Peer-review started: July 31, 2015
First decision: November 6, 2015
Revised: November 24, 2015
Accepted: December 13, 2015
Article in press: December 14, 2015
Published online: January 28, 2016
Processing time: 180 Days and 22.2 Hours
AIM: To investigating the relationship between thoracic and cardiac 18F-Natrium-Fluoride (18F-NaF) uptake, as a marker of ongoing calcification and cardiovascular risk factors.
METHODS: Seventy-eight patients (44 females, mean age 63, range 44-83) underwent whole body 18F-NaF positron emission tomography/computed tomography. Cardiovascular risk (CVR) was used to divide these patients in three categories: Low (LR), medium (MR) and high risk (HR). 18F-NaF uptake was measured by manually drawing volumes of interest on the ascending aorta, on the aortic arch, on the descending aorta and on the myocardium; average standardized uptake value was normalized for blood-pool, to obtain target-to-background ratio (TBR). Values from the three aortic segments were then averaged to obtain an index of the whole thoracic aorta.
RESULTS: A significant difference in whole thoracic aorta TBR was detected between HR and LR (1.84 ± 0.76 vs 1.07 ± 0.3, P < 0.001), but also between MR and HR-LR (1.4 ± 0.4, P < 0.02 and P < 0.01, respectively). Significance of this TBR stratification strongly varied among thoracic aorta subsegments and the lowest P values were reached in the descending aorta (P < 0.01). Myocardial uptake provided an effective CVR classes stratification (P < 0.001).Correlation between TBR and CVR was appreciable when the whole thoracic aorta was considered (R = 0.67), but it peaked when correlating the descending thoracic segment (R = 0.75), in comparison with the aortic arch and the ascending segment (R = 0.55 and 0.53, respectively).
CONCLUSION: Fluoride uptake within the thoracic aorta wall effectively depicts patients’ risk class and correlates with cardiovascular risk. Descending aorta is the most effective in CVR determination.
Core tip: We evaluated, in 78 patients who underwent whole body 18F-Natrium Fluoride (18F-NaF) positron emission tomography/computed tomography, the 18F-NaF uptake in different segments of thoracic aorta and within the myocardium, as a measure of ongoing molecular calcification. In particular, we tested the hypothesis of a correlation between thoracic aorta uptake and cardiovascular risk (CVR). We thus assessed 18F-NaF uptake (TBR) in different CVR groups (high, medium and low) and its correlation with absolute CVR value. TBR stratified the three CVR classes, mostly in the descending aortic segment and in the myocardium. Thoracic aorta uptake correlated with CVR and with myocardial uptake.
- Citation: Fiz F, Morbelli S, Bauckneht M, Piccardo A, Ferrarazzo G, Nieri A, Artom N, Cabria M, Marini C, Canepa M, Sambuceti G. Correlation between thoracic aorta 18F-natrium fluoride uptake and cardiovascular risk. World J Radiol 2016; 8(1): 82-89
- URL: https://www.wjgnet.com/1949-8470/full/v8/i1/82.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i1.82
Cardiovascular disease has been representing, for many decades an ever-ongoing pandemia; acute and chronic cardiovascular ailments, once a hallmark of western population and lifestyle, have seeped through the lower-income population strata[1]. The assessment of individual cardiovascular risk (CVR) allows the implementation of primary and secondary prevention measures[2-4], such as lifestyle modifications and the administration of drugs contrasting the development and progression of atherosclerosis[5].
However, CVR assessment does not provide any information on atherosclerotic plaque biology and progression; it neither can effectively tell whether vascular lesions are stabilized by therapy nor can identify those vascular segments that are at higher risk for occlusion.
To overcome this limitation, non-invasive imaging has been proposed. The most widespread application is coronary calcium scoring (CS), which uses the high attenuation coefficient of calcium to detect calcified plaques within the vascular tree by X-ray computed tomography (CT)[6]. This technique is able to thoroughly depict the total coronary calcification load, which is an expression of the underlying plaque burden; it is however unclear whether data provided by this type of imaging are able to accurately stratify the actual CVR[7-9].
Discrepancies between CS and CVR are often related to the relatively low sensitivity of the CT approach, which is unable to detect micro-calcifications[10] and thus tend to miss the earlier phases of plaque formation, which would probably benefit the most from clinical intervention. In addition, tomo-densitometric approaches are unable to reveal whether the plaque is at risk of rupture.
In order to overcome these limitations, radio-isotope plaque imaging was proposed in relatively recent times[8,11-14]. In particular, the use of bone-seeking positron-emitting tracer, such as 18F-Natrium Fluoride (18F-NaF) was attempted, given the great similarity in actors participating to bone apposition in the skeleton and plaque calcification in the arterial wall[15]. These studies confirmed the feasibility of 18F-NaF plaque imaging and the correlation of arterial 18F-NaF uptake with CVR[8]. Recent evidence points out that this approach is mostly helpful in the earlier phases of calcification, while the plaque is still actively concentrating mineral ions[16]. Moreover, 18F-NaF plaque imaging has shown potential even in the identification of the vulnerable plaque[17,18].
These considerations should theoretically bolster the application of 18F-NaF imaging in the coronary district. However, this type of approach is still limited by several positron emission tomography (PET)-related factors, such as the low spatial resolution and the continuous cardiac movement, which prevent an accurate depiction of coronary wall mineral metabolism and causes high variability in the evaluated uptake[14].
These issues can be overridden in at least two ways. First, there is strong evidence that thoracic aorta calcifications are strongly linked with coronary artery calcifications score[19,20]; moreover, aortic lesions have been shown to even precede those in the epicardial arteries[21]. Thus, processes underlying thoracic aorta mineral turnover could herald or mirror those observed in the coronary tree.
Alternatively, cardiac fluoride deposition could be measured on the entire myocardium, as proposed by Beheshti et al[22], thus including in the semi-quantitative analysis both the contribution from major vessels and the signal from the microvasculature. This approach could limit the impact of smear artifacts due to cardiac motion and small vessel diameter.
In the present study, we analyze the correlation between clinical CVR score and two indexes of thoracic aorta and global cardiac 18F-NaF uptake, aiming to test the feasibility of their use as surrogate markers of CVR.
The study included 78 patients with either breast or prostate cancer (44 females, mean age 63.3 ± 8.2 range 44-83) undergoing 18F-NaF PET/CT scan for evaluation of presence of bone metastases. Exclusion criteria included history of major cardiac adverse events, vasculitis, autoimmune or systemic inflammatory disease or chemotherapy in the preceding 8 wk, as previously proposed[8]. Cardiovascular risk stratification was performed according to a simplified version of the Framingham model (including age, diabetes, smoking, systolic blood pressure and body mass index)[4]. According to the Framingham score, the whole study group was subdivided into three risk categories: High (> 20%), intermediate (10%-20%) and low (< 10%) risk of cardiovascular events in the next 10 years.
Ongoing or previous treatment with statins was used as a further exclusion criterion to avoid influence of this treatment on the results[5]. The Internal Review Board evaluated and approved this retrospective study; all patients signed a written informed consent.
Patients underwent 18F-NaF PET/CT using two 16 slices PET/CT hybrid systems: (1) biograph 16 (Siemens Medical Solutions, Knoxville TN, United States); and (2) discovery LS (GE Medical Systems, Milwaukee, WI, United States). In both cases patients received an intravenous bolus injection of 18F-NaF (4.8-5.2 MBq per kilogram of body weight). PET/CT acquisition started 60-75 min thereafter, in the meantime the patient was hydrated and encouraged to void, as to diminish the unbound tracer fraction. The entire body was scanned from vertex to toes in an “arms down” position; emission scan lasted 120’’ per bed position. PET raw data were reconstructed by means of ordered subset expectation maximization (OSEM, 3 iterations, 16 subsets) and attenuation correction was performed using CT data. The transaxial field of view and pixel size of the reconstructed PET images were 58.5 cm and 4.57 mm, respectively, with a matrix size of 128 × 128 mm. As per standard PET/CT imaging protocol, 16-detector row helical CT scan was performed with non-diagnostic current and voltage settings (120 Kv, 80 mA), with a gantry rotation speed of 0.5 s and table speed of 24 mm per gantry rotation. No contrast medium was injected. The entire CT dataset was fused with the 3-dimensional (3D) PET images using an integrated software interface (Syngo; Siemens Erlangen, Germany) and was used for anatomical localization.
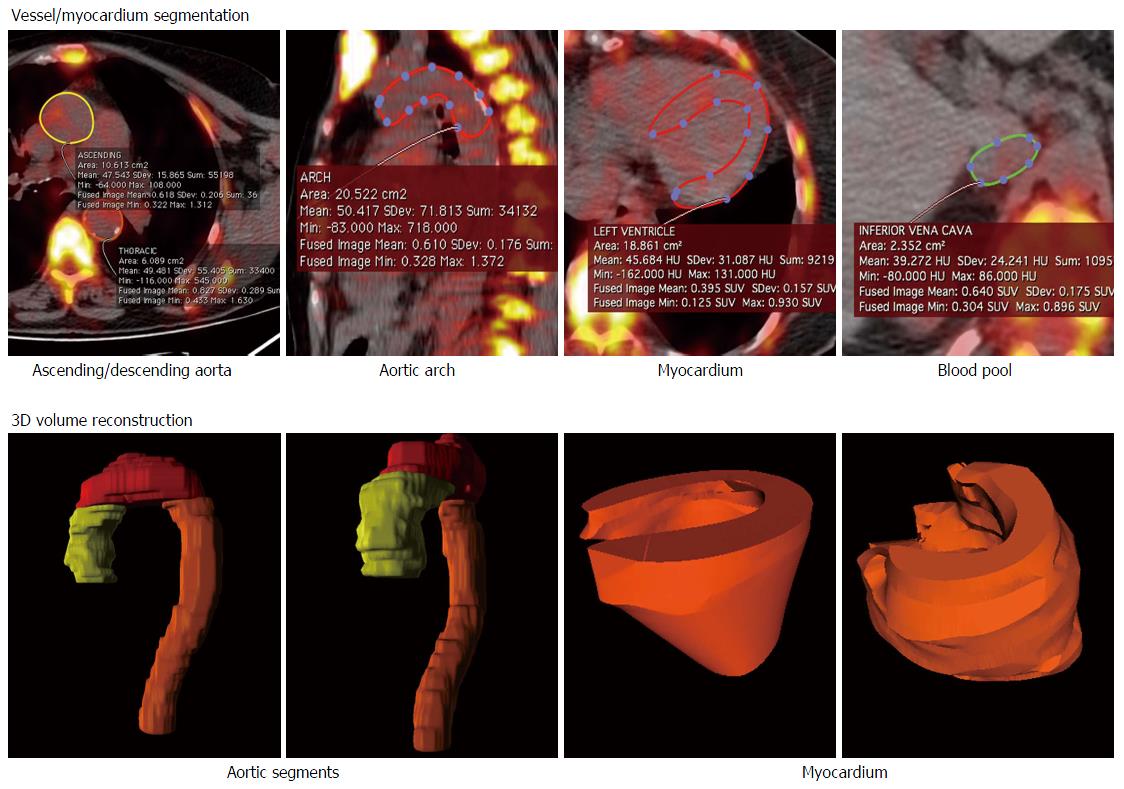
Reconstructed images were anonymized and analyzed offline on a dedicated workstation, running a DICOM image visualizer (Osirix 64-bit, Pixmeo, Geneva, CH). Volume of interest (VOI) were manually drawn on three segments of the thoracic aorta (ascending, arch, descending) using the co-registered CT images as anatomical reference and on the entire myocardium, as proposed by Beheshti et al[22]. The three aortic segments were averaged to obtain the overall thoracic aorta uptake. See Figure 1 for an example of VOI generation. Average standardized uptake value (SUV) was calculated and then normalized for background activity, obtained as mean SUV of a 10-slice thick VOI drawn on the inferior vena cava. Resulting figure was labeled target-to-background ratio (TBR); myocardial uptake was defined normalized global molecular calcification score (NGMCS), as proposed[22].
All data are reported as mean ± SD. Differences between groups were tested using one-way analysis of variance, with intergroup comparison afforded using Bonferroni test. Two-tailed Pearson R index was used to test the significance of correlations. Intra- and inter-rater agreement were afforded using Cohen’s kappa and two-tailed Pearson R for qualitative and quantitative variables, respectively. Statistical analyses were performed using a software application (SPSS, v. 21.0, IBM, Armonk NY, United States). The statistical review of the manuscript was performed by a biomedical statistician.
After stratification, 20, 36 and 22 patients belonged to the high, medium and low risk category, respectively. Mean ten-years CV risk was 29% ± 2.4% for the high-risk population, 14.3 ± 3 for the medium risk stratum and 6.6 ± 2.3 for the low risk group. The latter patients were also significantly younger with respect to the other two sub-populations (58.3 ± 8.2 vs 65 ± 9.5 - medium risk and 65 ± 9.1 - high risk) (Table 1).
| Patients number | Mean age | Gender m/f | Systolic bp | Bp treatment (Yes/No) | Smoke | Diabetes | BMI | Mean CVR | Whole thoracic aorta TBR | NGMCS | |
| Low CVR | 22 | 58.3 ± 8.2 | 7/15 | 114 ± 5 | 2/20 | 1/21 | 2/22 | 24 ± 1 | 6.6% ± 2.3% | 1.14 ± 0.34 | 104 ± 46 |
| Medium CVR | 36 | 65 ± 9.5 | 10/26 | 123 ± 6 | 12/24 | 14/22 | 5/31 | 25 ± 3 | 14.3% ± 3% | 1.4 ± 0.4 | 62 ± 21 |
| High CVR | 20 | 65 ± 9.1 | 10/10 | 128 ± 11 | 8/12 | 19/1 | 15/5 | 27 ± 2 | 29% ± 2.4% | 1.8 ± 0.75 | 43 ± 17 |
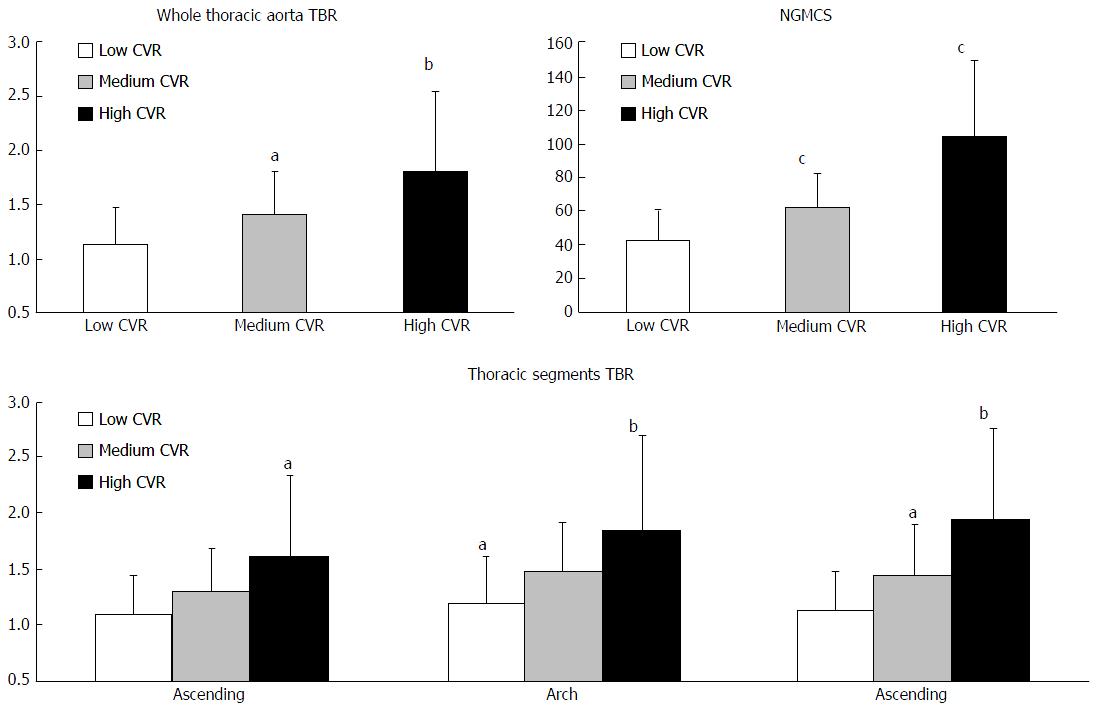
TBR effectively stratified the three risk categories, when considering the overall thoracic aorta (1.8 ± 0.75, 1.4 ± 0.4 and 1.14 ± 0.34 for high, medium and low risk, respectively, P < 0.05).
Significance of stratification varied among aortic subsegments, with descending being capable to tell apart the three CVR categories (TBR equal to 1.94 ± 0.8, 1.44 ± 0.45 and 1.14 ± 0.34, respectively, P < 0.02). Conversely, aortic arch and ascending aorta could discriminate high and low risk (P < 0.01 and P < 0.02) but provided less clear distinction between medium risk and the other two risk categories. Refer to Figure 2 and to Table 2 for the exact figures of TBR and significance.
| High | Medium | Low | |
| Thoracic aorta | |||
| High | - | 0.031 | 0.002 |
| Medium | 0.031 | - | 0.023 |
| Low | 0.002 | 0.023 | - |
| Ascending aorta | |||
| High | - | 0.085 | 0.013 |
| Medium | 0.085 | - | 0.056 |
| Low | 0.013 | 0.056 | - |
| Aortic arch | |||
| High | - | 0.066 | 0.007 |
| Medium | 0.066 | - | 0.033 |
| Low | 0.007 | 0.033 | - |
| Descending aorta | |||
| High | - | 0.012 | 0.001 |
| Medium | 0.012 | - | 0.018 |
| Low | 0.001 | 0.018 | - |
| Normalized global molecular calc. Score | |||
| High | - | 0.000 | 0.000 |
| Medium | 0.000 | - | 0.003 |
| Low | 0.000 | 0.003 | - |
NGMCS, resulted an effective index of risk assessment: Its values were 104 ± 45.6, 62 ± 20.8 and 42.7 ± 17.4 in high, medium and low CV risk groups, respectively (P < 0.001; Table 2 and Figure 2).
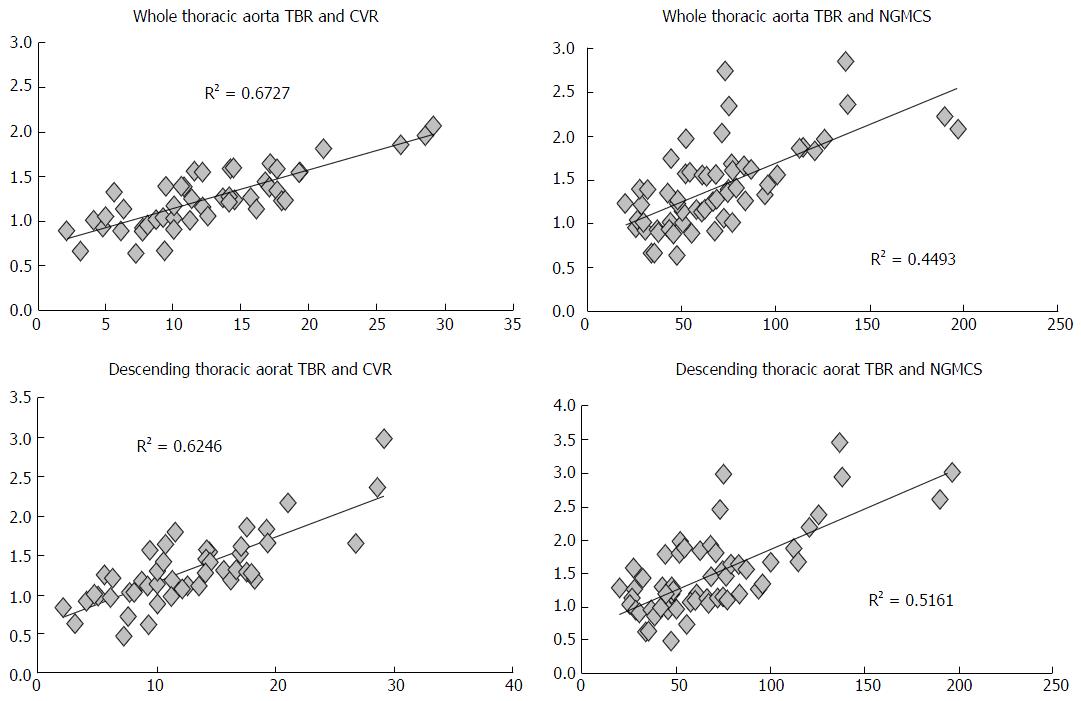
Overall, thoracic aorta TBR showed a significant correlation with CVR (R = 0.82, P < 0.001). Among segments, descending aorta displayed the closest correspondence to the clinical risk assessment (R = 0.79). Among single risk factors, age and hypertension were tightly associated with aortic TBR (R = 0.32, P < 0.05; R = 0.54, P < 0.01, respectively); moreover, patients with diabetes had a significantly higher TBR with respect to non-diabetics (1.7 ± 0.7 and 1.4 ± 0.5, P < 0.01).
Aortic TBR showed a significant correlation with NGMCS as well (whole thoracic aorta vs NGMCS produced an R-index of 0.67). However, this correlation was prevalently observed in descending aorta (R = 0.72) (Table 3 and Figure 3).
| Segment TBR | CVR | NGMCS | ||
| R2 | R | R2 | R | |
| Ascending | 0.52 | 0.72 | 0.27 | 0.52 |
| Arch | 0.48 | 0.70 | 0.35 | 0.59 |
| Descending | 0.62 | 0.79 | 0.52 | 0.72 |
| All segments | 0.67 | 0.82 | 0.45 | 0.67 |
Finally, measurement of TBR was accurate and relatively operator-independent: Intra- and inter-rater agreement was excellent (Cohen’s kappa value = 0.91; Pearson’s R = 0.95).
In recent years, non-invasive, functional imaging of the coronary district has received a large attention[17]. In particular, the widespread distribution of PET scanner with 3D detection technology has allowed grasping the signal produced by plaque metabolism[11-14,16]. The scientific background underlying this approach is still relatively young and its applications lie predominantly within the research terrain[23]. However, a few studies have provided a glimpse of future clinical application: The two main streaks are at present the detection of early (CT-negative) arterial plaque[16,22,24] and the identification of the vulnerable atherosclerotic lesion[18,25,26].
Although the issue of organ movement can be solved by gating[18], the main limiting factor in applying PET imaging on the study of the coronary district is the small size of these vessels[26], which fall below the resolution capability of most scanner[27] and can cause underestimation of PET signal due to partial volume effect.
In this context, the utilization of surrogate measures to estimate the coronary mineral turnover can provide an affordable method to interrogate calcium deposition in this district; our data show a tight correlation between activity of thoracic aorta, activity within the myocardium, depicting the process of arterial calcification as a systemic ailment, rather than a localized phenomenon. The concept of “plaque promoting environment” is further corroborated in the analysis of the whole myocardial volume: The application of the model by Beheshti et al[22], with subsequent normalization for background activity, provided an excellent stratification among risk classes. The evidence of increased macro and micro-vascular signal from high-risk patients opens new questions, which call for longitudinal studies, aimed to assess whether an increased 18F-NaF activity is the harbinger of future calcifications and/or adverse cardiovascular events.
Correlation between aortic uptake and CV risk was consistent in all three segments but relationship between thoracic and cardiac 18F-NaF accumulation was somewhat less steady and prevailed in the arch and in descending aorta. This is in agreement with our previous observation, which demonstrated that vessel fluoride uptake is “per se” a marker of CVR, regardless of the studied segment[8]. Conversely, intensity of this uptake tends to correlate in vessels that are subject to similar flow conditions. Specifically, these data parallel those reported in the literature for vascular calcification indicating that vascular regions characterized by vessel branching are more prone to plaque formation, due to the influence of regional flow alterations[28,29]. These considerations imply that, when using thoracic aorta as a surrogate for coronary artery vascular lesions, the analysis should be focused on the descending segment and on the arch.
This study presents some limitations. It is a retrospective study, performed on patients that were referred for an oncologic condition. Ethical consideration impeded radiotracer injection outside of validated clinical indications; recent studies have yet presented strong evidence that vascular and plaque 18F-NaF uptake is linked with active calcification processes[18,30]. Moreover, the strict inclusion criteria limited the size of the population; however, the sample size actually matched the one of several previous studies regarding 18F-NaF uptake within plaque and arterial vessels[10,14,18].
Finally, cardiac volume-of-interest was placed on an ungated PET; however, the large volume of the sampled tissue should have largely limited the influence of motion artifacts.
Altogether, the continuous improvement of PET/CT instrumentation has been opening a whole new perspective on plaque biology. The systematization of this analysis has the potential to allow the quantification of plaque mineral turnover, granting the possibility to stratify the evolutionary pattern of vascular lesions. In the coronary artery setting, where the small vessel size remains at the boundary of the PET diagnostic capability, the application of an indirect evaluation of active calcification might enable to derive a prognostic index and to measure the effectiveness of plaque treatments.
18F-Natrium-Fluoride (18F-NaF) is a bone seeking tracer that has been successfully proposed for the imaging of micro-calcification processes within the atherosclerotic plaque. Since coronary arteries are one of the most important targets of this kind of research, but tend to be subject to partial volume effects in images produced by commercial scanners. The authors hypothesized that thoraci aorta could serve as an ersatz, on the bases of the known correlation between thoracic aorta and coronary calcifications.
The analysis proved that thoracic aorta uptake is related to both the cardiovascular risk profile and the uptake in the whole myocardium (as a measure of the entire calcification activity occurring in the heart micro- and macro-vasculature).
Computational analysis of 18F-NaF uptake within the thoracic aorta vessel walls has the potential to become a robust, independent and non-invasive risk of cardiovascular risk. Differently from clinical scores, which give off a likelihood of acute events, 18F-NaF uptake actually depicts the activity of calcium deposition within arteries, as a marker of plaque growth.
This research should serve as a base to further perspective studies, used to identify the role of 18F-NaF uptake in predicting future cardiovascular events. This kind of analysis could be automated by applying simple mathematical approaches, so as to become faster and more operator-independent.
The authors present a retrospective analysis of correlation between different segments of thoracic aorta 18F-NaF uptake and cardiovascular risk, as well as to evaluation of correspondence between aortic and myocardial uptake in 78 oncologic patients. The study is interesting, even if there are several reports demonstrating the reliability of 18F-NaF positron emission tomography/computed tomography in imaging plaque inflammation in large arteries and the correlation between radiotracer uptake in atherosclerotic lesions and the presence of cardiovascular risk factors, identifying the patients at risk of future cardiovascular events.
P- Reviewer: Petix NR, Said SAM
S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | World Health Organization. “Top ten causes of death. 2012. Fact sheet N310: 3. [updated 2014; May] Available from: http://www.who.int/mediacentre/factsheets/fs310/en/. |
| 2. | Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46-51. [PubMed] |
| 3. | Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50-e103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1027] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 4. | Pencina MJ, D’Agostino RB, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119:3078-3084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 626] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 5. | Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825-1831. [PubMed] |
| 6. | Ichii M, Ishimura E, Shima H, Ohno Y, Ochi A, Nakatani S, Tsuda A, Ehara S, Mori K, Fukumoto S. Quantitative analysis of abdominal aortic calcification in CKD patients without dialysis therapy by use of the Agatston score. Kidney Blood Press Res. 2013;38:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Pen A, Yam Y, Chen L, Dennie C, McPherson R, Chow BJ. Discordance between Framingham Risk Score and atherosclerotic plaque burden. Eur Heart J. 2013;34:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Morbelli S, Fiz F, Piccardo A, Picori L, Massollo M, Pestarino E, Marini C, Cabria M, Democrito A, Cittadini G. Divergent determinants of 18F-NaF uptake and visible calcium deposition in large arteries: relationship with Framingham risk score. Int J Cardiovasc Imaging. 2014;30:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Canpolat U, Yorgun H, Aytemir K, Hazrolan T, Kaya EB, Ateş AH, Dural M, Gürses KM, Sunman H, Tokgözoğlu L. Cardiovascular risk and coronary atherosclerotic plaques detected by multidetector computed tomography: Framingham and SCORE risk models underestimate coronary atherosclerosis in the symptomatic low-risk Turkish population. Coron Artery Dis. 2012;23:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Pugliese G, Iacobini C, Blasetti Fantauzzi C, Menini S. The dark and bright side of atherosclerotic calcification. Atherosclerosis. 2015;238:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J, Klutmann S. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med. 2010;51:862-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | Derlin T, Wisotzki C, Richter U, Apostolova I, Bannas P, Weber C, Mester J, Klutmann S. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: correlation with atherogenic risk factors. J Nucl Med. 2011;52:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | George RT. 18F-sodium fluoride positron emission tomography: an in vivo window into coronary atherosclerotic plaque biology. J Am Coll Cardiol. 2012;59:1549-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, Richardson H, White A, McKillop G, van Beek EJ. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 15. | Doherty TM, Fitzpatrick LA, Inoue D, Qiao JH, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629-672. [PubMed] |
| 16. | Fiz F, Morbelli S, Piccardo A, Bauckneht M, Ferrarazzo G, Pestarino E, Cabria M, Democrito A, Riondato M, Villavecchia G. 18F-NaF Uptake by Atherosclerotic Plaque on PET/CT Imaging: Inverse Correlation Between Calcification Density and Mineral Metabolic Activity. J Nucl Med. 2015;56:1019-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Rosa GM, Bauckneht M, Masoero G, Mach F, Quercioli A, Seitun S, Balbi M, Brunelli C, Parodi A, Nencioni A. The vulnerable coronary plaque: update on imaging technologies. Thromb Haemost. 2013;110:706-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 742] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 19. | Tison GH, Guo M, Blaha MJ, McClelland RL, Allison MA, Szklo M, Wong ND, Blumenthal RS, Budoff MJ, Nasir K. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2015;9:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Adar A, Erkan H, Gokdeniz T, Karadeniz A, Cavusoglu IG, Onalan O. Aortic arch calcification is strongly associated with coronary artery calcification. Vasa. 2015;44:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Brodov Y, Gransar H, Rozanski A, Hayes SW, Friedman JD, Thomson LE, Dey D, Slomka PJ, Min JK, Shaw LJ. Extensive thoracic aortic calcification is an independent predictor of development of coronary artery calcium among individuals with coronary artery calcium score of zero. Atherosclerosis. 2015;238:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Beheshti M, Saboury B, Mehta NN, Torigian DA, Werner T, Mohler E, Wilensky R, Newberg AB, Basu S, Langsteger W. Detection and global quantification of cardiovascular molecular calcification by fluoro18-fluoride positron emission tomography/computed tomography--a novel concept. Hell J Nucl Med. 2011;14:114-120. [PubMed] |
| 23. | Hao L, Hao J, Fang W, Han C, Zhang K, Wang X. Dual isotope simultaneous imaging to evaluate the effects of intracoronary bone marrow-derived mesenchymal stem cells on perfusion and metabolism in canines with acute myocardial infarction. Biomed Rep. 2015;3:447-452. [PubMed] |
| 24. | Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, Brady TJ, Tawakol A. (18)F-NaF PET Imaging of Early Coronary Artery Calcification. JACC Cardiovasc Imaging. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, Brady TJ, Tawakol A. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging. 2010;3:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 26. | Johnson T, Ding H, Lipinski J, Molloi S. SU-E-I-25: Quantification of Coronary Artery Cross-Sectional Area in CT Angiography Using Integrated Density: A Simulation Study. Med Phys. 2015;42:3247. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Slomka PJ, Pan T, Berman DS, Germano G. Advances in SPECT and PET Hardware. Prog Cardiovasc Dis. 2015;57:566-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Craiem D, Chironi G, Casciaro ME, Graf S, Simon A. Calcifications of the thoracic aorta on extended non-contrast-enhanced cardiac CT. PLoS One. 2014;9:e109584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Shahcheraghi N, Dwyer HA, Cheer AY, Barakat AI, Rutaganira T. Unsteady and three-dimensional simulation of blood flow in the human aortic arch. J Biomech Eng. 2002;124:378-387. [PubMed] |
| 30. | Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennet MR. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 378] [Article Influence: 37.8] [Reference Citation Analysis (0)] |