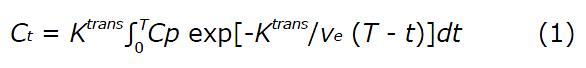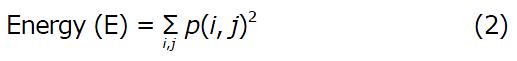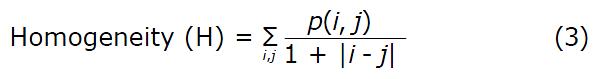Peer-review started: July 4, 2015
First decision: September 22, 2015
Revised: September 24, 2015
Accepted: November 23, 2015
Article in press: November 25, 2015
Published online: January 28, 2016
Processing time: 210 Days and 18.2 Hours
AIM: To investigate the merits of texture analysis on parametric maps derived from pharmacokinetic modeling with dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) as imaging biomarkers for the prediction of treatment response in patients with head and neck squamous cell carcinoma (HNSCC).
METHODS: In this retrospective study, 19 HNSCC patients underwent pre- and intra-treatment DCE-MRI scans at a 1.5T MRI scanner. All patients had chemo-radiation treatment. Pharmacokinetic modeling was performed on the acquired DCE-MRI images, generating maps of volume transfer rate (Ktrans) and volume fraction of the extravascular extracellular space (ve). Image texture analysis was then employed on maps of Ktrans and ve, generating two texture measures: Energy (E) and homogeneity.
RESULTS: No significant changes were found for the mean and standard deviation for Ktrans and ve between pre- and intra-treatment (P > 0.09). Texture analysis revealed that the imaging biomarker E of ve was significantly higher in intra-treatment scans, relative to pretreatment scans (P < 0.04).
CONCLUSION: Chemo-radiation treatment in HNSCC significantly reduces the heterogeneity of tumors.
Core tip: Head and neck squamous cell carcinoma (HNSCC) is a major form of cancer that still kills many cancer patients, and patients would certainly benefit with improved imaging methodology. The merits of texture analysis were investigated on parametric maps derived from pharmacokinetic modeling with dynamic contrast-enhanced magnetic resonance imaging as imaging biomarkers for the prediction of treatment response in patients with HNSCC, undergoing chemo-radiation treatment. Texture analysis revealed that the imaging biomarker energy of parameter ve was significantly higher in intra-treatment scans, relative to pretreatment scans. This indicates that chemo-radiation treatment in HNSCC significantly reduces the heterogeneity of tumors.
- Citation: Jansen JF, Lu Y, Gupta G, Lee NY, Stambuk HE, Mazaheri Y, Deasy JO, Shukla-Dave A. Texture analysis on parametric maps derived from dynamic contrast-enhanced magnetic resonance imaging in head and neck cancer. World J Radiol 2016; 8(1): 90-97
- URL: https://www.wjgnet.com/1949-8470/full/v8/i1/90.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i1.90
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) has become an important tool for the non-invasive quantification of tumor-associated vasculature[1]. By applying pharmacokinetic modeling on sequential T1-weighted images acquired after administration of a contrast agent, one can yield relevant quantitative tumor biomarkers, such as volume transfer rate (Ktrans) and volume fraction of the extravascular extracellular space (ve)[2,3]. For patients with advanced head and neck squamous cell carcinoma (HNSCC), DCE-MRI has shown potential for assessment of treatment response and outcome[4-6]. More specifically, measures derived from DCE-MRI, such as Ktrans and ve, were demonstrated to provide helpful additional information regarding characterization and prognosis of HNSCC with neck nodal metastases[7,8]. HNSCC has been shown to be heterogeneous, due to a chaotic and poorly organized tumor vasculature. Furthermore, tumor heterogeneity is linked with tumor malignancy[9,10]. Tumor heterogeneity of the blood supply may underlie treatment resistance as it prevents therapeutic efficacy. Thus, tumor heterogeneity is highly relevant for the assessment of tumor malignancy and the prediction of treatment outcome. Most studies often report summarizing measures, including mean, median, or standard deviation based on a selection of pixel-by-pixel values, to characterize the whole tumor. However, these often utilized parameters do not always completely characterize the morphologic nature (i.e., heterogeneity) of tumors. It is clinically highly relevant to discover or improve on imaging biomarkers that properly reflect tumor heterogeneity that may assist HNSCC treatment.
A suitable method to assess tumor heterogeneity is image texture analysis[11-15]. During texture analysis, an algorithm is utilized that quantifies spatial intensity coherence of an image, which yields a number of textural features (related to heterogeneity), that are independent of the above mentioned summarizing measures such as mean and standard deviation. An example is the gray-level co-occurrence matrix (GLCM), which is a popular algorithms for texture analysis[11]. Image texture analysis has shown potential in the application of tumor differentiation and treatment response prediction[16-18]. For example, Karahaliou et al[17] investigated the feasibility of using texture analysis to quantify the heterogeneity of lesion kinetics and differentiate malignant and benign breast lesions. El Naqa et al[12] employed the texture features from PET images to predict treatment response in cervix and head and neck cancers. Alic et al[11] found that the tumor response group in limb sarcoma had a high feature of image coherence in pretreatment DCE-MRI Ktrans maps. However, the texture analysis of DCE-MRI pharmacokinetic maps has not been investigated in head and neck cancers for predicting treatment response yet. The purpose of the current study is to assess the merits of GLCM-based texture analysis of parametric maps derived from DCE-MRI pharmacokinetic modeling for the prediction of treatment response in patients with HNSCC.
Our institutional review board approved and issued a waiver of informed consent for this retrospective study, which was compliant with the Health Insurance Portability and Accountability Act. Nineteen head and neck cancer patients with nodal metastases (M/F: 16/3; age: 41-74 years old; primary tumor site: Oropharynx) with histopathologically confirmed HNSCC were selected for this study. The characteristics of the patients are listed in Table 1. All patients were treated with intensity-modulated radiation therapy with dose prescriptions of 70 Gy for gross disease, 59.4 Gy for high-risk regions, and 50 to 54 Gy for low-risk regions[19,20]. Patients received cetuximab (400 mg/m2 loading dose, followed by 250 mg/m2 weekly), bevacizumab (15 mg/kg, days 1 and 22), and cisplatin (50 mg/m2, days 1, 2, 22, and 23). Locoregional control (LC) was determined by clinical and radiographic examination [MRI and/or positron emission/computed tomography (PET/CT)] using established criteria[21,22] with a median followup of 32 mo (range: 14.7-76.3). Patients with residual morphologic abnormalities on followup imaging were not classified as a locoregional failure (LF) unless recurrence was proven by biopsy or the abnormality progressed in size on serial imaging.
| Characteristics | Value |
| Total patients | 19 |
| Demographics | |
| Mean age (yr) | 57 |
| Age range (yr) | 41-74 |
| Male/female | 3/16 |
| Location of primary tumor | |
| Oropharynx | 19 |
| Stages | |
| Stage III | 15 |
| Stage IV | 4 |
| Tumor size (cm3) | 0.615-14.879 |
| Therapy type | Chemo-radiation |
| Outcome | |
| Locoregional control | 17 |
| Locoregional failure | 2 |
Patients underwent examination with MRI before (pretreatment) and 10 to 14 d (intra-treatment) after the commencement of chemo-radiation treatment. MRI was performed on a 1.5-Tesla scanner (General Electric, Milwaukee, WI, United States) using an 8-channel neurovascular phased-array coil. The MRI protocol consisted of the standard anatomic MRI scans (T1/T2 weighted images) and DCE-MRI scans.
A two-dimensional spoiled gradient echo (2D-SPGR) pulse sequence was used for DCE-MRI image acquisition. The data acquisition parameters for the 2D-SPGR pulse sequence were: Repetition time = 7.8 ms, echo time = 1.9 ms, temporal resolution = 6 s, phases = 50-60, number of excitation = 1, flip angle (α) = 30°, receive bandwidth = 15.63-kHz, field of view = 18-20 cm, slice thickness = 5-6 mm, yielding 3-8 slices, zero gap and a 256 × 128 matrix (zero-filled to 256 × 256 during image reconstruction).
The contrast of gadopentetic diethylene triamine penta acetic acid (Gd-DTPA) (Magnevist; Berlex Laboratories, Wayne, NJ, United States) was delivered by antecubital vein catheters at a bolus of 0.1 mmol/kg and 2 cc/s, followed by saline flush using a MR-compatible, programmable power injector (Spectris; Medrad, Indianola, PA, United States). The total acquisition time for obtaining the DCE-MRI data was approximately 5 min.
Pharmacokinetic modeling was performed using the Tofts model[23]:
Math 1
where Ct (mmol/L) is the concentration of contrast agent in the tumor tissue; Cp (mmol/L) is the concentration of contrast agent in the blood plasma [known as the arterial input function (AIF)]; Ktrans (min-1) is the volume transfer rate between the blood plasma and extravascular extracellular space (EES); ve is the volume fraction of EES; T is the acquisition time course (min); and t is the integration variable.
Pharmacokinetic measures (Ktrans, ve) were estimated by using a nonlinear least-square fitting method, of which the optimization procedure consisted of using a Matlab build-in subspace trust region algorithm[24]. Three-dimensional regions of interest were delineated for the metastatic node on all the 2D-SPGR slices containing the tumor by a neuroradiologist with more than 10 years of experience, as described previously[25]. The data was fitted on a voxel-by-voxel basis within the ROI, yielding values of average (mean) and standard deviation, as well as parametric maps of Ktrans and ve.
Image texture analysis was performed on parametric maps of Ktrans and ve at the tumor’s central slice. First, the noise on the maps was removed with a noise thresholding method which is based on the Ostu’s algorithm. Histogram equalization with 64 discrete levels was performed to enhance map contrast. GLCM was calculated with 16 gray levels by setting the distance of pixel of interest to the left of its neighbor to be one pixel[11]. Based on the GLCM obtained, two texture measures of parametric maps were computed:
Math 1
Where p(i, j) is the (i, j)th entry in a gray-tone spatial dependence matrix (I = 1:N; j = 1:N), and N is the number of distinct gray levels in the quantized image. E returns the sum of squared elements in the GLCM. E ranges from 0 to 1. For a constant image, E equals 1.
Math 1
H returns a value that measures the closeness of the distribution of elements in the GLCM to the GLCM diagonal. H ranges from 0 to 1. For a diagonal GLCM, H equals 1.
The measures derived from DCE-MRI pharmacokinetic modeling and image texture analysis were used for statistical analysis. The Lilliefors test was used to test the normality of all DCE-MRI-derived measures from the study patients. The differences in these measures between pretreatment and intra-treatment DCE-MRI scans were tested using a paired student’s t-test. The non-parametric Mann-Whitney U test was performed to assess the metric differences between LC and LF groups. The measures from pretreatment and intra-treatment scans, and the metric difference between pretreatment and intra-treatment scans, were used for the Mann-Whitney U test. To determine the measures that provide the best prediction of outcome, a forward sequential feature selection algorithm was used, followed by logistic regression analysis, which determined the probability of prediction. Statistical analysis was performed using Matlab R2008a on a Microsoft Windows system.
The clinical outcome for 19 patients with cancer of the oropharynx was assessed: 17 patients had local control of the disease (LC), and 2 patients had local failure (LF).
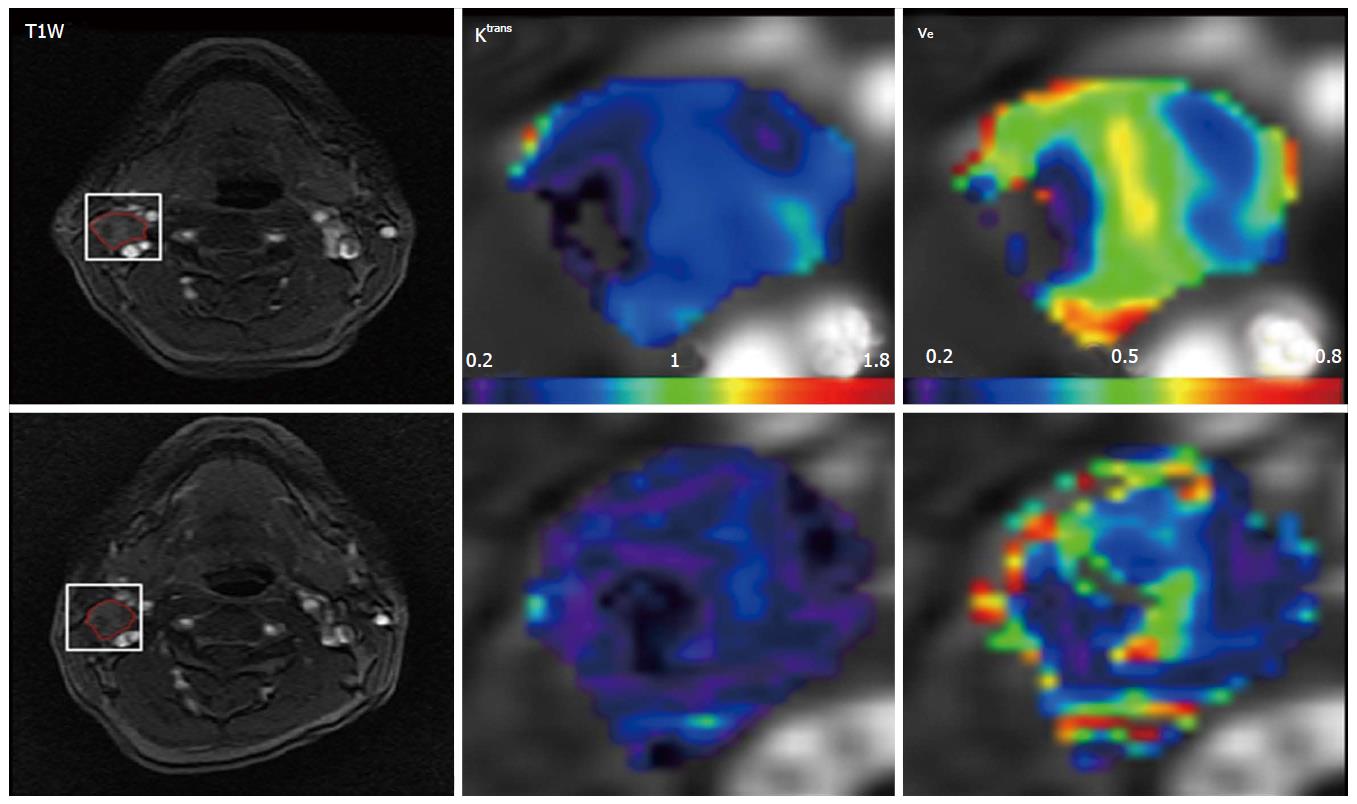
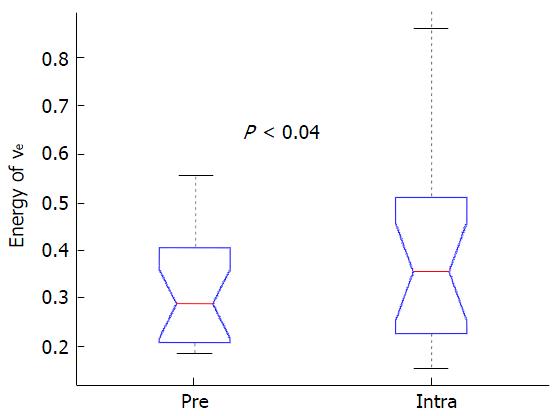
Figure 1 displays DCE-MRI images (pretreatment and intratreatment scans) and derived parametric maps (Ktrans and ve) for a representative patients with locoregional failure (male, 50 years). For comparing the difference of tumor volumes between pretreatment and intratreatment studies in 19 patients, there was a statistical significance (9.02 ± 6.87 cm3vs 7.23 ± 6.22 cm3; P < 0.02, Table 2), showing tumor volumes were smaller after the commencement of treatment. No significant changes were found for the common summarizing measures (mean and standard deviation) for Ktrans and ve (P > 0.09). However, texture analysis revealed that the imaging biomarker E of ve was significantly higher in the intra-treatment scans than in the pretreatment scans [0.41 ± 0.22 vs 0.30 ± 0.11 (no unit); P < 0.04], as shown in Table 2 and Figure 2.
| Parameters | Measures | Pre (n = 19) (mean ± SD) | Intra (n = 19) (mean ± SD) | P values |
| Tumor volume (cm3) | 9.02 ± 6.87 | 7.23 ± 6.22 | 0.02a | |
| Ktrans (volume transfer rate, min-1) | Mean | 0.34 ± 0.18 | 0.37 ± 0.22 | 0.36 |
| SD | 0.25 ± 0.11 | 0.25 ± 0.11 | 0.99 | |
| E | 0.34 ± 0.14 | 0.38 ± 0.19 | 0.18 | |
| H | 0.84 ± 0.04 | 0.83 ± 0.05 | 0.61 | |
| ve (volume fraction of the extravascular extracellular space) | Mean | 0.29 ± 0.12 | 0.34 ± 0.19 | 0.09 |
| SD | 0.15 ± 0.04 | 0.17 ± 0.07 | 0.26 | |
| E | 0.30 ± 0.11 | 0.41 ± 0.22 | 0.041 | |
| H | 0.84 ± 0.04 | 0.83 ± 0.06 | 0.78 |
No significant difference was found when comparing the measures between the LC and LF groups, either in the pretreatment or intra-treatment scans, as seen in Table 3. However, there was a trend towards greater elevation in the E of ve in the LF patients after treatment, relative to the LC patients [0.37 ± 0.23 vs 0.07 ± 0.16 (no unit); P = 0.07].
| Measures for patient groups (LC and LF) | Pre | Intra | Difference between intra and pre | ||
| Tumor volume (cm3) | LC | 9.68 ± 7.03 | 7.70 ± 6.45 | -1.97 ± 3.13 | |
| LF | 3.79 ± 0.73 | 3.42 ± 0.91 | -0.37 ± 0.17 | ||
| P value | 0.15 | 0.54 | 0.64 | ||
| Mean | LC | 0.34 ± 0.19 | 0.38 ± 0.23 | 0.04 ± 0.15 | |
| LF | 0.34 ± 0.01 | 0.26 ± 0.13 | -0.08 ± 0.14 | ||
| P value | P = 0.84 | P = 0.42 | P = 0.42 | ||
| Ktrans (min-1) | SD | LC | 0.26 ± 0.11 | 0.25 ± 0.11 | -0.007 ± 0.12 |
| LF | 0.20 ± 0.05 | 0.27 ± 0.16 | -0.08 ± 0.14 | ||
| P value | P = 0.49 | P = 0.74 | P = 0.49 | ||
| E | LC | 0.35 ± 0.15 | 0.38 ± 0.19 | 0.02 ± 0.11 | |
| LF | 0.27 ± 0.08 | 0.41 ± 0.30 | 0.14 ± 0.22 | ||
| P value | P = 0.74 | P = 1.00 | P = 0.57 | ||
| H | LC | 0.84 ± 0.04 | 0.83 ± 0.06 | -0.01 ± 0.04 | |
| LF | 0.82 ± 0.03 | 0.85 ± 0.04 | 0.03 ± 0.04 | ||
| P value | P = 0.35 | P = 0.57 | P = 0.35 | ||
| ve | Mean | LC | 0.29 ± 0.13 | 0.35 ± 0.20 | 0.05 ± 0.12 |
| LF | 0.28 ± 0.10 | 0.26 ± 0.01 | -0.01 ± 0.11 | ||
| P value | P = 0.94 | P = 0.42 | P = 0.35 | ||
| SD | LC | 0.16 ± 0.04 | 0.17 ± 0.07 | 0.01 ± 0.07 | |
| LF | 0.14 ± 0.01 | 0.22 ± 0.01 | 0.08 ± 0.02 | ||
| P value | P = 0.23 | P = 0.14 | P = 0.10 | ||
| E | LC | 0.31 ± 0.12 | 0.38 ± 0.21 | 0.07 ± 0.16 | |
| LF | 0.28 ± 0.02 | 0.65 ± 0.21 | 0.37 ± 0.23 | ||
| P value | P = 0.94 | P = 0.10 | P = 0.07 | ||
| H | LC | 0.84 ± 0.04 | 0.82 ± 0.06 | -0.01 ± 0.06 | |
| LF | 0.84 ± 0.02 | 0.91 ± 0.06 | 0.06 ± 0.06 | ||
| P value | P = 0.74 | P = 0.14 | P = 0.18 | ||
In this study, we assessed the benefit of GLCM texture analysis on pharmacokinetic DCE-MRI maps for the prediction of treatment response in patients with HNSCC with neck nodal metastasis. An early and reliable predictor of treatment outcome could be especially valuable in the management of advanced HNSCC. Whereas common summarizing measures (mean and standard deviation) for Ktrans and ve did not yield significant differences, a biomarker derived from texture analysis, E of ve, did yield significant results. Specifically, we observed a significantly higher intra-treatment energy E of ve, compared to pretreatment scans.
The GLCM-derived biomarker, E, can be interpreted as an inverse heterogeneity measure, in which lower values are indicative of greater heterogeneity[11]. Hence, as the energy E of ve was higher during treatment, compared with before treatment, it seems that treatment reduces the heterogeneity of the tumor. However, the treatment-induced reduction in heterogeneity seems more pronounced for patients with local failure, rather than patients with local control, which might indicate that ineffective treatment counter-intuitively yields more homogeneous, rather than heterogeneous, tumor characteristics. It is important to note that the low number of patients with LF observed in our cohort limits the reliability of this analysis. The low number of LF patients in this study is because at our center, patients with advanced locoregional cancer of the oropharynx who undergo chemo-radiation treatment have an approximately 90% local control rate. Future studies would require larger patient populations. If treatment-induced reduction of tumor heterogeneity in patients with local failure is validated in subsequent studies, this finding may have implications for the future design of adaptive chemo-radiation therapy trials in advanced head and neck cancers.
DCE-MRI provides a non-invasive method to probe the properties of tumor vasculature, such as perfusion, permeability of blood vessels, and volume of extracellular space. The ability of DCE-MRI to predict a patient’s response to chemo-radiation treatment has been previously investigated in head and neck cancers[5,7,8]. In a study by Cao et al[7] that followed head and neck cancer patients for a median of 10 mo (range, 5-27 mo), the blood volume in the primary gross tumor volume was increased significantly in local control patients after 2 wk of chemo-radiation, compared with local failure patients (P < 0.03)[7]. In a study of 33 patients with head and neck cancer treated with neoadjuvant chemo-radiation therapy, Kim et al[8] found that the average pretreatment Ktrans value of a group with complete response to treatment was significantly higher than that of a group with partial response (P = 0.001) at a 6 mo follow-up. Our findings add to these studies that in addition to standard descriptive measures of pharmacokinetic maps, texture analysis may provide potential biomarkers related to local disease control, and thus may help guide clinical management.
The results of our study are not necessarily consistent with other studies that applied image texture analysis on DCE-MRI-derived parametric maps[11,26]. For example, Alic et al[11] used the heterogeneity features (i.e., coherence and fractal dimension) from texture analysis on parametric maps derived from DCE-MRI scans in 18 patients with limb sarcomas and found that tumors that responded to treatment had a high coherence on the pretreatment scans of those patients[11]. Similarly, O’Connor et al[26] found that microvascular uniformity (assessed with the fractal measure box dimension) in pretreatment scans correlated with treatment response in 10 patients with colorectal cancer with liver metastases. However, different tumor types and treatment regimens may impact the results of DCE-MRI-derived parametric maps in different ways. Validation studies with histopathology[27] or other measures of tumor micro-environment[10,25,28,29] open up scope for future studies in this area.
The limitations of this study are that: (1) the study had a small cohort of patients with only 2 patients with loco-regional failures, therefore an investigation with a large population is needed; (2) in this study, we made an assumption of an accurate T1 and AIF measurement and ignorable patient movements, which may affect the values of pharmacokinetic metrics and generated texture features; and (3) We did not compare the sensitivity and specificity of our method with the results from other investigators. All these issues will be addressed in future studies.
Image texture analysis can provide biomarker’s of heterogeneity for the analysis of DCE-MRI parametric maps of head and neck cancers. Specifically, we observed that chemo-radiation treatment significantly reduces the heterogeneity of tumors. The findings from this study may have potential value in stratifying patients and designing individualized treatment plans during the early management of HNSCC patients.
The authors would like to thank the MRI technologists for their great efforts to help perform all MRI examinations and Ms. Dara Srisaranard for her kind contribution to patient enrollment and data management. We thank Ms. Sandhya George for editing the manuscript.
Head and neck squamous cell carcinoma (HNSCC) is a major form of cancer that still kills vast numbers of cancer patients, and patients would certainly benefit with improved imaging methodology for a better staging/diagnosis, treatment evaluation, and treatment response prediction.
Although magnetic resonance imaging (MRI) is often standardly applied in the clinical workup of patients newly diagnosed with HNSCC, advanced MRI methods [such as dynamic contrast-enhanced (DCE)-MRI] and advanced data analysis methods are currently being developed to optimize the added value of imaging.
In this study, novel texture analysis was applied to parametric maps derived from DCE-MRI. The results are very promising.
In principle, the applied image analysis can be readily applied on any parametric map, hence they can be incorporated in many clinical cancer trials that incorporate MRI imaging.
AIF: Arterial input function; DCE: Dynamic contrast-enhanced; EES: Extravascular extracellular space; Gd-DTPA: Gadopentetic diethylene triamine penta acetic acid; GLCM: Gray-level co-occurrence matrix; HNSCC: Head and neck squamous cell carcinoma; LC: Locoregional control; LF: Locoregional failure; MRI: Magnetic resonance imaging; NEX: Number of excitation; PET: Positron emission tomography; ROI: Region of interest; SPGR: Spoiled gradient echo; TR: Repetition time; TE: Echo time.
This paper describes a study to assess the treatment response in patients with HNSCC using quantitative texture metrics of energy and homogeneity. The authors observed that the heterogeneity of the tumors has been reduced after the treatment and the texture biomarkers can be used to evaluate the treatment response. Overall, the paper is well written and relative easy to follow.
P- Reviewer: Xian J, Zhang T
S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293-3298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 306] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 2. | Padhani AR, Husband JE. Dynamic contrast-enhanced MRI studies in oncology with an emphasis on quantification, validation and human studies. Clin Radiol. 2001;56:607-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 164] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Padhani AR, Khan AA. Diffusion-weighted (DW) and dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) for monitoring anticancer therapy. Target Oncol. 2010;5:39-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Asaumi J, Yanagi Y, Konouchi H, Hisatomi M, Matsuzaki H, Kishi K. Application of dynamic contrast-enhanced MRI to differentiate malignant lymphoma from squamous cell carcinoma in the head and neck. Oral Oncol. 2004;40:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Shukla-Dave A, Lee NY, Jansen JF, Thaler HT, Stambuk HE, Fury MG, Patel SG, Moreira AL, Sherman E, Karimi S. Dynamic contrast-enhanced magnetic resonance imaging as a predictor of outcome in head-and-neck squamous cell carcinoma patients with nodal metastases. Int J Radiat Oncol Biol Phys. 2012;82:1837-1844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Chawla S, Kim S, Dougherty L, Wang S, Loevner LA, Quon H, Poptani H. Pretreatment diffusion-weighted and dynamic contrast-enhanced MRI for prediction of local treatment response in squamous cell carcinomas of the head and neck. AJR Am J Roentgenol. 2013;200:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Cao Y, Popovtzer A, Li D, Chepeha DB, Moyer JS, Prince ME, Worden F, Teknos T, Bradford C, Mukherji SK. Early prediction of outcome in advanced head-and-neck cancer based on tumor blood volume alterations during therapy: a prospective study. Int J Radiat Oncol Biol Phys. 2008;72:1287-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Kim S, Loevner LA, Quon H, Kilger A, Sherman E, Weinstein G, Chalian A, Poptani H. Prediction of response to chemoradiation therapy in squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2010;31:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Jackson A, O’Connor JP, Parker GJ, Jayson GC. Imaging tumor vascular heterogeneity and angiogenesis using dynamic contrast-enhanced magnetic resonance imaging. Clin Cancer Res. 2007;13:3449-3459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Jansen JF, Koutcher JA, Shukla-Dave A. Non-invasive imaging of angiogenesis in head and neck squamous cell carcinoma. Angiogenesis. 2010;13:149-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Alic L, van Vliet M, van Dijke CF, Eggermont AM, Veenland JF, Niessen WJ. Heterogeneity in DCE-MRI parametric maps: a biomarker for treatment response. Phys Med Biol. 2011;56:1601-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | El Naqa I, Grigsby P, Apte A, Kidd E, Donnelly E, Khullar D, Chaudhari S, Yang D, Schmitt M, Laforest R. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit. 2009;42:1162-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 363] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 13. | Alic L, van Vliet M, Wielopolski PA, ten Hagen TL, van Dijke CF, Niessen WJ, Veenland JF. Regional heterogeneity changes in DCE-MRI as response to isolated limb perfusion in experimental soft-tissue sarcomas. Contrast Media Mol Imaging. 2012;8:340-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, Ganeshan B, Miles KA, Cook GJ, Goh V. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice. Insights Imaging. 2012;3:573-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 695] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 15. | Dominietto M, Lehmann S, Keist R, Rudin M. Pattern analysis accounts for heterogeneity observed in MRI studies of tumor angiogenesis. Magn Reson Med. 2013;70:1481-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Just N. Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer. 2014;111:2205-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 386] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 17. | Karahaliou A, Vassiou K, Arikidis NS, Skiadopoulos S, Kanavou T, Costaridou L. Assessing heterogeneity of lesion enhancement kinetics in dynamic contrast-enhanced MRI for breast cancer diagnosis. Br J Radiol. 2010;83:296-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Yang X, Knopp MV. Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a review. J Biomed Biotechnol. 2011;2011:732848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Setton J, Caria N, Romanyshyn J, Koutcher L, Wolden SL, Zelefsky MJ, Rowan N, Sherman EJ, Fury MG, Pfister DG. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27:3684-3690. [PubMed] |
| 21. | Goenka A, Morris LG, Rao SS, Wolden SL, Wong RJ, Kraus DH, Ohri N, Setton J, Lok BH, Riaz N. Long-term regional control in the observed neck following definitive chemoradiation for node-positive oropharyngeal squamous cell cancer. Int J Cancer. 2013;133:1214-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Mutter RW, Lok BH, Dutta PR, Riaz N, Setton J, Berry SL, Goenka A, Zhang Z, Rao SS, Wolden SL. Constraining the brachial plexus does not compromise regional control in oropharyngeal carcinoma. Radiat Oncol. 2013;8:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 24. | More JJ, Sorensen DC. Computing a Trust Region Step. SIAM J Sci Statist Comput. 1983;4:553-572. |
| 25. | Jansen JF, Schöder H, Lee NY, Stambuk HE, Wang Y, Fury MG, Patel SG, Pfister DG, Shah JP, Koutcher JA. Tumor metabolism and perfusion in head and neck squamous cell carcinoma: pretreatment multimodality imaging with 1H magnetic resonance spectroscopy, dynamic contrast-enhanced MRI, and [18F]FDG-PET. Int J Radiat Oncol Biol Phys. 2012;82:299-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | O’Connor JP, Rose CJ, Jackson A, Watson Y, Cheung S, Maders F, Whitcher BJ, Roberts C, Buonaccorsi GA, Thompson G. DCE-MRI biomarkers of tumour heterogeneity predict CRC liver metastasis shrinkage following bevacizumab and FOLFOX-6. Br J Cancer. 2011;105:139-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Jansen JF, Carlson DL, Lu Y, Stambuk HE, Moreira AL, Singh B, Patel SG, Kraus DH, Wong RJ, Shaha AR. Correlation of a priori DCE-MRI and (1)H-MRS data with molecular markers in neck nodal metastases: Initial analysis. Oral Oncol. 2012;48:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Jansen JF, Schöder H, Lee NY, Wang Y, Pfister DG, Fury MG, Stambuk HE, Humm JL, Koutcher JA, Shukla-Dave A. Noninvasive assessment of tumor microenvironment using dynamic contrast-enhanced magnetic resonance imaging and 18F-fluoromisonidazole positron emission tomography imaging in neck nodal metastases. Int J Radiat Oncol Biol Phys. 2010;77:1403-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Jansen JF, Parra C, Lu Y, Shukla-Dave A. Evaluation of Head and Neck Tumors with Functional MR Imaging. Magn Reson Imaging Clin N Am. 2016;24:123-133. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |