INTRODUCTION
Ultrasound is an essential modality within medical imaging, predominantly for assessing soft tissues. Recently, the additional tool of ultrasound elastography has become commercially available for further assessment of tissues, in addition to the standard B-mode and Doppler imaging. The elastic properties of tissues are different from the acoustic impedance used to create B mode imaging and the flow properties used within Doppler imaging, hence elastography provides a different form of tissue assessment and possibly showing pathology before it can be detected on B mode imaging. This may be of particular use in the musculoskeletal system where there is a wide spectrum of tissue specialisation. Elastography assesses the strain (stiffness) of these tissues in response to stress, through a variety of different methods which will be discussed.
Throughout history, the stiffness of tissues has been used as a marker of disease, through palpation. Generally, malignant tissues are stiffer or harder than benign tissues, a feature which can be distinguished through direct palpation, corresponding to manual compression. This concept has been extended within the field of ultrasound, with maps of tissue stiffness generated alongside anatomical images.
PRINCIPLES OF ELASTOGRAPHY
The term elastography was described by Ophir et al[1] as a method of portraying the strain properties of biological tissue. The strain of a tissue is its response to an applied stress, or pressure, with both longitudinal and shear components. A longitudinal strain occurs when a tissue is compressed or stretched, whilst a shear strain is the response to angular forces, such as twisting. Biological tissues have both viscous (liquid-like) and elastic (solid-like) properties, with a complex interplay between the two given the non-uniform nature of biological tissues. When a stress is applied to fluids, the pressure is the same in all directions, hence shear strain and shear waves do not exist in pure fluids. The Elastic modulus of a material gives an indication of how it responds to a change in applied stress and is defined as the slope of the stress-strain curve. The elastic modulus can be described as either Young’s modulus (E) (compressive stress/compressive strain) or the shear modulus (G) (shear stress/shear strain). The bulk modulus is a three dimensional extension of Young’s modulus and describes volumetric stress over volumetric strain[2]. Young’s modulus and shear modulus are the most applicable to biological tissues, with an approximation between them described as E 3G. The elastic modulus of a tissue is inversely proportional to the strain, i.e., the greater the elastic modulus, the less the tissue strain.
In medical usage, elastography requires the application of a mechanical stress to the tissues and then measurement of the displacement before and immediately after the stress as an estimate of the strain[3]. Soft tissues in the body have a high water content and are virtually incompressible, thus sophisticated equipment is necessary to detect small tissue displacements. There are currently two main elastography methods in general clinical usage, namely compression/strain elastography and shear wave elastography.
Strain elastography
In strain, or compression elastography, a force (i.e., stress) is applied from the transducer by repetitive manual pressure and the displacement (strain) is calculated from the return velocities of the tissues with respect to time. Motion intrinsic to the subject can also be used as the stress generator, such as aortic pulsation, however this is less useful in the musculoskeletal system where more superficial structures are of interest.
Measuring the displacement (strain) of the tissues secondary to an applied force (stress) gives a qualitative map of the elastic modulus distribution, termed an elastogram. This elastogram is colour-coded and often super-imposed on a grey-scale B mode image for anatomical localisation. True quantitative measures cannot be taken from this elastogram, as the applied force is unknown. A semi-quantitative evaluation, however, can be determined from the ratio of the displacement of the tissue of interest and an adjacent structure, such as subcutaneous fat.
Strain elastography has many potential disadvantages, including the variability in the pressure applied to the tissue. This can be partly compensated for by a graphical representation of the adequacy of the compression, however, the potential for inter-observer and intra-observer variation remains[4]. At least three cycles of compression and decompression have been recommended for optimum assessment[5], however, extensive preload of the tissues through repeated compressions can alter tissue elasticity. Thus, enough compression/decompression cycles must performed to obtain a representative assessment, however, excessive compression may adversely affect the resulting elastograms through pre-loading the tissues. Correct probe alignment is required to compensate for potential out of plane compression of the tissue and anisotropy. Compression along the longitudinal axis of the region of interest has been shown to be optimal as compression in the transverse plane gives rise to artefacts at the medial and lateral sides of the image and out-of-plane movement. Constraints from bony anatomy, such as around the ankle, can also make uniform compression difficult across the region of interest, in particular in the musculoskeletal system.
Shear wave elastography
Shear wave elastography applies a vibration to tissues through a focussed ultrasound pulse, generated by the transducer. This deposition of energy within the tissues creates transverse waves, or shear waves, which are perpendicular to the push pulse. The shear wave velocities can be measured from Doppler frequency modulation of simultaneously transmitted probing ultrasound waves. Young’s modulus can then be estimated as a function of the shear wave velocity. The stiffer the tissue is (the less compliant to shear forces), the faster the propagated shear waves within it[6].
Although shear wave elastography is likely to be more reproducible than strain elastography owing to the standardised applied stress, it still has limitations. Shear waves are attenuated at depth and thus very deep tissues (> 9 cm from the skin) cannot be assessed. Conversely, an adequate depth of tissue is required in order to generate shear waves, hence very superficial structures are difficult to assess. This can partly be compensated for by using a gel stand-off on the patient. Shear waves are not generated within fluids, thus cystic structures cannot be adequately analysed[7]. The size of the region of interest may also potentially affect the shear wave measurements. Similar to strain elastography, the amount of probe pressure applied to the skin has the potential to affect the tissue elasticity, through pre-loading the tissues, as shown in a study assessing muscle elastic properties with shear wave elastography and varying technical parameters[8]. Assessment of a tissue in the longitudinal vs transverse plane may potentially affect the shear wave measurements owing to anisotropy, similar to strain elastography. The acoustic radiation force impulse required to generate shear waves deposits energy in the tissues, manifest partly as heating. This should be considered with repeated measurements, as the heating effect may adversely alter the measurements or potentially cause tissue damage. Commercially available systems have an in-built cooling delay in the order of a few seconds, to reduce any potential heating, however, prolonged usage may still heat the tissues and alter the properties of the generated shear waves.
Measured values of shear waves are given as either a velocity in m/s or a stiffness in kPa The elasticity expressed in kPa can be approximated into shear wave velocity using the equation E = 3rc2, where E is Young modulus, r is density (estimated at 1000 kg/m3), and c is the speed of sound[9]. This method of converting the values is useful in isotropic tissues, such as the breast, however, it may not be applicable to tendons and muscles which are anisotropic, with potential for faster propagation of shear waves along the longitudinal axis of the tendon[10]. Thus, caution must be used when comparing values quoted in kPa with m/s as they may not be directly comparable.
A study assessing the differences between two commercially available systems for shear wave elastography has shown that the results in assessing liver stiffness are broadly similar, however, the measurements in kPa vs m/s are not directly interchangeable, thus, the machines should be kept constant if temporal follow-up is required[11]. Reference standards for shear wave elastography have been determined in many tissues, including the thyroid, submandibular glands, masseter, gastrocnemius and supraspinatus muscles and the Achilles tendon[12], however these should be used with caution given the potential for poor reproducibility between vendor equipment.
Transient elastography
Another form of elastography currently within the remit of clinical practice is transient elastography, however, its use is generally outside of the musculoskeletal field. Transient elastography generates a single short impulse of energy, from which the generated shear waves are measured. This is available commercially as a “Fibroscan” for assessing liver fibrosis, but is not currently used in the musculoskeletal system[13].
MUSCULOSKELETAL APPLICATIONS OF ELASTOGRAPHY
Muscles
Skeletal muscles are readily accessible for ultrasound assessment, being superficial in location. Strain elastography has been used to assess many muscle pathologies[14,15], including muscular dystrophy, with a case report showing diseased muscle fibres to be stiffer compared with a normal healthy volunteer[16]. In children with cerebral palsy, elastography has been used to guide botulinum toxin injections into contracted muscles, identified as harder on the elastogram[17]. In myositis, an alteration in the normal muscle elastogram has been shown, with pathological muscles predominantly appearing harder[18]. Within normal muscle, strain elastography has demonstrated greater stiffness in the biceps brachii muscle following exercise compared with pre-exercise elastograms[19]. The postulated cause for this is the physiological response to exercise with increased blood flow and capillary permeability. Between three observers, the authors showed good reliability. A further study reported differences in the stiffness of quadriceps muscle in female subjects with patellofemoral pain syndrome compared with healthy controls[20]. Elastography has also been used in muscular trauma, showing alteration in the normal elastograms with intra-muscular haemorrhage and fibrosis[21]. Both strain and shear wave elastography have been used to assess masseter muscle in healthy volunteers and patients with facial pain[22-25], with good reproducibility between the two techniques. Patients with facial pain and temporomandibular joint dysfunction have been shown to have a greater stiffness in masseter muscle compared with healthy controls. This correlates with the clinical finding of altered hardness of masseter muscle in symptomatic patients with facial pain, thus ultrasound elastography could become a useful adjunct to current techniques in the assessment of these patients[26].
Assessing skeletal muscle with ultrasound elastography, however, has many potential limitations regarding reproducibility. Ensuring a standardised state of contraction/relaxation between assessments is necessary, as is the state of the muscle regarding exercise and rest. Muscles are also subject to anisotropy, so the probe orientation when assessing different muscles must be kept in the same plane (Figure 1). The above factors generate significant challenges with standardisation. In the immediate future elastography alone or in combination with other imaging modalities is unlikely to replace muscle biopsy for the diagnosis of muscular pathologies, namely muscular dystrophy and myositis. The role of elastography in assessing functional muscle disorders is also in its infancy and more research is needed to assess whether it is truly of value or not, given the difficulty with standardisation.
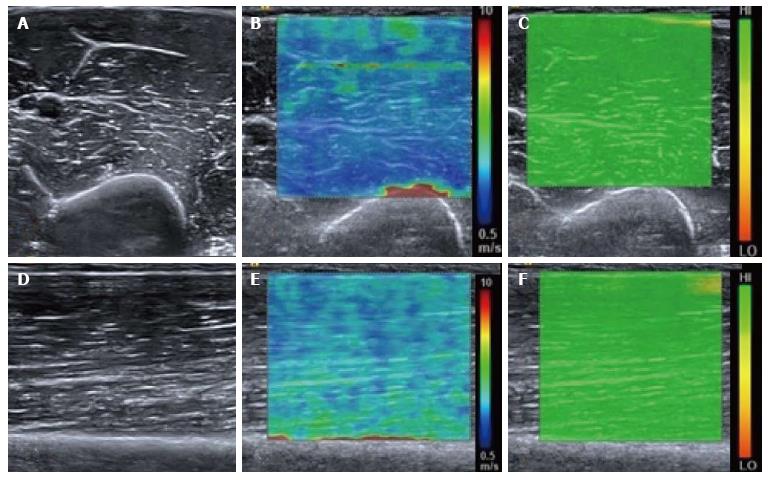
Figure 1 Normal relaxed biceps brachii muscle.
B mode transverse image of the mid biceps brachii muscle (A) with the corresponding shear wave elastography image (B) showing the shear wave velocity distribution. The velocity map is coloured such that blue represents the slowest waves and red the fastest, illustrated by the scale. Note the fast (red colour) shear waves at the interface with the hard humeral bone. A range of shear wave velocities are seen at the fascial interfaces within the muscle belly. The upper right image (C) shows the quality map with green colouring representing a high quality elastogram. Corresponding longitudinal B mode image of the mid biceps brachii muscle (D), shear wave velocity image (E) and quality map (F). The velocity distribution is slightly different in the longitudinal plane compared with the transverse plane owing to the greater effect of anisotropy in the transverse plane compared with the longitudinal plane.
Tendons
Elastography allows the possibility of assessing tendons for mucoid degeneration or small interstitial tears potentially before they become apparent on conventional B-mode imaging. When tendons degenerate, the collagen fibres break down and it is proposed that tendons become softer[27], thus a change in their elasticity can be detected with elastography. Conversely, if a tendon repairs with fibrosis, this may be seen as a hardening of the tendon substance, with a stiffer elastographic picture.
The Achilles tendon has received the most attention within elastography studies owing to its ease of accessibility, relatively large size and susceptibility to pathology. It is composed of tightly packed collagen fibres which appear as hypoechoic on B-mode ultrasound with areas of increased reflectivity corresponding to linear interfaces. In symptomatic Achilles tendons there is a disorganisation and breakdown of the collagen fibres, corresponding to mucoid degeneration, and it is proposed that this leads to a softer tendon structure compared with normal[28]. This is shown as alteration in the B-mode architecture, mainly as regions of hypoechogenicity within the tendon substance. The corresponding strain elastography images often show softer regions within the tendon in these areas[29-31]. In patients with surgically repaired Achilles tendons, the regenerative tissue has been shown to be harder and more heterogeneous compared with healthy controls, indicating the reparative tissue to be firmer than normal tendon tissue[30]. Furthermore, patients with ankylosing spondylitis have been shown to have changes in tendon elasticity in strain elastography correlating with B-mode changes, with a difference compared with healthy controls[32].
Shear wave elastography has also been used to study the Achilles tendon. Slower shear wave velocities have been demonstrated in tendinopathic tendons compared with healthy controls[33], also suggesting a softer tendon substance in pathology (Figure 2). As described previously, shear wave elastography measurements are not necessarily comparable between different manufacturers’ equipment, thus potentially limiting the reproducibility. This is evidenced by the large range of quoted normal shear wave values for the Achilles tendon within healthy subjects[34].
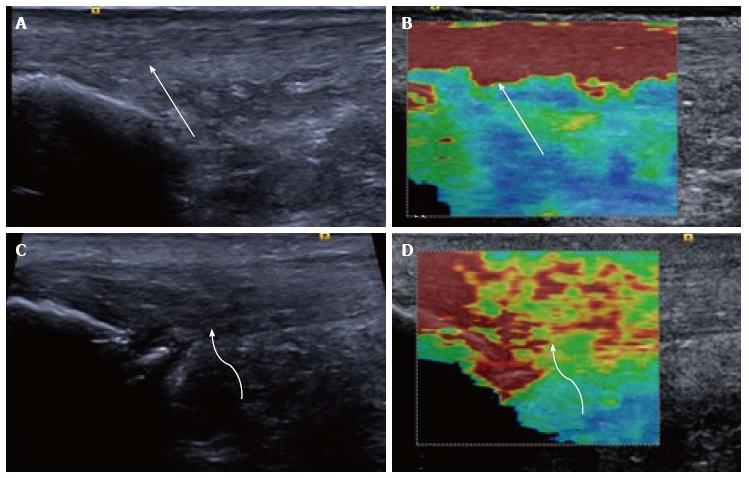
Figure 2 Longitudinal shear wave elastography of the Achilles tendon.
In a healthy volunteer the Achilles tendon is seen as smooth and homogeneous (arrow) on the B mode image (A) with a homogeneous elastogram (B, arrow). In a patient with symptomatic Achilles tendinopathy there is an alteration of the B mode echotexture with regions of hypoechogenicity (C, curved arrow) and dystrophic ossification at the calcaneal enthesis. The elastogram (D) is heterogeneous with regions of blue and yellow colouring (curved arrow) corresponding to a slower velocity and tendon softening.
The reproducibility of shear wave elastography has been assessed in tendons with in vivo and in vitro testing. In a study from 2013, repeatability was found to be better in the in vitro setting compared with in vivo, most likely because the in vitro conditions were more amenable to standardisation[35]. Also, the assessed tendon outside the body is static, compared with potential for movement and assessing a different position along the tendon or different probe orientation in human subjects compared with laboratory conditions. A different in vitro study of harvested equine tendons and strain elastography showed poor repeatability between experiments, reflecting the more variable nature of strain elastography compared with shear wave elastography[36].
Away from the Achilles tendon, ultrasound elastography has been used to assess supraspinatus muscle and tendon (Figure 3), with tendinosis predominantly being shown as tendon softening, but also regions of increased stiffness, purported to represent reparative fibrosis (Figures 4 and 5)[37-39]. Strain elastography has also been used around the elbow to assess the common extensor tendon. In a study comparing healthy volunteers with those who were symptomatic, the symptomatic common extensor tendons were shown to be softer compared with healthy volunteers[40].
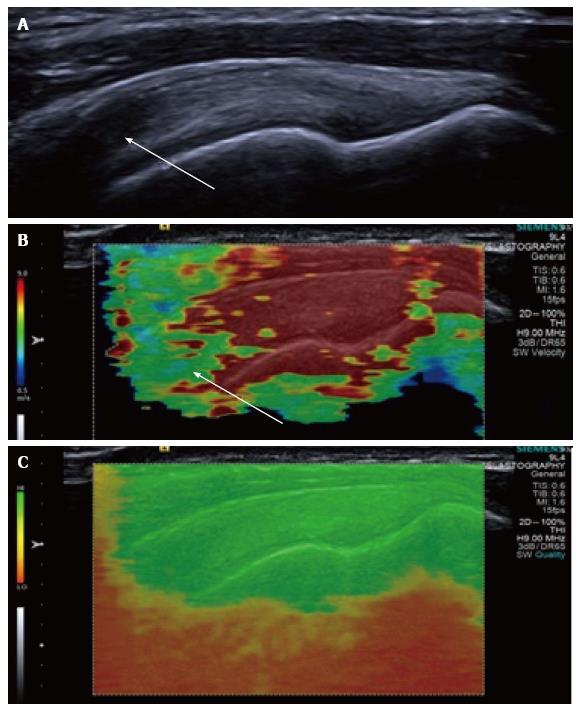
Figure 3 Longitudinal shear wave elastography of a normal supraspinatus tendon.
Normal B mode appearances (A) showing anisotropy (arrow) owing to the curved orientation of the tendon. The corresponding elastogram (B) shows heterogeneous stiffness at the region of anisotropy (arrow) and absence of measurements from deep in the humeral head. The quality map (C) shows a high quality elastogram in the tendon substance and poor quality in the bone, as would be expected for such a stiff structure with limited propagation of shear waves.
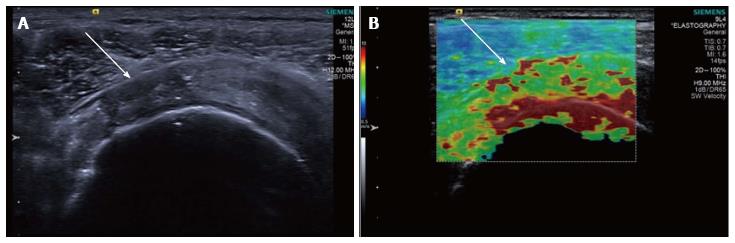
Figure 4 Longitudinal shear wave elastography of a tendinopathic supraspinatus tendon showing a disorganised, heterogeneous pattern, contrasted to the homogeneous appearance of the normal tendon in Figure 3.
Note the regions of interest (small boxes) where shear wave velocity measurements have been taken.
Figure 5 Longitudinal shear wave elastography of a tendinopathic supraspinatus tendon in a different patient to Figure 4.
The B mode image (A) shows a partial thickness tear involving the bursal surface fibres (arrow). The corresponding velocity elastogram (B) shows a disorganised pattern with the tear less well delineated (arrow) compared with the B mode image.
Thus, within the tendons the predominant elastographic picture of mucoid degeneration/tendinosis is softening, however, some studies have shown tendinosis as increased tendon stiffness, making a general statement of the elastic properties of tendinosis problematic. The future role of elastography may therefore be to identify areas that have a heterogeneous, disordered elastogram (either softening or hardening) corresponding to tendinosis, rather than the homogeneous elastogram of a normal tendon. This may allow preclinical detection of pathology, potentially of relevance to athletes allowing an alteration in training before injuries occur, or as a screening tool prior to any sporting commitments. Further research is also needed to ascertain if the changes identified on elastography resolve following treatment, or if the tendon becomes asymptomatic. If this were known, then potentially the appearances of a tendon on elastography could be used for prognosis to try and predict the time for tendon healing. Additionally, the elastograms of asymptomatic people of different ages has not been fully characterised and it is not known if the normal ageing process gives rise to different tendon stiffness, or the relevance of alterations in the elastograms in asymptomatic individuals.
Fascia and ligaments
A recent prospective study has shown that the coraco-humeral ligament is stiffer in patients with adhesive capsulitis compared with their unaffected contralateral shoulder. Interestingly, this study also demonstrated variations in the stiffness of the ligament depending on the degree of external rotation of the arm[41]. This is an exciting development, potentially adding additional criteria for diagnosing adhesive capsulitis in addition to the thickness of the coraco-humeral ligament.
Within the plantar fascia, a study using strain elastography found the plantar fascia in symptomatic patients to be thicker and more hypoechoic compared with controls, correlating with a loss of elasticity, or a harder fascia[42].
A further study looking at the stiffness of the A1 pulley in patients with trigger finger showed the pulley to be stiffer in symptomatic patients[43], corresponding with changes in the B-mode appearances too. A further preliminary study has assessed the transverse carpal ligament in the carpal tunnel and the effects of probe pressure on the resultant shear wave velocities. Variations were identified according to the applied probe pressure, indicating that the technique is still somewhat operator dependent[44]. The small size of the finger pulleys and carpal ligaments also significantly limits the use of this technique as the resolution of these small structures can be less than the resolution of the elastograms, particularly with regard to the region of interest for measurement of shear wave velocities within the currently available equipment.
Carpal tunnel
The median nerve at the wrist has been studied with both shear wave elastography and strain elastography[45]. Using strain elastography, the median nerve in patients with carpal tunnel syndrome has been shown to be stiffer compared with controls[46]. These findings are also replicated with shear wave elastography, with a greater shear wave stiffness shown in patients with carpal tunnel syndrome compared with controls[47]. Thus elastography is a useful addition to the B mode appearances and the cross sectional area of the median nerve in diagnosing carpal tunnel syndrome, with potential to replace nerve conduction studies as the diagnostic gold standard.
Joints
Very little information is available on elastography of the joints, in particular, the synovium. To date, there are no published clinical trials assessing elastography in the joints. Case reports of strain elastography in a small number of patients suggests that inflammatory synovitis may appear as firm, compared with infective synovitis which may appear as firm to soft. Thus, there is overlap in the elastographic appearances between infective and inflammatory synovitis and the technique is unable to replace the gold standard of tissue diagnosis for either infective or inflammatory synovitis[48,49].
There is no data published on the elastographic appearances of the synovium in normal subjects vs those with an inflammatory arthropathy (Figure 6). This is an area requiring further research, to determine if elastography can be as useful a tool as Doppler in the assessment of suspected inflammatory synovitis and potential response to disease modifying anti-rheumatic therapies.
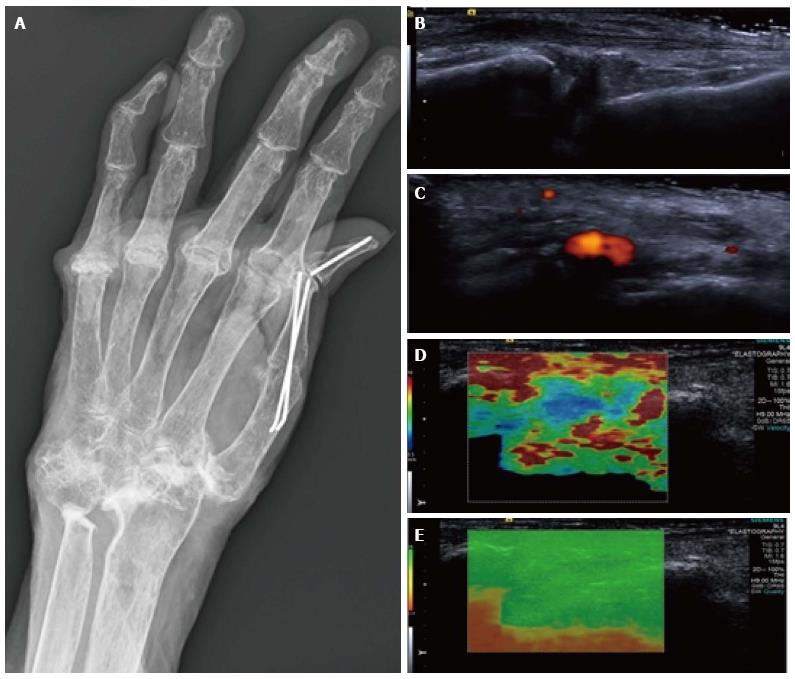
Figure 6 Synovitis at the carpus.
Radiograph (A) in a patient with long-standing rheumatoid arthritis and worsening wrist pain. The longitudinal B mode image (B) and colour Doppler image (C) show hypoechogenicity of the synovium with increased Doppler flow indicating an active synovitis. The corresponding longitudinal shear wave velocity elastogram (D) shows relatively uniformly soft synovium (blue colour) compared with the adjacent tissues which are more heterogeneous in their stiffness. The significance of this has not yet been adequately investigated. The elastogram is of good quality (E).
Tumours
Elastography in tumours has been used extensively outside the musculoskeletal system, predominantly within breast, thyroid and prostate lesions. Within the breast, elastography is proving to be useful to help differentiate between benign and malignant lesions (including threshold values for shear wave velocities in malignant lesions) however, within the thyroid there is significant overlap in the elastographic characteristics between the two.
In musculoskeletal soft tissue tumours, elastography has been used to prospectively assess lesions to attempt to differentiate between benign and malignant pathologies. Malignant lesions have been shown to be stiffer on strain elastography compared with benign lesions on a semi-quantitative scale, similar to lesions outside of the musculoskeletal system[50]. However, a recently published study by the group in Leeds assessing the role of shear wave elastography in musculoskeletal tumours has been unable to replicate these early findings, with the authors concluding that there is currently no additional role for shear wave elastography in soft tissue tumours compared with B mode imaging[51]. The authors found no statistically significant association between shear wave velocity and malignancy. According to the strain elastography study, one may have expected malignant tumours to be stiffer than benign tumours and thus have a tendency for higher shear wave velocities. Possibly the discordant results are accounted for by the tumour case mix, different elastography technique or study design. Soft tissue tumours are very heterogeneous on B mode imaging (Figure 7) compared with breast lesions and it is probable that further studies will replicate the findings of the Leeds group, showing a large overlap in the shear wave velocity measurements between benign and malignant soft tissue lesions. In the immediate future imaging characteristics alone, even with the addition of elastography, are unlikely to replace biopsy for the diagnosis of malignant vs benign lesions. Similar to tendons, the role of elastography in soft tissue tumours is likely to be in identifying a heterogeneous, disorganised internal substance suggesting an aggressive nature or de-differentiation, vs a smooth homogeneous pattern of a non-aggressive lesion. This could be used to guide biopsy by potentially identifying aggressive regions within a lesion, or to identify malignant degeneration in a previously diagnosed benign lesion.
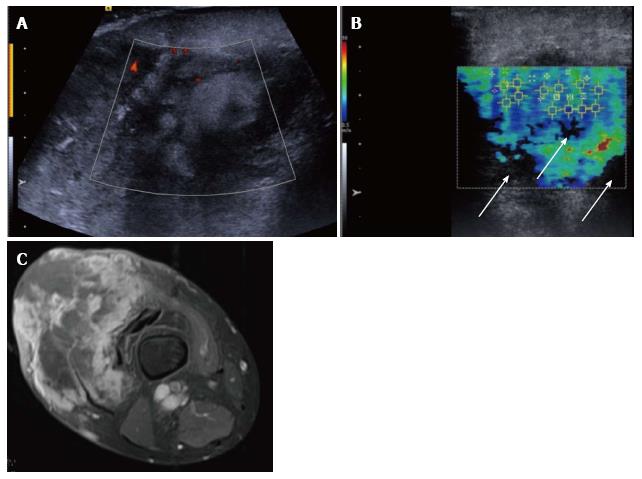
Figure 7 Pleomorphic sarcoma of the distal thigh.
Transverse B mode image with colour Doppler (A) shows a disorganised tumour with wide variation in echotexture and limited Doppler flow. The shear wave velocity elastogram (B) also shows a wide variation in stiffness with some regions of absent measurements, seen as the black areas on the image (arrow), possibly owing to the very dense/stiff nature of the tumour. The axial proton density fat suppressed magnetic resonance image (C) shows a large tumour in the anterior compartment of the thigh with very varied signal.
Another potential use of elastography in tumours may be to define the boundaries of a lesion, compared with normal tissue. In regions with poor B-mode contrast it can be hard to identify the margin of a lesion, however, the elastogram may show a sharp demarcation if the lesion has different elastic properties compared with the adjacent normal tissue (Figure 8). This is of value within breast[52] and prostate lesions and may prove to be the most useful application of elastography in soft tissue tumours.
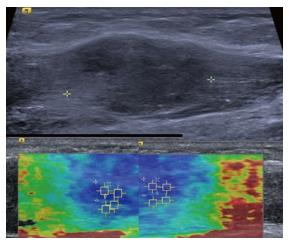
Figure 8 Intramuscular nodular fasciitis, longitudinal images.
The B mode image (upper image) shows that the margins of the lesion are poorly defined and relatively hard to discern. The shear wave velocity elastograms (lower images) show a clear difference in stiffness between the lesion and adjacent normal muscle fibres, as shown by the blue (slow) colour map of the lesion compared with the green and red (faster) colour map of the adjacent muscle fibres.
ELASTOGRAPHY OUTSIDE THE MUSCULOSKELETAL SYSTEM
Elastography has been extensively used in structures outside the musculoskeletal system, namely the liver, breast, thyroid and prostate[53-60]. Within the liver, it has now become standard in the assessment of fibrosis, replacing biopsy in many instances. In the breast, thyroid and prostate, elastography has become part of the non-invasive assessment of a lesion, being used in addition to the B mode and Doppler characteristics[61-63]. Generally, aggressive cancers with more malignant features have been shown to have a greater stiffness as measured by shear wave elastography compared with less aggressive tumours[64]. However, despite the recent advances in tumour characterisation with elastography and other imaging parameters, the gold standard of biopsy has not yet been replaced by imaging alone for the majority of lesions and it this is unlikely to be the case in the near future.
FUTURE DIRECTIONS
Technical
As with all technological advances, time will bring refinements in the current technology, perhaps allowing shear wave measurements at greater depths, or within very superficial structures such as skin.
Elastography adds another facet to the available ultrasound capabilities and may potentially be fused with other imaging modalities, namely CT and MRI. This could then create a navigable data set to aid with surgical planning or to guide tumour resection, particularly with reference to tumour margins.
Clinical applications
Within the musculoskeletal system ultrasound elastography has the potential to become as valuable a tool as within the liver, breast and thyroid. It may allow identification of preclinical tendinosis and thus modification of activity or prophylactic treatment prior to symptoms developing. It could be used to predict time to recovery following treatment and help guide the timing of return to activity, once the tendon elastogram returns to normal.
Elastography could ultimately obviate the need for biopsy in soft tissue tumours enabling differentiation between benign and malignant lesions, although as described above, further research is needed. Following tumour resection it may help distinguish post-surgical scarring from recurrent tumour, with the scarring being harder and more homogeneous than tumour.
Elastography could possibly be used to assess the state of cartilage in areas accessible to the ultrasound probe, such as the shoulder and talar dome. Cartilage which is degenerate may display different elastic properties, similar to DGEMRIC and T2 rho magnetic resonance imaging (MRI), thus providing a cheaper alternative to MRI. Within the synovium, if the synovitis of inflammatory arthropathies displays reliable alterations in elasticity compared with normal synovium, it may form part of the routine assessment and synovitis grading, similar to power Doppler.
Within current parameters, elastography data from bone is limited as it is virtually incompressible. If the technology could be refined to detect even the smallest deformation in bone or fracture callus, it could potentially be used to assess when a fracture callus has matured enough to withstand weight-bearing, or to similarly assess the fusion mass following spinal surgery.