Published online Oct 28, 2016. doi: 10.4329/wjr.v8.i10.846
Peer-review started: March 9, 2016
First decision: May 19, 2016
Revised: July 26, 2016
Accepted: August 17, 2016
Article in press: August 18, 2016
Published online: October 28, 2016
Processing time: 233 Days and 18.4 Hours
A 60-year-old man was admitted due to rectosigmoid colon cancer, and a hepatic mass was incidentally found during the staging work-up. The mass appeared cystic with a thick wall and contained multiple bizarre cord-like structures on ultrasound, computed tomography and magnetic resonance imaging. The differential diagnoses included organizing abscess/hematoma, foreign body granuloma and parasite infestation. Serologic study revealed anti-sparganum antibodies. Over 4-year follow-up, the patient did not complain of symptoms, and no changes in the characteristics of the liver mass were observed. Hepatic sparganosis is rare; only two cases have been clinically reported, and no detailed radiologic description was available until now. This case report presents a detailed radiologic description of a hepatic mass that could most likely represent hepatic sparganosis.
Core tip: Hepatic sparganosis is rare; only two cases have been clinically reported, and no detailed radiologic description was available until now. This report presents radiologic findings of a presumptive case of sparganosis manifesting as a hepatic mass. This hepatic mass showed nonenhancing low attenuation mass with bizarrely arranged calcified internal cord-like structures on computed tomography, a necrotic mass with internal serpiginous tubular structures on magnetic resonance imaging, and a well-defined mixed echoic mass with multiple cord-like structures on ultrasonography. The understanding of this case will help physicians to consider the possibility of hepatic sparganosis when they encounter hepatic masses with bizarrely arranged internal serpiginous structures.
- Citation: Jo GD, Lee JY, Hong ST, Kim JH, Han JK. Presumptive case of sparganosis manifesting as a hepatic mass: A case report and literature review. World J Radiol 2016; 8(10): 846-850
- URL: https://www.wjgnet.com/1949-8470/full/v8/i10/846.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i10.846
Sparganosis refers to a parasitic infestation of plerocercoid larva of Spirometra. Humans can serve as second intermediate hosts, paratenic hosts and occasionally definitive hosts. Three routes of infection are currently known. The first is the direct ingestion of live plerocercoid larva via the consumption of raw second intermediate hosts (amphibians, reptiles, avians, mammals, etc.). The second is the consumption of water containing Cyclops species infected with procercoid larva. The third method route of infection is the use of the flesh or skin of infected frogs or snakes as a poultice. Regions of high prevalence are located in Asian countries, including China, Japan, Thailand and South Korea[1]. Sparganosis frequently involves the abdominal wall, scrotum, lower extremities and thoracic wall. Less frequently involved areas include the abdominal cavity, pleural cavity, vertebral canal and orbit[2]. Regarding the liver, only two cases of sparganosis have previously been reported to the best of our knowledge. Khurana et al[3] reported a case of hepatic sparganosis in the English literature in 2012. The other case of hepatic sparganosis was reported in 1990 in the Korean literature[4]. Unfortunately, neither of these case reports contain sufficient radiologic findings of hepatic sparganosis because they did not contain radiologic images or descriptions of computed tomography (CT) or magnetic resonance (MR) imaging findings but only included ultrasonography (US) findings.
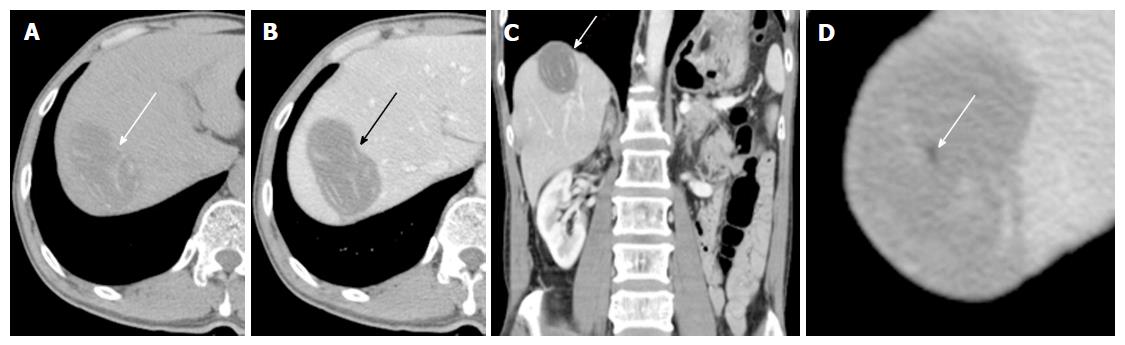
A 60-year-old man with rectosigmoid colon cancer was referred to our hospital for preoperative staging evaluation. He was diagnosed with rectosigmoid colon cancer at a regular health checkup. During an abdominal CT study performed for the staging of the rectosigmoid colon cancer, a large low-attenuation mass was incidentally detected in the liver. This mass had internal bizarrely arranged cord-like structures, calcifications, and a few focal areas of fat (Figure 1). Our primary radiologic impression was a teratoma. Angiomyolipoma and myxoid liposarcoma were included in the differential diagnosis. Cystic metastasis of colon cancer was described as the least likely differential diagnosis.
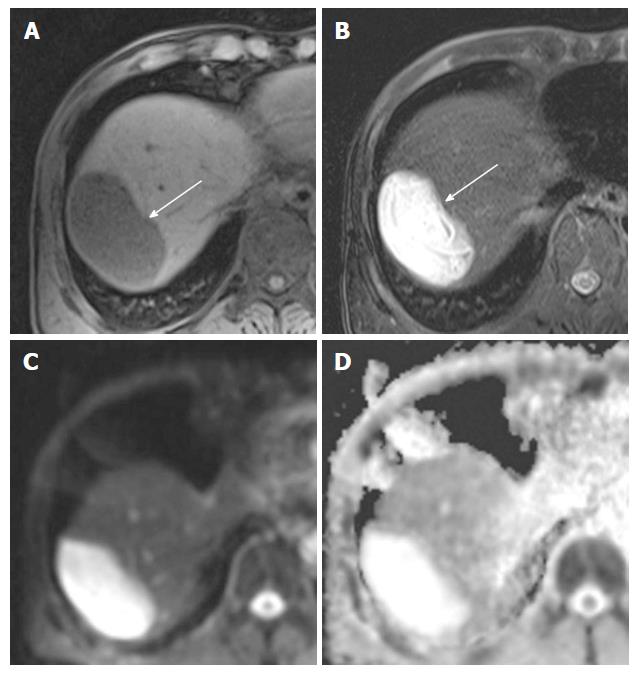
For further evaluation, gadolinium-enhanced liver MR imaging was performed (Figure 2). Serpiginous tubular structures were clearly observed on T2-weighted images (Figure 2B). Diffusion in the mass was not restricted (Figure 2C and D). Axial in-phase T1-weighted and opposed-phase T1-weighted images showed multiple small foci with signal drops in opposed-phase image that are indicative of the presence of microscopic fat deposits. These findings indicated a fat-containing totally necrotic mass or a cystic mass with internal dead tissue. Our differential diagnosis included an organizing abscess/hematoma, a foreign body granuloma, and parasite infestation. Colon cancer metastasis was considered unlikely.
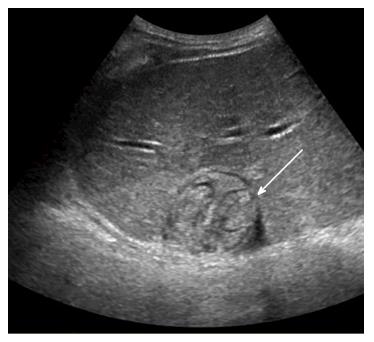
Liver US was performed to further evaluate the internal content of the liver mass. The mass was well demarcated upon US and contained echogenic material and serpiginous echogenic structures with a low echoic tubular rim (Figure 3). No vascularity was observed in the mass on color Doppler US. These findings led us to place foreign body granuloma and parasite-induced granuloma at the top of the list of differentials.
In a complete blood count including a differential cell count, no leukocytosis (white blood cell 5.02 × 103/μL) was observed, and the proportion of eosinophils was normal (2%). Because decreased liver excretory function was found on an indocyanine green excretion test, only an anterior resection of the rectosigmoid colon cancer without liver resection was conducted. The patient was discharged and followed up on an outpatient basis.
ELISA was performed two months after surgery to identify the parasite. The patient’s serum was tested for antibodies against Clonorchis, Paragonimus, Cysticercus and Sparganum. Only anti-sparganum antibodies were detected at a titer that was 1.4-fold of the cutoff value (positive and negative control sera were used to rule out the possibility of false negativity and false positivity). Although the treatment of choice for sparganosis is surgical removal, this approach was not employed due to the decreased liver excretory function. The hepatic mass was left untreated and regularly followed up.
The patient denied eating raw or undercooked snake or frog flesh and applying the skin or flesh of these animals to a wound as a poultice but admitted to drinking mountain water several times. Because he had a history of travel to the middle East for two years, which is a crucial diagnostic clue for echinococcosis[5], and serpiginous linear structures could also be observed in echinococcal cysts[6], an additional ELISA test was performed to differentiate between sparganosis and echinococcosis four years after surgery. At this time, the antibody titers for Sparganum and Echinococcus antigen were both below the cutoff values.
He underwent CT scans every 6 mo. During four years of follow-up, the mass in the liver exhibited no change in size or other aspects of appearance.
The radiologic characteristics of sparganosis in cerebral, scrotal, mammillary, and musculoskeletal areas have been described in several articles[7-13]. Serpiginous tubular structures have been reported in the breast, scrotal, and musculoskeletal sparganosis[11-13]. One reported scrotal sparganosis exhibited multiple serpiginous tubular echogenic structures surrounded by low echoic rims in an echogenic mass, and these findings were similar to the US findings from our case[11]. According to previous reports, serpiginous echogenic tubular structures and surrounding echogenic masses are indicative of sparganum larvae and granulomatous inflammation, respectively[11-13].
The two cases of hepatic sparganosis that have been reported thus far presented with large well-defined hepatic abscesses and sparganum larvae coming out of the drains[3,4]. The expelled larvae were flat, long and thread-like (35-40 cm long and 1.5-1.7 mm in width). Sparganum larvae may involve potentially all body tissues but the subcutaneous tissue is the most frequent site[14]. The phenomenon is speculated by the hypothesis that the plerocercoid larvae migrate to the tissue of rather low temperature. Another reason is that subcutaneous sparganosis is more easily detectable than that in deep viscera. In any reason, hepatic sparganosis is extremely rare.
The sensitivity and specificity of the ELISA used in this case are known to be 85.7% and 95.7% for sparganosis and 91.5% and 96% for Echinococcus, respectively[15,16]. The high specificity of the ELISA supports the notion that the hepatic mass resulted from sparganum. Overall, based on our imaging findings, initial ELISA test findings and the decreasing trend in the antibody titer for sparganum upon the additional ELISA, we concluded that our case involved a large organizing abscess with dead sparganum larva.
Hydatid cysts caused by Echinococcus infection can exhibit internal serpiginous structures due to the detachment of the pericysts and the collapsed membranes inside the cyst, which are collectively called the “snake sign”[6]. However, these collapsed membranes exhibit low signal intensity on all MR imaging sequences, which was not observed on any of the MR images in our case. Typical imaging findings related to hydatid cysts include daughter cysts, floating membranes, internal septa, and thickened walls that represent the pericyst; none of these findings were present in our case.
Calcification on CT images is a common finding of sparganosis, particularly in cerebral sparganosis; up to 75% of all cerebral sparganosis cases have been reported to have calcification[17]. Calcifications represent old lesions in late stages[10]. This finding also supports our diagnosis. The reason that our case had fat foci is unclear but might be related to the process of collagen degeneration in the dead worms.
In conclusion, our case may involve the very rare condition of hepatic sparganosis manifesting as a hepatic mass with calcified serpiginous structures and detailed US, CT, and MR imaging features. The understanding of this case will encourage radiologists and physicians to consider the possibility of hepatic sparganosis when they encounter hepatic masses with bizarrely arranged internal serpiginous structures.
A 60-year-old man with rectosigmoid colon cancer having incidentally detected hepatic mass during cancer staging evaluation.
Organizing abscess/hematoma, foreign body granuloma and parasite infestation.
Only anti-sparganum antibodies were detected on ELISA test.
Computed tomography (CT) scan demonstrated a large low-attenuation mass with internal bizarrely arranged cord-like structures, calcifications, and a few focal areas of fat. Liver magnetic resonance (MR) imaging showed serpiginous tubular structures on T2-weighted images. Diffusion in the mass was not restricted on diffusion-weighted MR images. Liver ultrasonography (US) demonstrated a well-demarcated mass with echogenic material and serpiginous echogenic structures.
This case report describes imaging features of rare hepatic sparganosis. Awareness of possibility of hepatic sparganosis can lead to proper diagnostic tests and earlier diagnosis.
The strength of this article is a detailed radiologic description of a presumptive case of hepatic sparganosis, including US, CT, and MR imaging findings.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cerwenka H, Huang CT, Torres US S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Lv S, Zhang Y, Steinmann P, Zhou XN, Utzinger J. Helminth infections of the central nervous system occurring in Southeast Asia and the Far East. Adv Parasitol. 2010;72:351-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Cho SY, Bae JH, Seo BS. Some Aspects Of Human Sparganosis In Korea. Kisaengchunghak Chapchi. 1975;13:60-77. [PubMed] [DOI] [Full Text] |
| 3. | Khurana S, Appannanavar S, Bhatti HS, Verma S. Sparganosis of liver: a rare entity and review of literature. BMJ Case Rep. 2012;2012:pii: bcr2012006790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Song CS, Moon WK, Kang PJ, Lee DW, Yang WS, Huh Y, Moon HK. A case of sparganosis combined with liver abscess. Korean J Med. 1990;39:686-690. |
| 5. | Byun SJ, Moon KC, Suh KS, Han JK, Chai JY. An imported case of echinococcosis of the liver in a Korean who traveled to western and central Europe. Korean J Parasitol. 2010;48:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Marrone G, Crino’ F, Caruso S, Mamone G, Carollo V, Milazzo M, Gruttadauria S, Luca A, Gridelli B. Multidisciplinary imaging of liver hydatidosis. World J Gastroenterol. 2012;18:1438-1447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 7. | Chang KH, Cho SY, Chi JG, Kim WS, Han MC, Kim CW, Myung H, Choi KS. Cerebral sparganosis: CT characteristics. Radiology. 1987;165:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Kradel J, Drolshagen LF, MacDade A. MR and CT findings in cerebral sparganosis. J Comput Assist Tomogr. 1993;17:989-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Moon WK, Chang KH, Cho SY, Han MH, Cha SH, Chi JG, Han MC. Cerebral sparganosis: MR imaging versus CT features. Radiology. 1993;188:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Song T, Wang WS, Zhou BR, Mai WW, Li ZZ, Guo HC, Zhou F. CT and MR characteristics of cerebral sparganosis. AJNR Am J Neuroradiol. 2007;28:1700-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Kim YJ, Lee MW, Jeon HJ, Yi JG, Paick SH, Kim HG, Lim SD, Hwang TS. Sparganosis in the scrotum: sonographic findings. J Ultrasound Med. 2007;26:129-131. [PubMed] |
| 12. | Hong SJ, Kim YM, Seo M, Kim KS. Breast and scrotal sparganosis: sonographic findings and pathologic correlation. J Ultrasound Med. 2010;29:1627-1633. [PubMed] |
| 13. | Cho JH, Lee KB, Yong TS, Kim BS, Park HB, Ryu KN, Park JM, Lee SY, Suh JS. Subcutaneous and musculoskeletal sparganosis: imaging characteristics and pathologic correlation. Skeletal Radiol. 2000;29:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Liu Q, Li MW, Wang ZD, Zhao GH, Zhu XQ. Human sparganosis, a neglected food borne zoonosis. Lancet Infect Dis. 2015;15:1226-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Kim H, Kim SI, Cho SY. Serological Diagnosis Of Human Sparganosis By Means Of Micro-ELISA. Kisaengchunghak Chapchi. 1984;22:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Jin Y, Anvarov K, Khajibaev A, Hong S, Hong ST. Serodiagnosis of echinococcosis by ELISA using cystic fluid from Uzbekistan sheep. Korean J Parasitol. 2013;51:313-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Chang KH, Chi JG, Cho SY, Han MH, Han DH, Han MC. Cerebral sparganosis: analysis of 34 cases with emphasis on CT features. Neuroradiology. 1992;34:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |