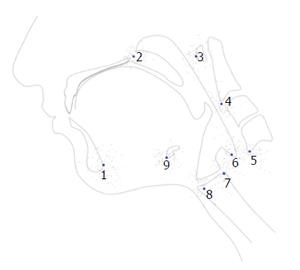
Kinematic data collected from video files included duration and displacement measurements representing the anterior and posterior slings. The movement of the hyoid towards the mandible represented the action of the anterior muscular sling (AMS). The movement of the larynx towards the cranial base represented the action of the posterior muscular sling (PMS). Each AMS and PMS time and distance measurement was measured at minimum and maximum elevation. For the AMS, the minimum position was defined as one frame prior to the first rostral movement of the hyoid related to pharyngeal swallowing and maximum position was defined as the most rostral movement of the hyoid during swallowing. For the PMS, the minimum position was defined as one frame prior to the first rostral movement of the larynx during pharyngeal swallowing and maximum position was defined as the frame where the larynx reaches its superior position during swallowing.
Duration of AMS and PMS movement was determined by dividing the difference in minimum and maximum frame numbers by 30 (fps). Displacement of the hyoid (representing the action of the AMS) and elevation of the posteroinferior edge of the cricoid toward the cranial base (representing the action of the PMS) were calculated using a coordinate mapping technique designed to track the components of the two-sling mechanism of hyolaryngeal elevation[16] (Figure 2). Displacement measurements of the hyoid and larynx were mathematically calculated from coordinate data. Timing and spatial measurements were compared using two-tailed t-tests with Bonferroni correction (α = 0.05, P < 0.025).
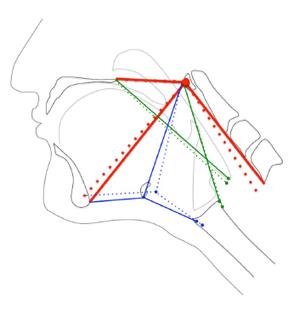
The nine coordinates mapping the elements of the anterior and PMSs in order to calculate kinematic variables were also used in a computational analysis of swallowing mechanics. The coordinate data set included nine coordinates for each subject at minimum and maximum excursion for the test group and control group. MorphoJ, software for geometric morphometric analysis, was used to perform a procrustes fit of all coordinates[17] (Figure 3). The procrustes fit resolved differences in scale and rotation between all subjects at both excursion points. Following the procrustes superimposition of coordinates, a canonical variate analysis was executed with classification variables assigned to each set of coordinates named by condition (test and control group) and excursion (minimum and maximum position of the hyolaryngeal complex).
To evaluate differences in pharyngeal constriction between test and control groups, the PCR was measured using the lateral view MBS studies[13]. PCR is a ratio of the area of the hypopharynx at maximum constriction to the area of the hypopharynx at rest. Significance of differences in PCR was determined using a two-tailed t-test.
All swallowing outcome variables indicated significantly worse swallowing in the rtHNC group than in the control group. PAS scores were highly significantly (P < 0.0001) greater in rtHNC (4.43 ± 2.42) than in the control (1.29 ± 0.56), NRRS scores for the valleculae were significantly (P = 0.002) greater in rtHNC (0.22 ± 0.20) than in the control (0.05 ± 0.11), and NRRS scores for the piriform sinuses were significantly (P = 0.003) greater in HNC (0.31 ± 0.36) than in the control (0.05 ± 0.14).
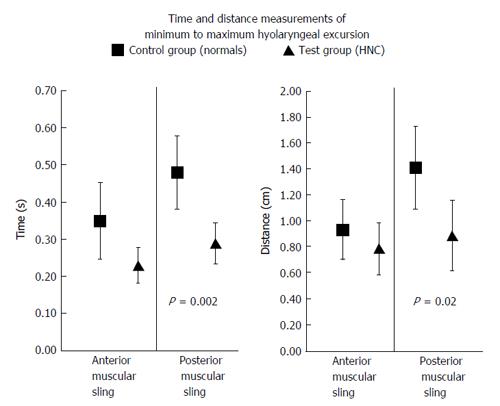
The duration of PMS elevating the larynx was significantly (P = 0.002) briefer in rtHNC (0.29 ± 0.11 s) than in the control (0.48 ± 0.23 s), and the displacement of the larynx by the posterior sling muscles is significantly (P = 0.02) reduced in rtHNC (0.89 ± 0.63 cm) than in the control (1.41 ± 0.73 cm). While these PMS measurements showed significant differences, the AMS measurements did not (Figure 4).
PCR was highly significantly (P = 0.0001) worse in rtHNC (0.16 ± 0.11) than in the control (0.05 ± 0.04).
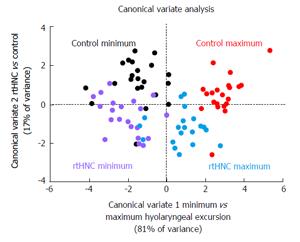
The scatter plot of canonical variate scores with classification variable highlighted indicated that CV1 is associated with the excursion of the hyolaryngeal complex and CV2 is associated the test and control groups (Figure 5). Swallowing mechanics indicated by differences in shape change was highly significantly (P < 0.0001) reduced in the rtHNC (D = 2.98) than in the control (D = 4.54). Eigenvectors for each coordinate demonstrate the direction and degree of covariant shape change of the control (Figure 6) and test (Figure 7) groups for visual comparison.
This study shows significant differences in swallowing safety and efficiency as measured by PAS and NRRS, respectively. Kinematic measurements showed that reduced function of the PMS which elevates the hyolaryngeal complex is associated with rtHNC. In contrast, the anterior sling was not significantly reduced in the test group. Finally, a computational analysis of swallowing mechanics confirms that the long pharyngeal muscles are implicated in this cohort of dysphagic patients, and that extending the head and neck while swallowing is presumably attempted to compensate for loss of pharyngeal function.
Poor swallowing outcomes among rtHNC patients, including increased penetration-aspiration and residue, are consistent with other findings in the literature[18,19]. However, this is the first time that the NRRS has been used to quantify residue in head and neck cancer patients. These findings confirm that the test and control groups used here are appropriate for a retrospective pseudo-experimental design for investigating underlying mechanisms of dysphagia, specifically focusing on the role of the two-sling mechanism of hyolaryngeal elevation.
Reduced hyolaryngeal elevation and hyoid movement has been noted among rtHNC patients[20,21], and reduced hyoid and laryngeal motility have also been associated with penetration-aspiration[10,22]. Association of the structures that underlie laryngeal elevation with poor swallowing outcomes has not been reported. Our data indicate a significantly diminished function of the PMS among rtHNC patients in both time and distance measurements with a large effect size changes for both timing and distance measurements (Cohen’s D = 1.46 for timing and 0.77 for distance). This is consistent with findings in the radiation oncology literature that the longitudinal PMS presumed to lie within the pharyngeal constrictor region of interest in computed tomography scans are more important than the anterior sling of muscles to swallowing function[18,23]. However, other published studies of rtHNC patients showed decreased hyoid displacement[20]. While our results do not reach statistical significance, timing and distance measurements of the hyoid was reduced when compared to the test group. The cohort did show a significant though small effect size change with Cohen’s D = 0.30 for distance and a medium effect size change for timing with Cohen’s D = 0.48. With these additional statistics, what can be said is that impairment of the posterior sling is much greater than the anterior sling in this cohort. The consensus in the literature is that the suprahyoid muscles (AMS) along with the thyrohyoid elevate the hyolaryngeal complex[24-26]. However, the results of this study indicate that the PMS appears to have a more significant role in swallowing dysfunction in rtHNC patients. Previous studies have not consistently taken into account the two-sling mechanism of hyolaryngeal elevation.
It is important to note that we did not include hyolaryngeal approximation, the movement of the anterior aspect of the larynx towards the hyoid bone, in this study. This movement is thought to be the unique function of the thyrohyoid muscle[27]. However, in a cohort of young healthy subjects the thyrohyoid was not found to be consistently active[4]. It has also been shown that the stylopharyngeus attaches to the larynx and elevates the thyroid cartilage toward the hyoid bone[5,28]. Conversely, it could be argued that the thyrohyoid (if active) assists the approximation of the posterior larynx towards the cranial base, which would in turn confound our PMS measurement. However, data show that the long pharyngeal muscles have a significantly greater mechanical advantage than the thyrohyoid in elevating the larynx[5].
Deglutition is a dynamic process of interrelated structures that covary in function, a fact that complicates univariate kinematic studies of swallowing. For example, the thyrohyoid membrane connects the hyoid and larynx and translates force to the larynx. A simple distance measurement cannot determine if the anterior sling muscles or the posterior sling muscles underlie elevation of the larynx. We propose a more informative approach using vectors to characterize how various elements interact as an integrated apparatus. In this present study we introduce a computational analysis of swallowing mechanics using a multivariate morphometric analysis of coordinates that map the two muscular slings elevating the hyoid, and the movement of the cranial base, mandible, and vertebrae as three skeletal levers from which the swallowing apparatus is suspended. Here we use a multivariate morphometric analysis of these coordinates to visualize vectors representing underlying muscle groups displacing the hyolaryngeal complex.
Canonical variate analysis of landmark coordinates showed overall shape changes judged on the basis of the Mahalanobis distance statistic (D-score). The Mahalanobis distance, also referred to as a generalized distance, is a dimensionless quantity that indicates how the covariance of one set of variables (in this study, a set of 9 coordinates) differs from the mean. A smaller D-score is interpreted as less overall shape change to the swallowing apparatus as mapped by 9 coordinates. As predicted, mean D-scores of the control group at maximum excursion were greater than the test group.
More interesting than the overall shape change scores are the eigenvector plots for each group (Figures 6 and 7). By comparing vectors of control and test groups it can be observed that there are small differences in the covariant distance of hyoid movement or pharyngeal shortening. The large differences are associated with laryngeal elevation and extension of the head and neck in the test group. This observation suggests that subjects with reduced hyolaryngeal elevation may attempt to compensate by hyper-extending the neck. More importantly, the vectors show that the long pharyngeal muscles, not the suprahyoid muscles, underlie this impairment. This visual analysis indicates that the long pharyngeal muscles are primary targets for rehabilitation[29]. These kinds of multivariate observations of an interrelated dynamic system are not possible using kinematic variables alone.
While this study demonstrates that the two-sling mechanism is important to hyolaryngeal elevation, we cannot say that reduced function of the two-sling mechanism is solely responsible for the poor swallowing outcomes. It is also possible that the functional difference between the groups could be explained by the significantly different degrees of pharyngeal constriction as measured by the PCR between the test and control groups (P = 0.0001). A higher PCR indicates weaker pharyngeal constriction[13]. A Spearman rank-order correlation of PCR with the PAS for the entire sample was r = 0.74, whereas distance measurements of the PMS correlated with PAS was r = -0.42, suggesting that poor swallowing outcomes in rtHNC patients are more strongly correlated with PCR than with PMS function. A recent study shows the correlation between PCR and residue as measured by NRRS[30]. However, it is likely that a reduced function of the posterior sling contributes to higher PCR. Leonard et al[31] have suggested that reduced hyolaryngeal approximation, a function that can be related to the PMS, is associated with weaker pharyngeal constriction.
It remains unclear how to best characterize differences in the two-sling mechanism associated with disordered swallowing. While diminished PMS function is indicated by kinematic measurements, eigenvectors of coordinates also demonstrate differences in the skeletal elements of the two-sling mechanism (Figures 6 and 7). Computational analysis of swallowing is currently being developed to include tongue base retraction and pharyngeal wall movement to provide a comprehensive approach to determining swallowing mechanics underlying effective and disordered swallowing. Registration of soft tissue landmarks is a confounding factor in this development.
In conclusion, this study demonstrates significant differences in swallowing outcomes between rtHNC patients and “normal” controls. We demonstrate reduced laryngeal elevation kinematics attributable to deficits in the PMS in the rtHNC group compared to the control group. Furthermore, multivariate computational analysis of swallowing reveals functional differences in the two-sling mechanism associated with pathology including different positioning of skeletal levers in addition to reduced laryngeal elevation. Whether the shape changes in the test group represent compensatory or maladaptive behaviors remains unclear. While videofluoroscopic imaging has been in use for some time, the investigative and diagnostic power of this modality is likely underutilized.
Dysphagia is a comorbidity head and neck cancer radiation treatment and presents a serious quality of life issue. The most important consideration for patients receiving radiation treatment second to cancer remission is swallowing function. Radiation damage to tissues can reduce salivary flow, and fibrosis impacts swallowing mechanics. Swallowing exercises have been shown to help mitigate the impact of fibrosis, and targeted treatment is thought to improve swallowing safety and facilitate patient compliance. A thorough understanding of swallowing mechanics associated with impairment enables clinicians to provide effective dysphagia management. In this paper multiple methods were used to document the impact of head and neck cancer treatment on swallowing safety, efficiency, and mechanics.
Modified barium swallows (MBS) studies are the diagnostic standard for dysphagia. These imaging studies are often underutilized to merely establish penetration-aspiration status. However, this singular assessment does not provide useful information to clinicians managing swallowing rehabilitation. Many methods have been developed to address this problem. Analytical tools applied to high quality imaging are essential to achieve better outcomes for patients.
Kinematic methods that document timing and movement of swallowing structures have been in use in research for some time and are now clinically accessible. In recent years the MBS impairment profile was developed allowing for a standardized, reliable and valid approach for the clinical assessment of swallowing physiology. In this paper coordinate mapping of muscle functional groups underlying oropharyngeal swallowing, coordinates are reliably collected using digital analysis tools such as ImageJ. These coordinates are used to calculate displacement measurements and for multivariate morphometric analysis using computational tools such as MorphoJ.
Computational analysis of swallowing mechanics allows for visualization of impaired functional anatomy of swallowing and can guide rehabilitation efforts along with other useful measurements. Future directions include patient specific analysis to guide dysphagia management.
Computational analysis of swallowing mechanics is a multivariate morphometric analysis of coordinates mapping the functional anatomy of swallowing and swallowing impairment using MBS imaging.
This is a very good manuscript studying the effects of radiotherapy in the swallowing mechanism of patients. The method used, the evaluation of data and the presentation is excellent (very instructive figures, extensive statistics, etc.).
P- Reviewer: Gao BL, Tsalafoutas IA S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: a combined manometric and videofluoroscopic study. Gastroenterology. 1992;103:128-136. [PubMed] |
| 2. | Eisenhuber E, Schima W, Schober E, Pokieser P, Stadler A, Scharitzer M, Oschatz E. Videofluoroscopic assessment of patients with dysphagia: pharyngeal retention is a predictive factor for aspiration. AJR Am J Roentgenol. 2002;178:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Langmore SE, Krisciunas GP. Dysphagia After Radiotherapy for Head and Neck Cancer: Etiology, Clinical Presentation, and Efficacy of Current Treatments. Perspectives on Swallowing and Swallowing Disorders (Dysphagia). 2010;19:32-38. |
| 4. | Pearson WG, Hindson DF, Langmore SE, Zumwalt AC. Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2013;85:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Pearson WG, Langmore SE, Yu LB, Zumwalt AC. Structural analysis of muscles elevating the hyolaryngeal complex. Dysphagia. 2012;27:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Pearson WG Jr, Zumwalt AC. Visualizing Hyolaryngeal Mechanics in Swallowing Using Dynamic MRI. Comput Methods Biomech Biomed Eng Imaging Vis. 2013;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Okuda S, Abe S, Kim HJ, Agematsu H, Mitarashi S, Tamatsu Y, Ide Y. Morphologic characteristics of palatopharyngeal muscle. Dysphagia. 2008;23:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Choi DY, Bae JH, Youn KH, Kim HJ, Hu KS. Anatomical considerations of the longitudinal pharyngeal muscles in relation to their function on the internal surface of pharynx. Dysphagia. 2014;29:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Jaffer NM, Ng E, Au FW, Steele CM. Fluoroscopic evaluation of oropharyngeal dysphagia: anatomic, technical, and common etiologic factors. AJR Am J Roentgenol. 2015;204:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Steele CM, Bailey GL, Chau T, Molfenter SM, Oshalla M, Waito AA, Zoratto DC. The relationship between hyoid and laryngeal displacement and swallowing impairment. Clin Otolaryngol. 2011;36:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Kendall KA, McKenzie S, Leonard RJ, Gonçalves MI, Walker A. Timing of events in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 201] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Leonard RJ, Kendall KA, McKenzie S, Gonçalves MI, Walker A. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Leonard R, Rees CJ, Belafsky P, Allen J. Fluoroscopic surrogate for pharyngeal strength: the pharyngeal constriction ratio (PCR). Dysphagia. 2011;26:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1676] [Cited by in RCA: 1899] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 15. | Pearson WG, Molfenter SM, Smith ZM, Steele CM. Image-based measurement of post-swallow residue: the normalized residue ratio scale. Dysphagia. 2013;28:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Thompson TZ, Obeidin F, Davidoff AA, Hightower CL, Johnson CZ, Rice SL, Sokolove RL, Taylor BK, Tuck JM, Pearson WG. Coordinate mapping of hyolaryngeal mechanics in swallowing. J Vis Exp. 2014;(87). [PubMed] |
| 17. | Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. 2011;11:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2995] [Cited by in RCA: 2116] [Article Influence: 151.1] [Reference Citation Analysis (0)] |
| 18. | Feng FY, Kim HM, Lyden TH, Haxer MJ, Feng M, Worden FP, Chepeha DB, Eisbruch A. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 355] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 19. | Gillespie MB, Brodsky MB, Day TA, Lee FS, Martin-Harris B. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004;114:1362-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Kendall KA, McKenzie SW, Leonard RJ, Jones C. Structural mobility in deglutition after single modality treatment of head and neck carcinomas with radiotherapy. Head Neck. 1998;20:720-725. [PubMed] |
| 21. | Pauloski BR, Rademaker AW, Logemann JA, Newman L, MacCracken E, Gaziano J, Stachowiak L. Relationship between swallow motility disorders on videofluorography and oral intake in patients treated for head and neck cancer with radiotherapy with or without chemotherapy. Head Neck. 2006;28:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Bingjie L, Tong Z, Xinting S, Jianmin X, Guijun J. Quantitative videofluoroscopic analysis of penetration-aspiration in post-stroke patients. Neurol India. 2010;58:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, Marsh R, Pameijer FA, Balm AJ. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 480] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 24. | Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, Brasseur JG, Hogan WJ. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol. 1989;257:G748-G759. [PubMed] |
| 25. | Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19:691-707, vii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 472] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 26. | Shaw SM, Martino R. The normal swallow: muscular and neurophysiological control. Otolaryngol Clin North Am. 2013;46:937-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Mepani R, Antonik S, Massey B, Kern M, Logemann J, Pauloski B, Rademaker A, Easterling C, Shaker R. Augmentation of deglutitive thyrohyoid muscle shortening by the Shaker Exercise. Dysphagia. 2009;24:26-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Meng H, Murakami G, Suzuki D, Miyamoto S. Anatomical variations in stylopharyngeus muscle insertions suggest interindividual and left/right differences in pharyngeal clearance function of elderly patients: a cadaveric study. Dysphagia. 2008;23:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Miloro KV, Pearson WG, Langmore SE. Effortful pitch glide: a potential new exercise evaluated by dynamic MRI. J Speech Lang Hear Res. 2014;57:1243-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Stokely SL, Peladeau-Pigeon M, Leigh C, Molfenter SM, Steele CM. The Relationship Between Pharyngeal Constriction and Post-swallow Residue. Dysphagia. 2015;30:349-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Leonard R, Kendall KA, McKenzie S. Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia. 2004;19:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |