Published online Dec 28, 2015. doi: 10.4329/wjr.v7.i12.501
Peer-review started: June 15, 2015
First decision: October 21, 2015
Revised: November 11, 2015
Accepted: December 8, 2015
Article in press: December 11, 2015
Published online: December 28, 2015
Processing time: 198 Days and 10 Hours
AIM: To measure the common bile duct (CBD) diameter by magnetic resonance cholangiopancreatography (MRCP) in a large asymptomatic population and analyze its some affecting factors.
METHODS: This study included 862 asymptomatic subjects who underwent MRCP. The CBD diameter was measured at its widest visible portion on regular end-expiration MRCP for all subjects. Among these 862 subjects, 221 volunteers also underwent end-inspiration MRCP to study the effect of respiration on the CBD diameter. The age, sex, respiration, body length, body weight, body mass index (BMI), portal vein diameter (PVD), length of the extrahepatic duct and CBD, cystic junction radial orientation and location were recorded. The subjects were divided into 7 groups according to age. All of the above factors were compared with the CBD diameter on end-expiration MRCP.
RESULTS: Among the 862 subjects, the CBD diameter was 4.13 ± 1.11 mm (range, 1.76-9.45 mm) and was correlated with age (r = 0.484; P < 0.05), with a dilation of 0.033 mm per year. The upper limit of the 95% reference range was 5.95 mm, resulting in a reasonable upper limit of 6 mm for the asymptomatic population. Respiration and other factors, including sex, body length, body weight, BMI, PVD, length of the extrahepatic duct and CBD, cystic junction radial orientation and location, were not related to the CBD diameter.
CONCLUSION: We established a reference range for the CBD diameter on MRCP for an asymptomatic population. The CBD diameter is correlated with age. Respiration did not affect the non-dilated CBD diameter.
Core tip: We measured the common bile duct (CBD) diameter by magnetic resonance cholangiopancreatography (MRCP) for a large asymptomatic population and suggested the normal upper limit of the duct be set at 6 mm on MRCP. The CBD diameter was correlated with age, and gradually dilates 0.033 mm per year. Respiration didn’t effect on the non-dilated CBD diameter on MRCP. The significant changes of CBD diameter between inspiration and expiration may suggest a dilation of CBD.
- Citation: Peng R, Zhang L, Zhang XM, Chen TW, Yang L, Huang XH, Zhang ZM. Common bile duct diameter in an asymptomatic population: A magnetic resonance imaging study. World J Radiol 2015; 7(12): 501-508
- URL: https://www.wjgnet.com/1949-8470/full/v7/i12/501.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i12.501
A dilated common bile duct (CBD) suggests obstructive causes, which may require invasive imaging or remedial procedures[1]. However, an accurate reference range for CBD size remains debatable[1-9]. Thus, to determine whether a spontaneous abnormality or atypical dilation is important, there needs to be a reference range such that CBD diameters exceeding the upper limit can be classified as abnormal.
With the widespread use of cross-sectional imaging and improvements in cross-sectional imaging technology, the diameter of the CBD is being detected incidentally with increasing frequency when using ultrasound, computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP)[1-9]. MRCP is a technique that uses T2 sequence magnetic resonance imagery to perform a noninvasive evaluation of the anatomy and pathology of the pancreatobiliary system[10]. MRCP can be used to measure the diameter of the CBD[11]. MRCP is the principal diagnostic modality that determines whether endoscopic retrograde cholangiopancretography is needed, particularly when ultrasound findings are equivocal[12]. Chen et al[1] measured the normal CBD diameter in 187 patients by MRCP and found that the CBD diameter was significantly correlated only with age.
The diameter of the CBD changes in response to various factors, including age[1-3], cholecystectomy[2,3], measurement location[4], respiration[5], and body mass index (BMI, which was calculated as weight in kilograms divided by the square of height in meters)[3]. For some of these factors, such as age and gender, the effect on the CBD is not clear. More than 30 years ago, Wu et al[6] utilized ultrasound to determine that the CBD diameter increases by 1 mm every decade. Later, other studies supported this observation[1-4,9]. However, Horrow et al[7] obtained controversial results by ultrasound; they found that age was not associated with the size of the extrahepatic bile duct in 258 asymptomatic adults. Some studies[1,3,8] have suggested that gender has no significant effect on CBD diameter by ultrasound and MRCP, but Matcuk et al[9] reported that the extrahepatic bile duct was larger in females after performing an ultrasound on 1484 normal individuals. There has been only one study[3] concerning the effect of BMI on the CBD diameter. The anomalous junction of the cystic duct with the common bile duct may cause stagnation of bile[13]. Cystic duct anatomic variants (such as the cystic junction radial orientation variant) can be a source of confusion during surgery if unrecognized[14]. Low-junction patients with a short CBD experience several complications, including congenital dilation of the cystic duct[13]. Choledochocele is a cystic or diverticular dilatation of the lower bile duct and is sometimes associated with cholangitis or pancreatitis[15]. To the best of our knowledge, there is no report concerning the relationship between the diameter and length of the extrahepatic duct and the CBD, the cystic junction radial orientation or the cystic junction location.
The purpose of our study was to evaluate the CBD diameter in a large cohort of asymptomatic patients using MRCP and to determine the normal size range of the CBD in this population. In addition, this study aimed to determine the effects of age, sex, respiration, body length, body weight, BMI, portal vein diameter (PVD), extrahepatic duct and CBD length, cystic junction radial orientation and cystic junction location on the CBD diameter as measured by MRI.
This retrospective study was approved by our institutional review board. Patient informed consent was waived. During the period of January 2010 to March 2014, we recruited all the patients who underwent an abdominal MRI in our hospital for our study. We recorded the age, sex, medical history, list of medications, total serum cholesterol, liver function tests, and hepatitis status of each patient. In addition, body length, body weight and BMI were recorded for the volunteers.
The following search criteria were used: (1) normal abdomen; (2) hepatic cysts; (3) hepatic or splenic hemangiomas; and (4) renal cysts.
The exclusion criteria were the following: (1) pre-existing hepatobiliary and pancreatic surgery; (2) intra- or retroperitoneal tumors, inflammation or hemorrhagic diseases; (3) biliary tract stones; (4) cholecystitis; (5) cirrhosis of the liver; (6) ascites; (7) abnormal liver function tests (total bilirubin, aspartate aminotransferasea, alanine aminotransferase); (8) current use of medication that causes relaxation of smooth muscle (e.g., calcium blockers and papaverine hydrochloride); and (9) abnormal total serum cholesterol.
We identified 5792 patients who underwent abdominal MR imaging at our hospital. Of these patients, 167 were excluded because of artifacts. A total of 4763 patients met the exclusion criteria and were not included in the study. The final study cohort consisted of 862 consecutive patients, including 450 male and 412 female patients aged 5 to 87 years (mean age ± SD, 46.10 ± 16.38 years). Among these 862 people, 221 were volunteers, including 108 males and 113 females aged 17 to 80 years (mean age ± SD, 37.80 ± 17.77 years).
The patients were divided into 7 groups according to their age: Group I, ≤ 20 years; Group II, 21-30 years; Group III, 31-40 years; Group IV, 41-50 years; Group V, 51-60 years; Group VI, 61-70 years; and Group VII, > 70 years.
The patients were divided into normal weight (BMI < 25 kg/m2), overweight (25 kg/m2≤ BMI < 28 kg/m2) and obesity (BMI ≥ 28 kg/m2) groups according to their BMI[16,17].
MR imaging was performed on the patients after an overnight fast of at least 8 h prior to the MR examination. All the examinations were performed with a 1.5-T MR scanner with 38 mT/M gradients and a 120 mT/M-per-second slope (Signa Excite; GE Medical Systems, Milwaukee, WI, United States) using a phased-array torso-pelvis coil. The imaging sequences, including two-dimensional coronal and axial single-shot fast spin-echo (SSFSE) T2-weighted imaging, axial respiratory gating fast-recovery fast-spin echo (FRFSE) T2-weighted imaging with fat suppression, fast-spoiled gradient-echo T1-weighted imaging with fat suppression, axial spoiled dual gradient-echo T1-weighted in- and out-of-phase MR imaging, axial slab three-dimensional (3D) spoiled gradient-echo dynamic contrast-enhanced MR imaging with fat suppression, and SSFSE radial series slab MRCP, were performed when all the patients were at the end of expiration and were holding their breath. End-expiration MRCP was considered conventional MRCP for each patient. The volunteers also underwent MRCP at the end of inspiration.
Coronal and axial SSFSE T2-weighted images were obtained during breath-holding with the following parameters: echo time (TE) = 90-100 ms; 2 s between slice acquisitions; section thickness = 5 mm; intersection gap = 0.5 mm; matrix = 384 × 224; one-half signal acquired; and field of view (FOV) = 33 cm × 33 cm. FRFSE T2-weighted images were obtained with the following parameters: repetition time (TR) ms/TE ms = 10000-12000/90-100, with TR determined by the frequency of respiration; section thickness = 5 mm; intersection gap = 0.5 mm; matrix = 256 × 192; number of signals acquired (NSA) = 3; and FOV = 34 cm × 34 cm. The acquisitions were completed in approximately 3-4 min.
Radial oblique slab SSFSE images were obtained for end-expiration and end-inspiration MRCP with the following parameters: TE = 1300 ms; 6 s between image acquisitions; section thickness = 40 mm; matrix = 384 × 224; one-half signal acquired; and FOV = 30 cm × 30 cm.
All of the other routine sequences mentioned above were not used in the analysis presented in this article; thus, we have not listed the parameters for those sequences.
It took approximately 30 min to complete all of the non-contrast MRI sequences and 35 min to complete the contrast-enhanced MR imaging.
The original MRI data were loaded onto a workstation (GE, AW 4.1, Sun Microsystems, Palo Alto, CA, United States) for review. Two observers (with 4 and 6 years of experience interpreting abdominal MR images) retrospectively and individually reviewed the coronal and transverse T2-weighted and MRCP images to evaluate the CBD.
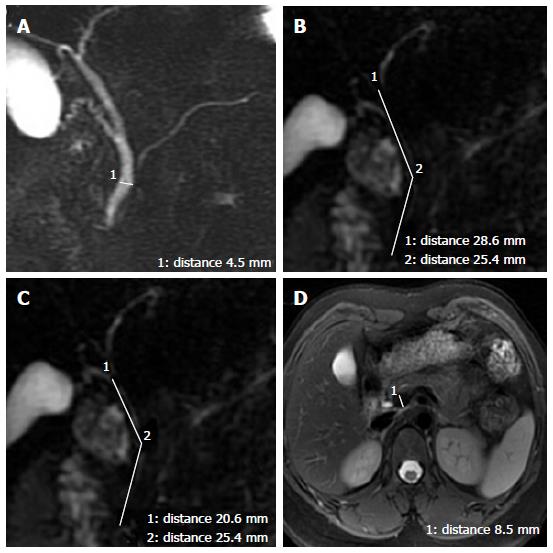
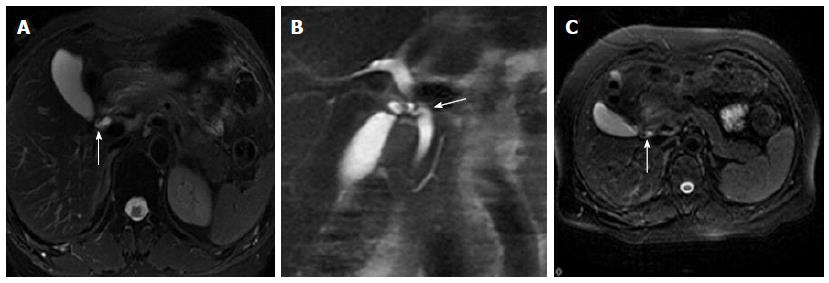
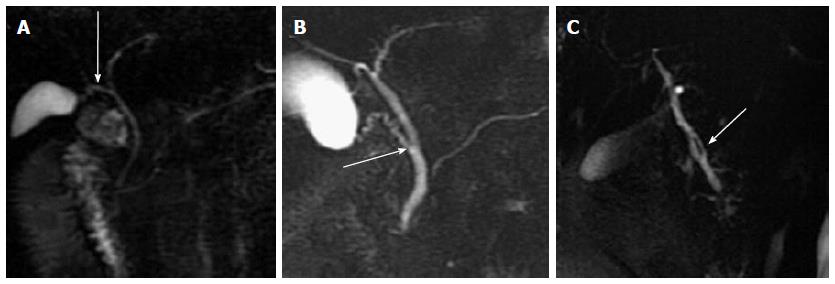
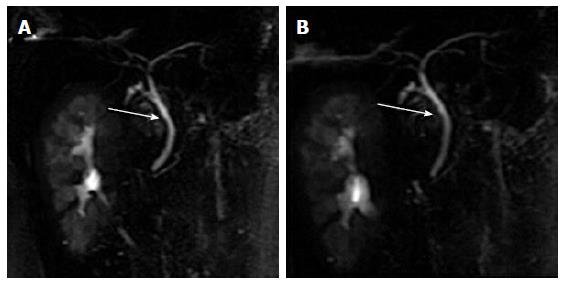
The widest diameter of the CBD was measured by placing an electronic caliper perpendicular to the long axis at the widest visible portion of the CBD on end-expiration MRCP for all the patients (Figure 1A). To study the effect of breath on the diameter of the CBD, the volunteers also underwent end-inspiration MRCP. The measurements on end-inspiration MRCP were taken at the same location as those on end-expiration. Because the CBD frequently exhibits a tortuous or serpentine course, the length of the extrahepatic bile duct is the sum of the length from the hepatic hilum to the tortuous portion and from the tortuous portion to the ampulla (Figure 1B). Similarly, the length of the CBD is the sum of the length from the cystic duct insertion to the tortuous portion and from the tortuous portion to the ampulla (Figure 1C). The anteroposterior diameters of the portal vein were measured by placing the electronic caliper at the splenic veins into the portal vein on T2-weighted images (Figure 1D). The radial orientation of the cystic junction was defined as lateral (insertion diagonally from the right), medial (insertion into the left side of the common hepatic duct), or posteroanterior (overlap of the junction with the bile duct in the posteroanterior view)[14] (Figure 2). Proximal, middle and low insertion of the cystic duct into the bile duct was defined when the cystic junction was detected in the proximal, middle or distal third, respectively, of the bile duct between the hepatic hilum and the ampulla of Vater (Figure 3).
Data derived from the MR images were expressed as the average of the two observers’ findings. Any discrepancies in the discrete data were discussed by the two observers until a consensus was reached.
The inter-rater agreement for the prevalence of the cystic junction radial orientation and cystic junction location was assessed using the kappa (κ) statistic. This statistic is generally interpreted as follows: A κ value equal to or greater than 0.81 indicates very good agreement, a κ value ranging from 0.80 to 0.61 indicates good agreement, a kappa value ranging from 0.60 to 0.41 indicates moderate agreement, and a κ value of less than 0.41 indicates poor agreement.
The results of the CBD diameter, body length, body weight, BMI, PVD, and extrahepatic duct and CBD length were expressed as the mean ± SD. The upper limit of the 95% reference range for the CBD diameter was defined as the mean + 1.64 SD.
The independent t test was used to compare the CBD diameter between patients younger and older than 60 years and between genders. CBD diameters were analyzed based on age, body length, body weight, PVD, and extrahepatic duct and CBD length using Pearson correlations. The CBD diameters in the end-inspiration and end-expiration phases were analyzed using paired t tests. Analysis of variance (ANOVA) was used to compare the diameter by BMI, cystic junction radial orientation and cystic junction location. Linear regressions were used to confirm the relationships between the CBD diameters and age.
The data analysis was performed using Statistical Package for Social Sciences (SPSS) for Windows (Version 13.0, Chicago, IL, United States). P values ≤ 0.05 were considered statistically significant.
Agreement between the two radiologists was good regarding the prevalence of the cystic junction location (κ = 0.79) and moderate concerning the prevalence of the cystic junction radial orientation (κ = 0.53).
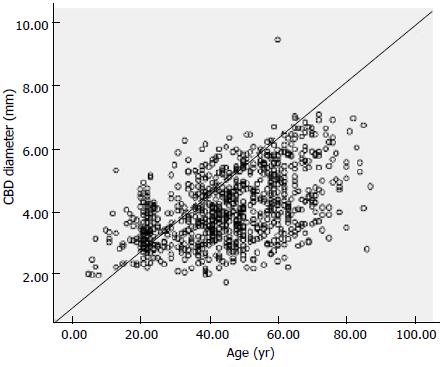
Among the 862 subjects, the mean diameter of the CBD on end-expiration MRCP was 4.13 ± 1.11 mm (1.76-9.45 mm). There was a significant correlation between the CBD diameter and age (r = 0.484, P < 0.05; Figure 4). According to the linear periodic model, the regression equation for diameter was as follows: 0.033 × age + 2.624. Thus, the duct gradually dilated by 0.033 mm per year. Table 1 lists the mean CBD diameters of the subjects in each group. The upper limit of the 95% reference range for the CBD diameter was 5.95 mm, resulting in the reasonable upper limit of 6 mm for the asymptomatic population. The CBD diameter in people older than 61 years of age (4.93 ± 1.15 mm) was significantly different than that in subjects younger than 60 years of age (3.93 ± 0.99 mm; t = -11.364, P = 0.000).
| Groupnumber | Age (yr) | Patient number | Common bile duct diameterMean ± SD (mm) |
| I | ≤ 20 | 42 | 3.23 ± 0.77 |
| II | 21-30 | 123 | 3.45 ± 0.67 |
| III | 31-40 | 137 | 3.80 ± 0.97 |
| IV | 41-50 | 234 | 4.01 ± 0.89 |
| V | 51-60 | 155 | 4.50 ± 1.11 |
| VI | 61-70 | 113 | 4.83 ± 1.18 |
| VII | > 70 | 58 | 5.12 ± 1.10 |
In the cohort of 862 subjects, the mean CBD diameter in females was slightly larger than that in males (4.18 ± 1.09 mm vs 4.09 ± 1.13 mm), although this difference was not statistically significant (t = -1.252, P = 0.211).
Among the 221 volunteers, the mean CBD diameter was slightly larger on end- inspiration MRCP (3.90 ± 0.96 mm) than on end-expiration MRCP (3.88 ± 0.96 mm), but the difference was not statistically significant (t = -0.896, P = 0.371) (Figure 5).
In the cohort of 221 volunteers, the normal weight subjects (83.7%; 185/221) had a CBD diameter of 3.85± 0.95 mm, the overweight subjects (14.5%; 32/221) had a CBD diameter of 4.09 ± 1.00 mm, and the obese subjects (1.8%; 4/221) had a CBD diameter of 3.61± 1.14 mm. The CBD diameters are not significantly different among the normal weight, overweight and obese groups (F = 1.034, P = 0.357).
In the 221 volunteers, the mean CBD diameters were not significantly related to body length or body weight (Table 2). In the 862 subjects, the mean CBD diameters were not significantly related to the PVD, extrahepatic bile duct length or CBD length (Table 2).
| Patient number | Mean ± SD | R value | P value | |
| Body length (m) | 221 | 1.60 ± 0.07 | -0.067 | 0.325 |
| Body weight (kg) | 221 | 57.01 ± 9.17 | 0.041 | 0.548 |
| Portal vein diameter (mm) | 862 | 8.79 ± 0.91 | 0.034 | 0.318 |
| Length of the extrahepatic bile duct (mm) | 862 | 63.75 ± 9.07 | 0.045 | 0.185 |
| Length of the CBD (mm) | 862 | 47.53 ± 10.44 | 0.003 | 0.922 |
Based on the different cystic junction radical orientations, subjects (74.8%; 645/862) with a lateral junction had a CBD diameter of 4.09 ± 1.10 mm, subjects with a medial junction (7.9%; 68/862) had a CBD diameter of 4.25 ± 1.30 mm, and subjects with a posteroanterior junction (17.3%; 149/862) had a CBD diameter of 4.24 ± 1.03 mm. The CBD diameters were not significantly different between the subjects grouped based on cystic junction radial orientation (F = 1.559, P = 0.211). Based on the cystic junction location, subjects with a proximal insertion (23.5%; 203/862) had a CBD diameter of 4.04 ± 1.17 mm, subjects with a middle insertion (73.8%; 636/862) had a CBD diameter of 4.30 ± 1.08 mm, and subjects with a low insertion (2.7%; 23/862) had a CBD diameter of 4.16 ± 1.08 mm. The CBD diameters were not significantly different among the groups based on cystic junction location (F = 1.683, P = 0.186).
In this study, we found that the mean diameter of the CBD on end-expiration MRCP was 4.13 ± 1.11 mm, with a range of 1.76 to 9.45 mm. The CBD diameters were significantly different between patients younger and older than 60 years of age (P < 0.05). The CBD diameter was correlated with age (r = 0.484; P < 0.05) and gradually dilated 0.033 mm per year. We suggest that the normal upper limit of the duct should be set at 6 mm. The CBD diameters were not significantly related to gender, body length, body weight, BMI, PVD, the length of the extrahepatic duct or the CBD, the cystic junction radial orientation or location. Respiration did not affect the non-dilated CBD diameter. Our results established a reference range for the CBD diameter on MRCP in an asymptomatic population that will be useful for evaluating suspected biliary tract disease.
Previous studies have shown that the mean diameter of the CBD is between 3.4 and 7.39 mm, with a range of 1.0 to 15.0 mm[1,2,4-8,18,19], and our results were well within the reported range. In our study, the upper limit of the 95% reference range for the CBD diameter was 5.95 mm, and the upper limit was 6 mm; these values are comparable to those from ultrasound[4] and CT[2]. The upper limit in our study was lower than that reported by Chen et al[1], possibly because of the larger population and wider age range in our study.
A few reports have considered the important age-dependent variations in the CBD diameter[1-4,6,8,9,18,19]. Some studies have revealed a slight increase in duct diameter with advancing age[6,9]. It has also been shown that the CBD diameter is directly proportional to age after patients were divided into two groups with 65 years as the cut-off age[1]. Park et al[18] reported that the CBD diameter by CT in people older than 51 years of age was significantly different than that in subjects younger than 50 years of age. Additionally, Kaim et al[19] reported that the CBD diameter in asymptomatic elderly subjects (> 75 years) was considerably higher compared with the recommended borderline values in the ultrasound literature. However, Horrow et al[7] found no increase in the size of the extrahepatic bile duct with increasing age in an adult population, and their data do not support the rule of a 1-mm-per-decade increase in the size of the bile duct by ultrasound. In this study, we found that the CBD diameter increases with age and gradually dilates 0.033 mm per year. CBD diameters are significantly different between patients who are younger or older than 60 years of age, perhaps because longitudinal smooth muscle bands and their intervening connective tissue fragments with increasing age accompanied by the loss of the reticulo-endothelial network of the ductal wall[20], resulting in age-related biliary dilatation of the CBD.
Some previous studies have reported that gender has no significant effect on the CBD diameter[1,3,8]. However, Matcuk et al[9] found that the extrahepatic bile duct increases with female sex by ultrasound. Our studies support the notion that gender has no significant effect on the CBD diameter.
Wachsberg[5] demonstrated that the maximal bile duct measurement can increase during deep inspiration by ultrasonography. However, their study included thirty subjects with a maximal anteroposterior CBD diameter of 5 mm or greater, some of whom presented with biliary obstruction. An MRCP study[21] found that the mean maximal diameter of the extrahepatic bile duct was significantly larger on end-inspiratory MRCP in the group of subjects with an extrahepatic bile duct diameter of less than 10 mm. However, their study included 102 patients with suspected biliary abnormalities by ultrasonography or computed tomography. Our results showed that the mean CBD diameters between end-inspiratory MRCP and end-expiratory MRCP were not statistically different. Our study is unique in that MRCP was used to evaluate the effect of respiration on the “normal” diameter of the CBD. Our results indicate that respiration does not affect the non-dilated CBD diameter. We speculate that the significant changes in the CBD diameter between inspiration and expiration[5,21] may suggest dilation of the CBD.
Previous studies have suggested that body length and body weight have no significant effect on the CBD diameter[3,8]. Our studies support these observations. Daradkeh et al[3] reported that the CBD diameter was correlated with BMI by ultrasound. In this study, we found that BMI had no significant effect on the CBD diameter, perhaps because ultrasound has limitations regarding overweight persons[22]. In our study, 14% (32/221) of the patients were overweight, and 1.8% (4/221) were obese. Ultrasound may have certain limitations in measuring the CBD diameter in these 15.8% of the patients, thereby resulting in measureable differences.
In our study, we also found that the PVD was not associated with the CBD diameter on MRCP, a finding that is similar to that reported by Chen et al[1].
The most common or ‘‘normal’’ way of entry (up to 65%) involves draining the cystic duct from the right lateral position[23]; however, in other series, a lateral junction was observed in only 31.8% of the cases[14]. In our study, lateral insertion of the cystic duct was detected in 74.8% of the cases, whereas medial and posteroanterior insertions accounted for the remainder. Our study of the cystic junction radial orientation supports the report by Turner et al[23]. The cystic duct usually joins the common hepatic duct about halfway between the porta hepatis and the ampulla of Vater (in 75% of cases)[23]. We found that the cystic duct joins the common hepatic duct about halfway between the porta hepatis and the ampulla of Vater in 73.8% of cases, a rate similar to that reported by Turner et al[23]. We found no relationships among the diameter and length of the extrahepatic duct, length of the CBD, cystic junction radial orientation or cystic junction location.
There are some limitations to this retrospective study. First, the variation in the depth of individual patient inspiration may have affected the length and maximal diameter of the extrahepatic bile duct during respiratory MRCP, although all of the patients were instructed before the examinations to take a deep breath or to completely exhale. Second, there were only a few patients older than 70 (6.7%) or younger than 20 (4.9%) years. This may have introduced bias regarding the imaging review and analysis.
In conclusion, in this study, we established a reference range for the CBD diameter on MRCP for an asymptomatic population. The CBD diameter is correlated with age, and its normal upper limit can be set at 6 mm. Respiration and other factors, such as gender, body length, body weight, BMI, PVD, extrahepatic duct and CBD length, and the cystic junction radial orientation and location, do not affect the non-dilated CBD diameter. The significant changes in the CBD diameter between inspiration and expiration may suggest dilation of the CBD. This is a useful reference for evaluating suspected biliary tract disease.
A dilated common bile duct (CBD) suggests obstructive. An accurate reference range for CBD size remains debatable. Magnetic resonance cholangiopancreatography (MRCP) can be used to measure the diameter of the CBD.
An accurate reference for CBD size on imaging.
To measurement the CBD diameter in a large cohort of asymptomatic patients (862) using MRCP.
The CBD diameter is correlated with age, and its normal upper limit can be set at 6 mm. Respiration and other factors, such as gender, body length, body weight, body mass index, portal vein diameter, extrahepatic duct and CBD length, and the cystic junction radial orientation and location, do not affect the non-dilated CBD diameter.
This work is alright to publish. However, more relationships of diameter other than age should be presented. Relationships of diameter and say, gender, patient weight and height are suggested.
P- Reviewer: Chow J S- Editor: Wang JL L- Editor: A E- Editor: Wu HL
| 1. | Chen T, Hung CR, Huang AC, Lii JM, Chen RC. The diameter of the common bile duct in an asymptomatic Taiwanese population: measurement by magnetic resonance cholangiopancreatography. J Chin Med Assoc. 2012;75:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Senturk S, Miroglu TC, Bilici A, Gumus H, Tekin RC, Ekici F, Tekbas G. Diameters of the common bile duct in adults and postcholecystectomy patients: a study with 64-slice CT. Eur J Radiol. 2012;81:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Daradkeh S, Tarawneh E, Al-Hadidy A. Factors affecting common bile duct diameter. Hepatogastroenterology. 2005;52:1659-1661. [PubMed] |
| 4. | Bowie JD. What is the upper limit of normal for the common bile duct on ultrasound: how much do you want it to be? Am J Gastroenterol. 2000;95:897-900. [PubMed] |
| 5. | Wachsberg RH. Respiratory variation of extrahepatic bile duct diameter during ultrasonography. J Ultrasound Med. 1994;13:617-621. [PubMed] |
| 6. | Wu CC, Ho YH, Chen CY. Effect of aging on common bile duct diameter: a real-time ultrasonographic study. J Clin Ultrasound. 1984;12:473-478. [PubMed] |
| 7. | Horrow MM, Horrow JC, Niakosari A, Kirby CL, Rosenberg HK. Is age associated with size of adult extrahepatic bile duct: sonographic study. Radiology. 2001;221:411-414. [PubMed] |
| 8. | Mahour GH, Wakim KG, Ferris DO. The common bile duct in man: its diameter and circumference. Ann Surg. 1967;165:415-419. [PubMed] |
| 9. | Matcuk GR, Grant EG, Ralls PW. Ultrasound measurements of the bile ducts and gallbladder: normal ranges and effects of age, sex, cholecystectomy, and pathologic states. Ultrasound Q. 2014;30:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Uysal F, Obuz F, Uçar A, Seçil M, Igci E, Dicle O. Anatomic variations of the intrahepatic bile ducts: analysis of magnetic resonance cholangiopancreatography in 1011 consecutive patients. Digestion. 2014;89:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 11. | Chiu NC, Chiou YY. Role of MRCP in the measurement of the CBD diameter. J Chin Med Assoc. 2012;75:423-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Shanmugam V, Beattie GC, Yule SR, Reid W, Loudon MA. Is magnetic resonance cholangiopancreatography the new gold standard in biliary imaging? Br J Radiol. 2005;78:888-893. [PubMed] |
| 13. | Uetsuji S, Okuda Y, Komada H, Yamamura M, Kamiyama Y. Clinical evaluation of a low junction of the cystic duct. Scand J Gastroenterol. 1993;28:85-88. [PubMed] |
| 14. | Tsitouridis I, Lazaraki G, Papastergiou C, Pagalos E, Germanidis G. Low conjunction of the cystic duct with the common bile duct: does it correlate with the formation of common bile duct stones? Surg Endosc. 2007;21:48-52. [PubMed] |
| 15. | Kamisawa T, Yoshiike M, Egawa N, Tsuruta K, Okamoto A, Matsukawa M. Classification of choledochocele. Hepatogastroenterology. 2005;52:29-32. [PubMed] |
| 16. | Salas-Salvadó J, Rubio MA, Barbany M, Moreno B. SEEDO 2007 Consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria. Med Clin (Barc). 2007;128:184-196; quiz 1 p following 200. [PubMed] |
| 17. | Adeboye B, Bermano G, Rolland C. Obesity and its health impact in Africa: a systematic review. Cardiovasc J Afr. 2012;23:512-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Park JS, Lee DH, Jeong S, Cho SG. Determination of Diameter and Angulation of the Normal Common Bile Duct using Multidetector Computed Tomography. Gut Liver. 2009;3:306-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kaim A, Steinke K, Frank M, Enriquez R, Kirsch E, Bongartz G, Steinbrich W. Diameter of the common bile duct in the elderly patient: measurement by ultrasound. Eur Radiol. 1998;8:1413-1415. [PubMed] |
| 20. | Kialian GP, Aznaurian AV. The age-related characteristics of the muscular layer of the common bile duct in man. Morfologiia. 1995;108:10-12. [PubMed] |
| 21. | Ito K, Shimizu A, Tanabe M, Matsunaga N. Respiratory variation of the extrahepatic bile duct: evaluation with deep inspiratory and expiratory MRCP. Magn Reson Imaging. 2012;30:579-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 22. | Ongoïba N, Sissoko F, Ouologuem I, Bérété S, Diop AK, Sidibé S, Touré M, Kéita AD, Koumaré AK. The size of the bile duct by echograph. A study. Morphologie. 2012;96:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Turner MA, Fulcher AS. The cystic duct: normal anatomy and disease processes. Radiographics. 2001;21:3-22; questionnaire 288-294. [PubMed] |