Published online Jan 28, 2016. doi: 10.4329/wjr.v8.i1.109
Peer-review started: May 4, 2015
First decision: October 27, 2015
Revised: November 12, 2015
Accepted: December 3, 2015
Article in press: December 4, 2015
Published online: January 28, 2016
Processing time: 275 Days and 22.7 Hours
AIM: To test the incremental value of 3T magnetic resonance neurography (MRN) in a series of unilateral radiculopathy patients with non-contributory magnetic resonance imaging (MRI).
METHODS: Ten subjects (3 men, 7 women; mean age 54 year and range 22-74 year) with unilateral lumbar radiculopathy and with previous non-contributory lumbar spine MRI underwent lumbosacral (LS) plexus MRN over a period of one year. Lumbar spine MRI performed as part of the MRN LS protocol as well as bilateral L4-S1 nerves, sciatic, femoral and lateral femoral cutaneous nerves were evaluated in each subject for neuropathy findings on both anatomic (nerve signal, course and caliber alterations) and diffusion tensor imaging (DTI) tensor maps (nerve signal and caliber alterations). Minimum fractional anisotropy (FA) and mean apparent diffusion coeffcient (ADC) of L4-S2 nerve roots, sciatic and femoral nerves were recorded.
RESULTS: All anatomic studies and 80% of DTI imaging received a good-excellent imaging quality grading. In a blinded evaluation, all 10 examinations demonstrated neural and/or neuromuscular abnormality corresponding to the site of radiculopathy. A number of contributory neuropathy findings including double crush syndrome were observed. On DTI tensor maps, nerve signal and caliber alterations were more conspicuous. Although individual differences were observed among neuropathic appearing nerve (lower FA and increased ADC) as compared to its contralateral counterpart, there were no significant mean differences on statistical comparison of LS plexus nerves, femoral and sciatic nerves (P > 0.05).
CONCLUSION: MRN of LS plexus is useful modality for the evaluation of patients with non-contributory MRI of lumbar spine as it can incrementally delineate the etiology and provide direct objective and non-invasive evidence of neuromuscular pathology.
Core tip: Magnetic resonance neurography of the lumbosacral plexus is a useful modality for the evaluation of patients with non-contributory magnetic resonance imaging of the lumbar spine. It can incrementally delineate the etiology and provides direct objective and non-invasive evidence of neuromuscular pathology.
- Citation: Chhabra A, Farahani SJ, Thawait GK, Wadhwa V, Belzberg AJ, Carrino JA. Incremental value of magnetic resonance neurography of Lumbosacral plexus over non-contributory lumbar spine magnetic resonance imaging in radiculopathy: A prospective study. World J Radiol 2016; 8(1): 109-116
- URL: https://www.wjgnet.com/1949-8470/full/v8/i1/109.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i1.109
Radiculopathy refers to pain, weakness, numbness or tingling along the nerve distribution, due to inflammation or compression of the nerve roots. Electro-diagnostic (ED) testing, including electromyography and nerve conduction velocity are commonly used to confirm the presence of radiculopathy with high specificity, but with limited sensitivity[1]. Magnetic Resonance imaging is the preferred non-invasive imaging technique in the evaluation of patients with radiculopathy, and serves as a valuable adjunct to ED testing. Magnetic resonance imaging (MRI) also shows excellent interobserver agreement for detection of nerve root compression in patients with radiculopathy[2]. It helps in delineation of the site and cause of radiculopathy, and has been shown to have a greater sensitivity than ED testing, though with limited specificity[3]. Management dilemma, however, frequently occurs when a patient with clinical features of radiculopathy has normal or non-contributory MRI findings. Magnetic resonance neurography (MRN) is an imaging technique optimized for evaluation of the peripheral nerves and their associated pathologies. It uses high resolution imaging with combined 2-Dimensional (2D) and 3D inversion recovery (IR) turbo spin echo (TSE) sequences for multiplanar depiction of small and large peripheral nerves emanating from the lumbosacral (LS) plexus and its tributaries. Currently available 3.0 Tesla (3T) scanners provide increased signal-noise ratio and superior spatial resolution over 1.5T scanners, with improved fluid conspicuity. This translates into better anatomic and pathological characterization of the nerves. The utility of MRN has been shown in evaluating patients with cervical and lumbar radiculopathy[4-7]. However, its incremental value over conventional MR imaging has not yet been demonstrated. In this study, we tested the role of 3T MRN in a prospective series of patients with unilateral radiculopathy and a prior non-contributory MR imaging of the lumbar spine.
Institutional review board approval was taken for this health insurance portability and accountability act compliant prospective study. Informed consent was obtained from all subjects. The subjects presented with unilateral radiculopathy symptoms, positive straight leg raising test, and had prior normal or non-contributory MR imaging of the lumbar spine. No patient had known tumor, neurofibromatosis, hereditary neuropathy or prior nerve surgery.
All patients were studied on 3T, 60 cm bore research MR Scanner (Trio, Siemens, Erlangen, Germany) recruited over one year period (Table 1). The technique employed 2D axial T1W, Axial T2 spectral adiabatic inversion recovery (SPAIR) and isotropic 3D short tau inversion recovery (STIR) sampling perfection with application optimized contrasts using variable flip angle evolutions (SPACE) (focused on whole abdomen and pelvis from T12-L1 level to lesser trochanters for the imaging for nerves); isotropic 3D T2 TSE focused on LS spine; and diffusion tensor imaging (DTI) technique from L3-lesser trochanters (single shot EPI, 12 encoding directions; b-values 0, 800 and 1000 s/mm2, TR/TE/SL - 6000/68/4). The total acquisition time was 45 min.
| Pulse sequence | 2D/3D | TR (ms) | TE (ms) | Slice thickness (mm) | Coverage |
| Axial T1W | 2D | 700 | 8 | 4 | T12-L1 to lesser trochanters |
| Axial T2 SPAIR | 2D | 4800 | 65 | 4 | T12-L1 to lesser trochanters |
| Coronal STIR SPACE | 3D | 2000 | 78 | 1.5 isotropic | T12-L1 to lesser trochanters |
| Sagittal T2 SPACE | 3D | 2000 | 120 | 0.9 isotropic | T12-L1 to sacrum |
| Axial DTI | 2D | 6000 | 65 | 4 | T12-L1 to lesser trochanters |
Ten subjects were recruited over the course of the year (3 men, 7 women; mean age 54 year, range 22-74 year). The symptoms and signs included pain (10/10), sensory alterations (2/10), motor symptoms (5/10), Tinel sign (0/10), prior trauma (3/10) and prior bony surgery in the field of view (2/10).
Two readers, experienced in musculoskeletal research imaging (Chhabra, Thawait) assessed the image quality in consensus on a scale of 1-3 (1 - poor; 2 - good; 3 - excellent) on a picture archiving and communication system workstation (UV, Emageon). The readers were blinded to the clinical information from the patients and final reports of the prior imaging studies. Lumbar spine MR imaging performed as part of the MRN LS protocol as well as bilateral L4-S1 nerves, sciatic, femoral and lateral femoral cutaneous nerves were evaluated in each subject for neuropathy findings indicated by qualitative signal intensity and size alterations on anatomic and DTI tensor maps. Lowest fractional anisotropy (FA) and mean apparent diffusion coeffcient (ADC) values of L4-S2 nerve roots, sciatic and femoral nerves on tensor calculated images were recorded in consensus.
Descriptive analysis for qualitative imaging findings performed. All data were stored on a spreadsheet (Excel 2010, Microsoft, Seattle, WA). Paired t-test was performed for the analysis of side to side differences among FA and mean ADC values of L4-S2 nerve roots, sciatic and femoral nerves. P value of < 0.05 was considered statistically significant.
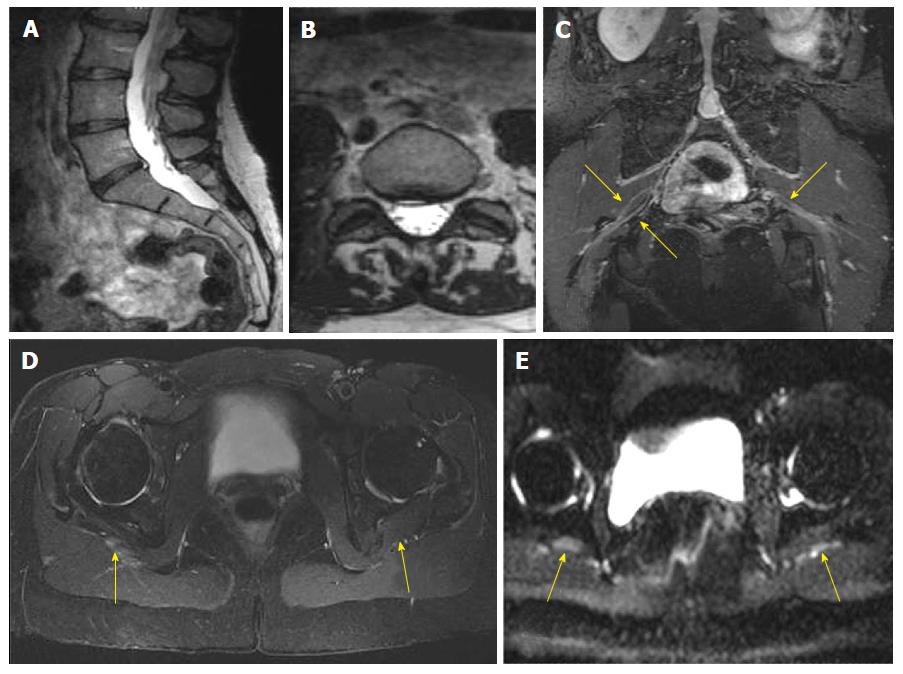
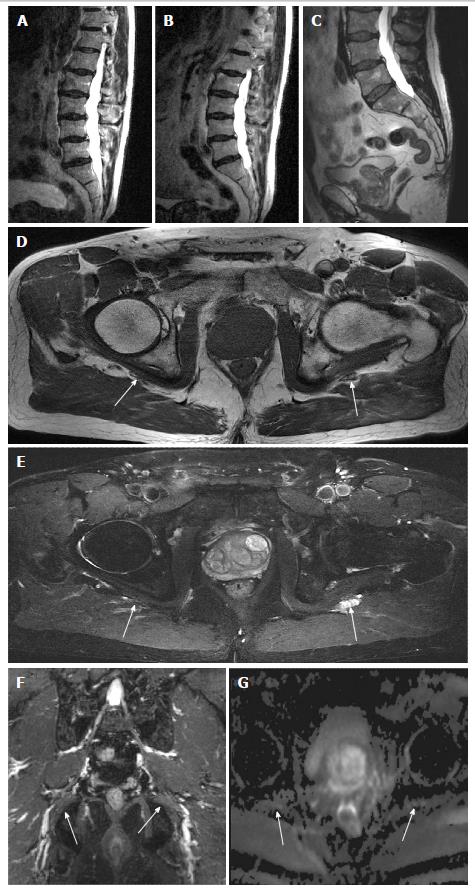
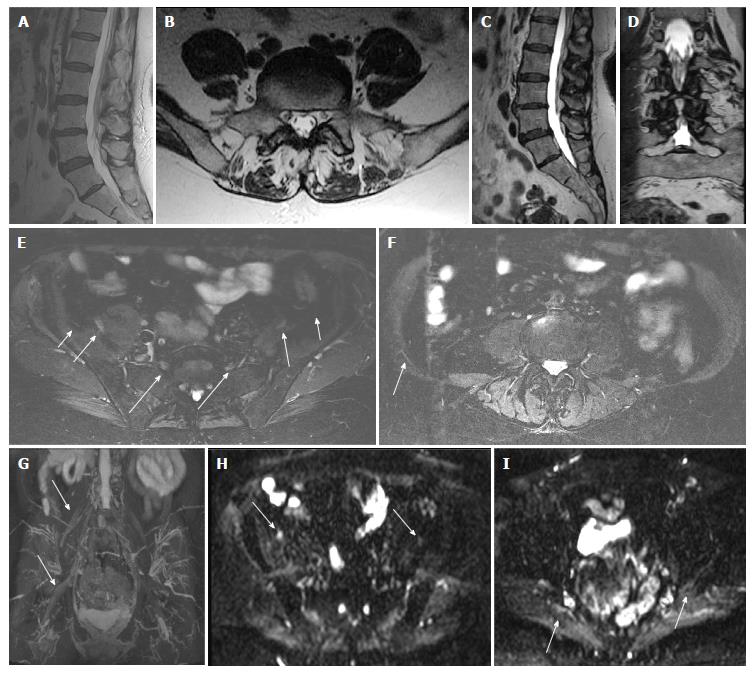
All anatomic studies (10/10) received a quality grading of excellent quality and DTI studies received different quality grading (excellent 5/10, good 3/10 and poor 2/10). All DTI studies were included in analysis. In a blinded evaluation, all 10 examinations demonstrated neural and/or neuromuscular abnormality corresponding to the site of radiculopathy. The spectrum of abnormalities included imaging findings of contributory femoral neuropathy (2/10) (Figure 1), lateral femoral cutaneous neuropathy (2/10), lumbosacral nerve root abnormality (3/10), sciatic neuropathy (9/10) (Figures 2 and 3), obturator neuropathy (1/10), iliohypogastric neuropathy (1/10) (Figure 4), and double crush syndrome with ipsilateral S2 and sciatic neuropathy (1/10). The variants included split femoral nerve (1/10) and split sciatic nerves (3/10). Contributory lumbar disc herniation was seen in 2/10 cases. Other incidental abnormalities included hip labral tear (1/10), low grade hamstring tear (1/10), prostate hypertrophy (1/10), sacral insufficiency fractures (1/10), renal cysts (1/10), transitional lumbosacral vertebra (1/10), trochanteric bursitis (2/10), ischiofemoral impingement (1/10) and colonic diverticulosis (1/10). On DTI, the nerve signal alterations were more conspicuous. Although individual differences were observed between the abnormally hyperintense nerve as compared to its contralateral counterpart (Figures 1 and 4), there were no significant mean differences in FA and ADC on statistical comparison of LS plexus nerves, femoral and sciatic nerves (P > 0.05).
The LS plexus pathology can be a significant source of neuropathic pain (radiculopathy) which poses a great diagnostic challenge to the clinician and the radiologist, due to the deep location of the nerves and their variable regional innervation. LS spine related abnormalities also frequently mimic or contribute to the clinical features of radiculopathy[8]. Traditionally, the diagnosis of LS radiculopathy is investigated using ED tests and MR lumbar spine findings. The high sensitivity of MRI combined with high specificity of ED tests often provide very good complementary information[1]. However, ED tests are operator dependent and the results depend on the technical expertise of the examiner. Such studies are also invasive and not recommended in patients on anticoagulation therapy[9]. MR imaging of LS spine is a reproducible and non-invasive diagnostic modality, but its value may be limited by a number of factors, such as the presence of multiple disc herniations or multilevel nerve impingements findings in middle aged-older age groups, commonly performed low resolution imaging with lack of thin slice 3D evaluation, absence of sacroiliac joint or pelvis inclusion in the field of view, and finally, reader focus on evaluation of the cause (disc herniation) vs effect (nerve inflammation or entrapment).
This preliminary prospective study has supports the incremental value of MRN LS plexus over lumbar spine MR imaging and ED studies. Since the protocol includes 3D imaging of lumbar spine, disc herniations and transitional bony anatomy can be easily assessed on multiplanar reconstructions. Although, the transitional segment could have been overlooked by the inexperienced outside reader in Figure 4, 3D imaging has the potential to make its identification more apparent and easier.
MRN of LS plexus is a different way of looking at the spine, with the conventional MR spine sagittal T1W and STIR imaging replaced by axial T1W, axial T2 SPAIR and coronal 3D IR TSE imaging. This allows comprehensive coverage of the whole lower abdomen and pelvis. Thereby, high resolution multi-planar depiction of normal and abnormal peripheral nerves is enabled[10-11]. For side to side comparison, uniform fat suppression is essential. Therefore, authors use inversion recovery type fat suppression. Chemical selective fat suppression does not work well in the large field of view. On the other hand, STIR SPACE and axial SPAIR provide more uniform fat suppression in such a large field of view for optimal comparison. With 3D imaging, there is an additional advantage of vascular signal suppression with superior delineation of the nerves.
3D imaging clearly brings out subtle differences in the nerve signal intensity as shown in Figures 3 and 4, which are otherwise inconspicuous on the axial 2D images. DTI images further enhance the endoneurial fluid signal intensity alterations and confirm the side to side differences with effective vascular signal suppression on higher b values or tensor calculated images. This study shows that MRN produces high quality anatomic images of the LS plexus in all cases and in about 80% cases of DTI, and confirms the clinical diagnosis. It can be potentially an important for patient satisfaction, since MRN objectively demonstrates the findings corresponding to the side of symptoms when other tests do not reveal the cause or confirm the clinical suspicion of radiculopathy. Additionally, it provides additional anatomic and lesion assessment which is not possible with other modalities[6,12].
Due to the high resolution (1.5 mm isotropic voxel for 3D imaging and 0.5-0.6 mm in plane resolution of 2D imaging), MRN affords comprehensive assessment of lumbar spine, pelvis, sacroiliac joints, hips and regional muscles as a one-stop shop. It is reflected in the number of incidental findings observed in this small study.
MRN showed pathology in the LS plexus nerves on the side of symptoms. Additionally, it also showed nerve variants in bilateral femoral and sciatic nerves. Similar to pathologic findings of disc herniations not impinging the nerves, mere presence of developmental variants does not mean that the latter is the cause of symptoms. Direct visualization of neuromuscular abnormality is an indispensable finding.
The MRN with DTI imaging protocol takes approximately 45 min on 3T scanner using front XL torso coil linked to spine coils in the back. We didn’t administer intravenous contrast, since we didn’t include any cases of known tumor. Normal spine MR imaging takes about 30 min on 3T scanner. We do not advocate replacing conventional LS MR imaging with MRN of LS plexus on a routine basis, since it takes longer to read MRN examinations and acquisition time is also longer. However, the incremental value over the conventional spine MRI examinations is important to appreciate and MRN exams should be considered in the setting of non-contributory MRI in radiculopathy patients.
Our study has several limitations. First, being a small pilot study, we did not obtain inter- or intra-observer performance assessment. It should be kept in mind that prospective studies are difficult and expensive to accomplish and we could perform only 10 examinations over one year period from the neurosurgery clinic. Second, although there was good correlation of imaging findings, it is not an accuracy study and we do not yet have outcomes data, which remains a topic for further research. Third, there is a spectrum and selection bias, but it could not be avoided, since we wanted to study incremental value of MRN over MR spine studies. Finally, we did not test cervical radiculopathy cases, we cannot comment on whether it provides any incremental value over MRI in those cases.
In conclusion, MRN of LS plexus is useful modality for the evaluation of patients with non-contributory MR imaging of lumbar spine as it can delineate the etiology and provide direct objective and non-invasive evidence of neuromuscular pathology.
Magnetic resonance imaging (MRI) is the preferred non-invasive imaging technique in the evaluation of patients with radiculopathy, and serves as a valuable adjunct to electro-diagnostic testing. However, a diagnostic dilemma may occur when a patient with clinical features of radiculopathy has normal or non-contributory MRI findings.
Magnetic resonance neurography (MRN) is an imaging technique optimized for evaluation of the peripheral nerves and their associated pathologies. The purpose of this study was to test the role of 3.0 Tesla MRN in a prospective series of patients with unilateral radiculopathy and a prior non-contributory MR imaging of the lumbar spine.
All examinations in the present study demonstrated neural and/or neuromuscular abnormality corresponding to the site of radiculopathy, thus proving that MRN is a useful modality for the evaluation of patients with non-contributory MR imaging of lumbar spine.
This study serves as evidence that MRN can be used to delineate the cause of radiculopathy in patients with non-contributory MRI findings.
In this preliminary study, the authors had investigated the role of MRN in the lumbosacral plexus neuropathy and found that MRN is useful modality for the evaluation of patients with non-contributory MRI of lumbar spine as it can incrementally delineate the etiology and provide direct objective and non-invasive evidence of neuromuscular pathology.
P- Reviewer: Shen J
S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Barr K. Electrodiagnosis of lumbar radiculopathy. Phys Med Rehabil Clin N Am. 2013;24:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Kuijper B, Beelen A, van der Kallen BF, Nollet F, Lycklama A Nijeholt GJ, de Visser M, Tans JT. Interobserver agreement on MRI evaluation of patients with cervical radiculopathy. Clin Radiol. 2011;66:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Reza Soltani Z, Sajadi S, Tavana B. A comparison of magnetic resonance imaging with electrodiagnostic findings in the evaluation of clinical radiculopathy: a cross-sectional study. Eur Spine J. 2014;23:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Erdem CZ, Erdem LO, Cağavi F, Kalayci M, Gündoğdu S. High resolution MR neurography in patients with cervical radiculopathy. Tani Girisim Radyol. 2004;10:14-19. [PubMed] |
| 5. | Dailey AT, Tsuruda JS, Goodkin R, Haynor DR, Filler AG, Hayes CE, Maravilla KR, Kliot M. Magnetic resonance neurography for cervical radiculopathy: a preliminary report. Neurosurgery. 1996;38:488-492, discussion 492. [PubMed] |
| 6. | Soldatos T, Andreisek G, Thawait GK, Guggenberger R, Williams EH, Carrino JA, Chhabra A. High-resolution 3-T MR neurography of the lumbosacral plexus. Radiographics. 2013;33:967-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Chen YY, Lin XF, Zhang F, Zhang X, Hu HJ, Wang DY, Lu LJ, Shen J. Diffusion tensor imaging of symptomatic nerve roots in patients with cervical disc herniation. Acad Radiol. 2014;21:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Nardo L, Alizai H, Virayavanich W, Liu F, Hernandez A, Lynch JA, Nevitt MC, McCulloch CE, Lane NE, Link TM. Lumbosacral transitional vertebrae: association with low back pain. Radiology. 2012;265:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Chémali KR, Tsao B. Electrodiagnostic testing of nerves and muscles: when, why, and how to order. Cleve Clin J Med. 2005;72:37-48. [PubMed] |
| 10. | Chhabra A. Peripheral MR neurography: approach to interpretation. Neuroimaging Clin N Am. 2014;24:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Chhabra A, Lee PP, Bizzell C, Soldatos T. 3 Tesla MR neurography--technique, interpretation, and pitfalls. Skeletal Radiol. 2011;40:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Moore KR, Tsuruda JS, Dailey AT. The value of MR neurography for evaluating extraspinal neuropathic leg pain: a pictorial essay. AJNR Am J Neuroradiol. 2001;22:786-794. [PubMed] |