Published online Dec 28, 2015. doi: 10.4329/wjr.v7.i12.494
Peer-review started: May 9, 2015
First decision: July 27, 2015
Revised: August 13, 2015
Accepted: October 12, 2015
Article in press: October 12, 2015
Published online: December 28, 2015
Processing time: 233 Days and 16.8 Hours
AIM: To investigate the time course of testosterone (T) recovery after cessation of androgen deprivation therapy (ADT) in patients treated with brachytherapy.
METHODS: One-hundred and seventy-four patients treated between June 1999 and February 2009 were studied. Patients were divided into a short-term usage group (≤ 12 mo, n = 91) and a long-term usage group (≥ 36 mo, n = 83) according to the duration of gonadotropin-releasing hormone agonist therapy. Median follow-up was 29 mo in the short-term group and was 60 mo in the long-term group.
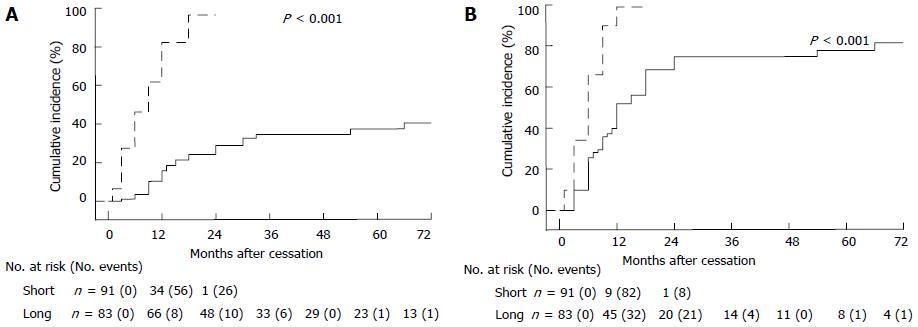
RESULTS: Cumulative incidence rates of T recovery to normal and supracastrate levels at 24 mo after cessation were 28.8% and 74.6%, respectively, in the long-term usage group, whereas these values were 96.4% and 98.8% in the short-term usage group. T recovery to normal and supracastrate levels occurred significantly more rapidly in the short-term than in the long-term usage group (P < 0.001 and P < 0.001, respectively). Five years after cessation, 22.6% of patients maintained a castrate T level in the long-term usage group. On multivariate analysis, lower T levels (< 10 ng/dL) at cessation of ADT was significantly associated with prolonged T recovery to supracastrate levels in the long-term usage group (P = 0.002).
CONCLUSION: Lower T levels at cessation of ADT were associated with prolonged T recovery in the long-term usage group. Five years after cessation of long-term ADT, approximately one-fifth of patients still had castrate T levels. When determining the therapeutic effect, especially biochemical control, we should consider this delay in T recovery.
Core tip: We evaluated the time course of testosterone recovery and the prognostic factors associated with prolonged testosterone recovery after the cessation of long-term (≥ 36 mo) androgen deprivation therapy in patients treated with brachytherapy. Five years after cessation, 22.6% of patients maintained a castrate testosterone level. We should consider this delay when determining therapeutic effects. Lower testosterone levels at cessation were significantly associated with prolonged testosterone recovery.
- Citation: Tsumura H, Satoh T, Ishiyama H, Hirano S, Tabata KI, Kurosaka S, Matsumoto K, Fujita T, Kitano M, Baba S, Hayakawa K, Iwamura M. Recovery of serum testosterone following neoadjuvant and adjuvant androgen deprivation therapy in men treated with prostate brachytherapy. World J Radiol 2015; 7(12): 494-500
- URL: https://www.wjgnet.com/1949-8470/full/v7/i12/494.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i12.494
Gonadotropin-releasing hormone (GnRH) agonists are widely used in various radiotherapies for the management of prostate cancer. The intended purpose and duration of hormonal therapy vary depending on the local extent of the cancer and the type of radiotherapy[1-3]. According to several randomized controlled studies, the use of 6 to 36 mo of hormonal therapy with external beam radiotherapy (EBRT) contributed to overall survival or cancer-specific survival in men with locally advanced or localized unfavorable-risk prostate cancer compared with radiotherapy alone[4-7]. After the cessation of androgen deprivation therapy (ADT), serum testosterone (T) levels usually recover from castrate levels to normal levels. However, some patients maintain the castrate T levels for several years after cessation, especially if hormonal manipulation is used for prolonged periods. In these cases, clinicians cannot assess whether radiotherapy controls prostate-specific antigen (PSA) levels because there is a possibility that prolonged effects of ADT simply control the disease. Thus, clinicians should assess the recovery of T levels after cessation of ADT when they interpret PSA relapse-free survival rates. Although some studies have documented the time course of recovery of T levels after cessation of long-term ADT, these studies were intended for patients who had received less than 36 mo of continuous GnRH agonist therapy or who were observed for shorter follow-up periods[8,9].
In this retrospective study, we estimated the time course of recovery of T levels after cessation of long-term use (≥ 36 mo) of ADT and short-term use (≤ 12 mo) of ADT in patients treated with prostate brachytherapy. In addition, the factors associated with T recovery were analyzed to determine which patients have the potential for prolonged time until recovery to supracastrate and/or normal T levels after cessation of ADT.
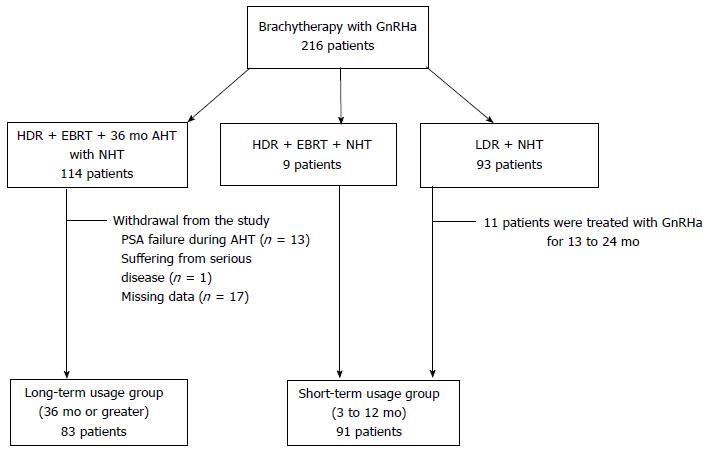
There were 216 candidates for this study who received either 192Ir high-dose rate (HDR) brachytherapy or 125I permanent low-dose rate (LDR) brachytherapy for prostate cancer with neoadjuvant hormonal therapy (NHT) or adjuvant hormonal therapy (AHT) using GnRH agonists between June 1999 and February 2009 at our institution. Patients were divided into two groups according to the duration of GnRH agonist therapy: short-term (neoadjuvant) usage group (duration 3 to 12 mo) and long-term (neoadjuvant and adjuvant) usage group (≥ 36 mo). A normal level of T and a castrate T level were defined as ≥ 207 ng/dL and ≤ 50 ng/dL, respectively. A T level of > 50 ng/dL was defined as a supracastrate level. Both supracastrate and normal levels were used for the definition of T recovery.
The T level of each patient was measured 1 mo before the cessation of GnRH agonist therapy (baseline levels) and until it recovered to a normal level. A follow-up examination after the cessation of ADT was scheduled every 3 mo for the first year, and then every 6 mo thereafter. Patients were removed from the study if PSA failure was observed during AHT because they needed to continue the administration of GnRH agonist therapy to maintain the castrate T level. All patients underwent a complete history and physical examination at the time of brachytherapy, including body mass index and the presence or absence of diabetes and hypertension. ADT consisted of GnRH agonist as a 1-mo or 3-mo formulation with or without an oral anti-androgen. Either flutamide (375 mg/d) or bicalutamide (80 mg) was used as the nonsteroidal anti-androgen agent. Either goserelin (3.6 or 10.8 mg) or leuprorelin (3.75 or 11.25 mg) was administrated as the GnRH agonist. Serum T levels were measured by immunoradiometric assay. Approval was granted by the ethics committee of our institution. Median follow-up times from cessation were 29 and 60 mo for the short-term and long-term groups, respectively.
Patients with low-risk or intermediate-risk prostate cancer were candidates for LDR brachytherapy. The prescribed dose to the periphery of the prostate was 145 Gy using a prostate implant technique that was described previously[10,11]. Patients who had large glands or who were at intermediate risk were treated with combined androgen blockade for 3 to 12 mo as NHT. Neither EBRT nor AHT was administered.
We previously mentioned about our protocol and procedure for HDR brachytherapy and hormonal therapy in high-risk prostate cancer[12,13]. Briefly, the mean dose to 90% of the planning target volume was 6.3 Gy/fraction of 192Ir HDR brachytherapy. After five fractions of HDR treatment, EBRT with 10 fractions of 3 Gy was administered. Patients received EBRT using a dynamic-arc conformal technique, administered with high-energy photons comprising 10-MV X-rays. The radiation field was limited to the prostate gland with or without proximal seminal vesicles with a 7-mm leaf margin using multileaf collimators. Testicular dose was not computed. All patients initially underwent 6 mo or more of neoadjuvant ADT. In patients who had high-risk cancer, adjuvant ADT was continued for 36 mo after EBRT. Low-risk or intermediate-risk patients were treated without adjuvant ADT D’Amico criteria were used for risk group stratification[14].
The Kaplan-Meier method was used to estimate the cumulative incidence of T recovery. A Log-Rank test was performed to compare these estimates. Multivariate Cox regression models were created based on the covariates that were significant in univariate analysis. Differences were regarded as statistically significant at P < 0.05. Analyses were performed using SPSS version 11.0 for Windows (SPSS, Inc., Chicago, IL, United States), GraphPad Prism, version 5 (GraphPad Software, Inc., CA, United States), and Microsoft Excel (Microsoft, Redmond, WA, United States).
Figure 1 provides the characteristics of the 216 patients who were candidates for the present study. Data for the 174 who were eligible for inclusion in the efficacy analysis were analyzed, and 42 patients (19.4%) were removed for reasons detailed in Figure 1: PSA failure during AHT (n = 13), severe disease (n = 1), missing data (n = 17), and ADT duration deviation from study protocol (n = 11). All patients reached castrate T levels at cessation of ADT. Table 1 shows the patient background data for the short-term and long-term usage groups (n = 91 and n = 83, respectively).
| Short-term usagegroup (n = 91) | Long-term usageGroup (n = 83) | |||
| Median | Range | Median | Range | |
| Age at cessation (yr) | 69 | 54-78 | 73 | 53-90 |
| Body mass index | 24.1 | 18.6-32.5 | 24.3 | 17.1-32.5 |
| Duration of GnRHa (mo) | 6 | 3-12 | 47 | 36-66 |
| Follow-up duration from cessation of GnRHa (mo) | 29 | 3-52 | 60 | 3-94 |
| n | % | n | % | |
| Diabetes, yes | 10 | 10.9 | 8 | 9.6 |
| Hypertension, yes | 36 | 39.5 | 33 | 39.7 |
We compared the cumulative incidence of T recovery to normal levels (Figure 2A) and to supracastrate levels (Figure 2B) between the short-term and long-term usage groups. A Log-Rank test showed that T recovery to normal levels occurred significantly more rapidly in the short-term than in the long-term usage group (HR = 9.180; 95%CI: 5.883-14.32; P < 0.001). T recovery to supracastrate levels also occurred significantly more rapidly in the short-term than in the long-term usage group (HR = 5.051; 95%CI: 3.346-7.624; P < 0.001).Cumulative incidences of T recovery to normal and supracastrate levels at 24 mo after cessation were 28.8% and 74.6%, respectively, in the long-term usage group, whereas these values were 96.4% and 98.8% in the short-term usage group. Five years after cessation, 22.6% of patients maintained a castrate T level in the long-term usage group.
Table 2 provides the univariate and multivariate results of factors that may influence T recovery to supracastrate levels in the long-term usage group. Age 73 years or older at cessation (n = 47; 57%) was significantly associated with slower recovery to supracastrate levels in the long-term usage group (multivariate analysis, P = 0.009). T level < 10 ng/dL at baseline (n = 39; 47%) was also significantly associated with slower recovery to supracastrate levels in this group (multivariate analysis, P = 0.002). Both age 73 years or older at cessation and T level < 10 ng/dL at baseline were also significantly associated with slower recovery to normal levels in the long-term usage group on multivariate analysis (P = 0.005 and P = 0.001, respectively). There were no significant factors associated with slower T recovery in the short-term usage group (data not shown).
| Factor | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| At brachytherapy | ||||||
| Body mass index | ||||||
| < 25 | 1.249 | 0.733-2.130 | 0.413 | - | - | - |
| ≥ 25 | 1.000 | (reference) | - | - | - | |
| Diabetes | ||||||
| No | 1.262 | 0.505-3.156 | 0.687 | - | - | - |
| Yes | 1.000 | (reference) | - | - | - | |
| Hypertension | ||||||
| No | 0.959 | 0.572-1.608 | 0.873 | - | - | - |
| Yes | 1.000 | (reference) | - | - | - | |
| At cessation | ||||||
| Age | ||||||
| < 73 yr | 1.98 | 1.182-3.317 | 0.009 | 2.020 | 1.190-3.429 | 0.009 |
| ≥ 73 yr | 1.000 | (reference) | 1.000 | (reference) | ||
| T level at baseline1 | ||||||
| < 10 ng/dL | 1.000 | (reference) | 1.000 | (reference) | ||
| ≥ 10 ng/dL | 2.261 | 1.316-3.883 | 0.003 | 2.327 | 1.354-4.000 | 0.002 |
| Drug formulation | ||||||
| Duration of activity | ||||||
| 1 mo | 1.000 | (reference) | - | - | - | |
| 3 mo | 1.419 | 0.821-2.454 | 0.209 | - | - | - |
| Material | ||||||
| Goserelin | 0.973 | 0.579-1.635 | 0.917 | - | - | - |
| Leuprorelin | 1.000 | (reference) | - | - | - | |
To examine the influence of different GnRH agonist agents on T recovery in the long-term usage group, patients were divided into two groups according to drug material: Goserelin (n = 34; 41%) and leuprorelin (n = 49; 59%). Patients were also divided into two groups according to the duration of drug activity: 1-mo formulation (n = 34; 41%) and 3-mo formulation (n = 35; 42%). Fourteen patients (17%) were switched from the 1-mo formulation to the 3-mo formulation for various reasons and were removed from this analysis. There was no significant difference regarding the time course of T recovery to supracastrate levels between goserelin and leuprorelin (univariate analysis, P = 0.917). The 1-mo formulation was not significantly associated with more rapid recovery to supracastrate levels, nor was the 3-mo formulation (univariate analysis, P = 0.209).
In the present study, we estimated the time course of recovery of T levels after cessation of long-term use (≥ 36 mo) of ADT in high-risk prostate cancer patients treated with brachytherapy. More than half the patients who received long-term ADT did not experience recovery to normal T levels at 5 years after cessation. In addition, approximately one-fifth of the patients who received long-term ADT still had castration levels at 5 years after cessation. In these cases, we have difficulty judging whether cure is attributable to radiotherapy, to sustained castration, or to both.
Several studies showed that the longer the ADT treatment, the more time that was required for T recovery[8,15-17]; some studies reported prolonged sustainment of castrate T levels after cessation of long-term ADT. Giberti et al[18] performed testicular biopsies in seven patients who received long-term ADT. This revealed impaired Leydig cell masses with tubular derangement and fibrosis. The findings suggested that long-term ADT induces not only functional inhibition of testicular androgenesis but also anatomical testicular damage that is likely irreversible. We previously investigated the changes in serum T and luteinzing hormone (LH) levels after withdrawal of long-term ADT in patents with intermittent endocrine therapy. Patients who maintained castrate T levels after long-term follow-up had above-normal LH levels[16]. This indicated that the feedback system of the hypothalamo-pituitary responded normally to the low levels of T after cessation. Thus, the prolonged sustainment of castrate T levels after cessation of long-term ADT may be attributable to the testicular damage, which is likely irreversible.
Shahidi et al[19] reported serum T levels were restored to normal levels in the majority of patients (88%) after short-term (3 to 6 mo) GnRH agonist administration and radiotherapy. Murthy et al[20] found that T was maintained at normal levels 5 years after the combination of a short course of GnRH agonist therapy (median, 97 d; range, 28-167 d) and EBRT. Our findings also suggest that the suppression of T levels after short-term ADT is reversible, because the majority of men who underwent prostate brachytherapy had T that recovered to normal levels. Although prolonged sustainment of castrate T levels after cessation of ADT is of little concern for the short-term usage group (≤ 12 mo), this sustainment occurred in approximately 20% of patients in the long-term usage group (≥ 36 mo) in the present study. Yoon et al[9] reported that approximately 10% of patients maintained castrate T levels after cessation of long-term use (2 years) of ADT. The rates of prolonged sustainment have a tendency to increase with the duration of the use of ADT. The longer the ADT treatment, the more patients were unlikely to recover from castrate T levels. The use of more than 2 years of ADT is likely to increase the incidence of this prolonged sustainment of castrate T levels.
The prolonged sustainment of castrate T levels not only could affect the biochemical control rates in patients treated with prostate radiotherapy but also could maintain the adverse long-term effects in patients. This could put some men at risk for cardiovascular events, diabetes, and osteoporotic fracture[21-23]. Fracture rates increased with increasing cumulative GnRH dose. The osteoporotic fracture caused by long-term ADT could affect the prognosis in prostate cancer patients, and the mortality rate doubled for men experiencing a fracture after their diagnosis compared with that for men who did not experience a fracture[24]. Thus, the management of bone health and T recovery is important in those patients[21,25].
In accordance with our findings, previous studies reported that older age was a significant factor associated with slower T recovery when GnRH agonist therapy was used for at least 24 mo[9,17,26]. The production of T decreases with age[27,28]. This decline might also be related to later T recovery in older men treated with long-term ADT[9].
The present study has certain shortcomings. Previous studies suggested the impact of scatter radiation on T levels and Leydig cell function in men treated with EBRT[29,30]. It is still unclear how HDR or LDR brachytherapy influences T levels. Thus, the cumulative incidence of T recovery might be incommensurable among men undergoing different kinds of radiotherapy. Unlike previous studies[9,17], we could not evaluate the impact of pre-ADT T levels on T recovery because some patients had already received ADT when they began treatment at our institution. In addition, we did not investigate how the prolonged sustainment of castrate T levels had an impact on patient quality of life. However, the present study is the first to find that a lower T level at cessation of ADT (≤ 10 ng/dL) is one significant factor that affected the slower T recovery to supracastrate levels in patients treated with long-term GnRH agonist therapy.
In men treated with long-term ADT, 22.6% of the patients maintained castrate T levels at 5 years after cessation. When determining the therapeutic effects, especially biochemical control, we should consider this delay in time to T recovery. Older age (73 years or older) and lower T levels (< 10 ng/dL) at ADT cessation were significantly associated with slower T recovery to supracastrate levels in men treated with long-term ADT.
Some patients maintain the castrate testosterone (T) levels for several years after cessation of androgen deprivation therapy (ADT), especially if hormonal manipulation is used for prolonged periods. In these cases, clinicians cannot assess whether radiotherapy controls prostate-specific antigen (PSA) levels because there is a possibility that prolonged effects of ADT simply control the disease. Thus, clinicians should assess the recovery of T levels after cessation of ADT when they interpret PSA relapse-free survival rates.
Some studies have documented the time course of recovery of T levels after cessation of long-term ADT. These studies were intended for patients who had received less than 36 mo of continuous gonadotropin-releasing hormone (GnRH) agonist therapy. The present study is the first to evaluate the time course of recovery of T levels after cessation of ≥ 36 mo use of ADT.
Previous studies reported that older age was a significant factor associated with slower T recovery when GnRH agonist therapy was used for at least 24 mo. The present study is the first to find that a lower T level at cessation of ADT (≤ 10 ng/dL) is one significant factor that affected the slower T recovery to supracastrate levels in patients treated with long-term GnRH agonist therapy.
Five years after cessation of long-term ADT (≥ 36 mo), approximately one-fifth of patients still had castrate T levels. When determining the therapeutic effect of radiotherapy, especially biochemical control, researchers should consider this delay in T recovery.
ADT: Prostate cancer usually requires androgen hormones such as T. GnRH agonists are widely used as ADT for the management of prostate cancer. GnRH agonists reduce the levels of serum T.
Very well written paper and the authors provided the important and clear message that GnRHa hormone therapy might cause long lasting androgen suppression.
P- Reviewer: Vinh-Hung V S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Monroe AT, Faricy PO, Jennings SB, Biggers RD, Gibbs GL, Peddada AV. High-dose-rate brachytherapy for large prostate volumes (& gt; or =50cc)-Uncompromised dosimetric coverage and acceptable toxicity. Brachytherapy. 2008;7:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Bittner N, Merrick GS, Butler WM, Galbreath RW, Lief J, Adamovich E, Wallner KE. Long-term outcome for very high-risk prostate cancer treated primarily with a triple modality approach to include permanent interstitial brachytherapy. Brachytherapy. 2012;11:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Zumsteg ZS, Spratt DE, Pei X, Yamada Y, Kalikstein A, Kuk D, Zhang Z, Zelefsky MJ. Short-term androgen-deprivation therapy improves prostate cancer-specific mortality in intermediate-risk prostate cancer patients undergoing dose-escalated external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:1012-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103-106. [PubMed] |
| 5. | Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, Venkatesan V, Lawton CA, Rosenthal SA, Sandler HM. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 515] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 6. | Denham JW, Steigler A, Lamb DS, Joseph D, Mameghan H, Turner S, Matthews J, Franklin I, Atkinson C, North J. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005;6:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 387] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Pickles T, Agranovich A, Berthelet E, Duncan GG, Keyes M, Kwan W, McKenzie MR, Morris WJ. Testosterone recovery following prolonged adjuvant androgen ablation for prostate carcinoma. Cancer. 2002;94:362-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Yoon FH, Gardner SL, Danjoux C, Morton G, Cheung P, Choo R. Testosterone recovery after prolonged androgen suppression in patients with prostate cancer. J Urol. 2008;180:1438-1443; discussion 1443-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Ishiyama H, Kitano M, Satoh T, Niibe Y, Uemae M, Fujita T, Baba S, Hayakawa K. Difference in rectal dosimetry between pre-plan and post-implant analysis in transperineal interstitial brachytherapy for prostate cancer. Radiother Oncol. 2006;78:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Sekiguchi A, Ishiyama H, Satoh T, Tabata K, Komori S, Tsumura H, Kawakami S, Soda I, Iwamura M, Hayakawa K. 125Iodine monotherapy for Japanese men with low- and intermediate-risk prostate cancer: outcomes after 5 years of follow-up. J Radiat Res. 2014;55:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Ishiyama H, Kitano M, Satoh T, Kotani S, Uemae M, Matsumoto K, Okusa H, Tabata K, Baba S, Hayakawa K. Genitourinary toxicity after high-dose-rate (HDR) brachytherapy combined with Hypofractionated External beam radiotherapy for localized prostate cancer: an analysis to determine the correlation between dose-volume histogram parameters in HDR brachytherapy and severity of toxicity. Int J Radiat Oncol Biol Phys. 2009;75:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Ishiyama H, Satoh T, Kitano M, Tabata K, Komori S, Ikeda M, Soda I, Kurosaka S, Sekiguchi A, Kimura M. High-dose-rate brachytherapy and hypofractionated external beam radiotherapy combined with long-term hormonal therapy for high-risk and very high-risk prostate cancer: outcomes after 5-year follow-up. J Radiat Res. 2014;55:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969-974. [PubMed] |
| 15. | Nejat RJ, Rashid HH, Bagiella E, Katz AE, Benson MC. A prospective analysis of time to normalization of serum testosterone after withdrawal of androgen deprivation therapy. J Urol. 2000;164:1891-1894. [PubMed] |
| 16. | Egawa S, Okusa H, Matsumoto K, Suyama K, Baba S. Changes in prostate-specific antigen and hormone levels following withdrawal of prolonged androgen ablation for prostate cancer. Prostate Cancer Prostatic Dis. 2003;6:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Kaku H, Saika T, Tsushima T, Ebara S, Senoh T, Yamato T, Nasu Y, Kumon H. Time course of serum testosterone and luteinizing hormone levels after cessation of long-term luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Prostate. 2006;66:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Giberti C, Barreca T, Martorana G, Truini M, Franceschini R, Rolandi E, Giuliani L. Hormonal pattern and testicular histology in patients with prostatic cancer after long-term treatment with a gonadotropin-releasing hormone agonist analogue. Eur Urol. 1988;15:125-127. [PubMed] |
| 19. | Shahidi M, Norman AR, Gadd J, Huddart RA, Horwich A, Dearnaley DP. Recovery of serum testosterone, LH and FSH levels following neoadjuvant hormone cytoreduction and radical radiotherapy in localized prostate cancer. Clin Oncol (R Coll Radiol). 2001;13:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Murthy V, Norman AR, Barbachano Y, Parker CC, Dearnaley DP. Long-term effects of a short course of neoadjuvant luteinizing hormone-releasing hormone analogue and radical radiotherapy on the hormonal profile in patients with localized prostate cancer. BJU Int. 2007;99:1380-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Zhumkhawala AA, Gleason JM, Cheetham TC, Niu F, Loo RK, Dell RM, Jacobsen SJ, Chien GW. Osteoporosis management program decreases incidence of hip fracture in patients with prostate cancer receiving androgen deprivation therapy. Urology. 2013;81:1010-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448-4456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1079] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 23. | Keating NL, O’Malley A, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2012;104:1518-1523. [PubMed] |
| 24. | Beebe-Dimmer JL, Cetin K, Shahinian V, Morgenstern H, Yee C, Schwartz KL, Acquavella J. Timing of androgen deprivation therapy use and fracture risk among elderly men with prostate cancer in the United States. Pharmacoepidemiol Drug Saf. 2012;21:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Skolarus TA, Caram MV, Shahinian VB. Androgen-deprivation-associated bone disease. Curr Opin Urol. 2014;24:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Wilke DR, Parker C, Andonowski A, Tsuji D, Catton C, Gospodarowicz M, Warde P. Testosterone and erectile function recovery after radiotherapy and long-term androgen deprivation with luteinizing hormone-releasing hormone agonists. BJU Int. 2006;97:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Plymate SR, Tenover JS, Bremner WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. J Androl. 1989;10:366-371. [PubMed] |
| 28. | Morley JE, Kaiser FE, Perry HM, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46:410-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 518] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 29. | Izard MA. Leydig cell function and radiation: a review of the literature. Radiother Oncol. 1995;34:1-8. [PubMed] |
| 30. | Zagars GK, Pollack A. Serum testosterone levels after external beam radiation for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1997;39:85-89. [PubMed] |