Published online Nov 28, 2015. doi: 10.4329/wjr.v7.i11.394
Peer-review started: July 5, 2015
First decision: July 31, 2015
Revised: August 22, 2015
Accepted: September 29, 2015
Article in press: September 30, 2015
Published online: November 28, 2015
Processing time: 153 Days and 10.8 Hours
AIM: To define the magnetic resonance imaging (MRI) parameters differentiating urethral hypermobility (UH) and intrinsic sphincter deficiency (ISD) in women with stress urinary incontinence (SUI).
METHODS: The static and dynamic MR images of 21 patients with SUI were correlated to urodynamic (UD) findings and compared to those of 10 continent controls. For the assessment of the urethra and integrity of the urethral support structures, we applied the high-resolution endocavitary MRI, such as intraurethral MRI, endovaginal or endorectal MRI. For the functional imaging of the urethral support, we performed dynamic MRI with the pelvic phased array coil. We assessed the following MRI parameters in both the patient and the volunteer groups: (1) urethral angle; (2) bladder neck descent; (3) status of the periurethral ligaments, (4) vaginal shape; (5) urethral sphincter integrity, length and muscle thickness at mid urethra; (6) bladder neck funneling; (7) status of the puborectalis muscle; (8) pubo-vaginal distance. UDs parameters were assessed in the patient study group as follows: (1) urethral mobility angle on Q-tip test; (2) Valsalva leak point pressure (VLPP) measured at 250 cc bladder volume; and (3) maximum urethral closure pressure (MUCP). The UH type of SUI was defined with the Q-tip test angle over 30 degrees, and VLPP pressure over 60 cm H2O. The ISD incontinence was defined with MUCP pressure below 20 cm H2O, and VLPP pressure less or equal to 60 cm H2O. We considered the associations between the MRI and clinical data and UDs using a variety of statistical tools to include linear regression, multivariate logistic regression and receiver operating characteristic (ROC) analysis. All statistical analyses were performed using STATA version 9.0 (StataCorp LP, College Station, TX).
RESULTS: In the incontinent group, 52% have history of vaginal delivery trauma as compared to none in control group (P < 0.001). There was no difference between the continent volunteers and incontinent patients in body habitus as assessed by the body mass index. Pubovaginal distance and periurethral ligament disruption are significantly associated with incontinence; periurethral ligament symmetricity reduces the odds of incontinence by 87%. Bladder neck funneling and length of the suprapubic urethral sphincter are significantly associated with the type of incontinence on UDs; funneling reduced the odds of pure UH by almost 95%; increasing suprapubic urethral sphincter length at rest is highly associated with UH. Both MRI variables result in a predictive model for UDs diagnosis (area under the ROC = 0.944).
CONCLUSION: MRI may play an important role in assessing the contribution of hypermobility and sphincteric dysfunction to the SUI in women when considering treatment options.
Core tip: Magnetic resonance imaging (MRI) allows visualization of the female urethra and periurethral tissues relevant to stress urinary incontinence (SUI). The role of MRI in the specific diagnosis of SUI caused by urethral hypermobility (UH) and/or intrinsic sphincter deficiency (ISD) has not been documented. The purpose of this pilot study was to define the MRI parameters differentiating UH and ISD types of incontinence, and assess their ability to predict the type of SUI when urodynamic (UD) results are used as a reference standard. Bladder neck funneling and length of the suprapubic urethral sphincter on MRI were significantly associated with the type of incontinence on UDs.
- Citation: Macura KJ, Thompson RE, Bluemke DA, Genadry R. Magnetic resonance imaging in assessment of stress urinary incontinence in women: Parameters differentiating urethral hypermobility and intrinsic sphincter deficiency. World J Radiol 2015; 7(11): 394-404
- URL: https://www.wjgnet.com/1949-8470/full/v7/i11/394.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i11.394
Stress urinary incontinence (SUI) is the observation of involuntary urinary loss from the urethra synchronous with exertion, sneezing, or coughing. Urodynamic stress incontinence is noted during urodynamic testing and is defined as the involuntary leakage of urine during increases in abdominal pressure in the absence of a detrusor contraction. SUI is one of the most common conditions among women with a significant impact on the quality of life due to psychosocial and hygienic problems[1]. Two main etiologic factors have been implicated in the urethral dysfunction leading to SUI, urethral hypermobility (UH) and intrinsic sphincter deficiency (ISD)[2]. In UH, it is the weakness of pelvic floor support that results in a rotational descent of the vesical neck and urethra during increases in abdominal pressure with subsequent leakage. In ISD, there is malfunction of the urethral sphincter which leads to low urethral closure pressures[3].
The type of urinary incontinence determines choice of surgical treatment, to prevent UH by repositioning the urethra into the pelvis to equalize pressure transmission between the bladder and urethra, and for women with low urethral resistance in ISD to increase the urethral closure pressures. Studies investigating the effects of UH and ISD on the outcome of the commonly performed procedures, such as transobturator tape (TOT) used to treat UH reported that the lack of UH as a contributing factor to SUI may be a risk factor for TOT failure[4]. Also, Sand et al[5] studied women who failed retropubic suspension and found a higher failure rate in those with ISD. It has been shown that SUI caused by ISD is the most challenging to treat; with failure rates as high as 54%[6]. These failures were attributed to the correction of UH without concomitant increase of urethral closure pressures.
Traditionally, the diagnosis of SUI is made based on history, clinical exam and urodynamics (UDs) or videourodynamics. Urethral pressure profilometry [which allows to measure maximum urethral closure pressure (MUCP)] may be combined with videourodynamics. This indirect method has limitations, as only the physiologic effect of sphincteric dysfunction can be assessed, without the evaluation of any morphological defects leading to SUI.
With its excellent soft tissue contrast and multiplanar acquisition, magnetic resonance imaging (MRI) allows visualization of the female urethra and periurethral tissues relevant to SUI[7,8]. MRI findings related to SUI caused by UH and ISD in women have been described[9]. Previous studies of MRI in female patients with SUI were focused on the assessment of lesions of the urethral support mechanism[10], defects of the levator ani muscle[11,12], and paravaginal fascia[13], as well as on the kinematics of pelvic floor muscles function[14].
To date, however, the role of MRI in the specific diagnosis of SUI caused by UH and/or ISD has not been documented. Therefore, the purpose of this pilot study was to define the MRI parameters differentiating UH and ISD types of incontinence, and assess their ability to predict the type of SUI with UDs as a reference standard.
The study was approved by the Institutional Review Board. The study was compliant with the Health Insurance Portability and Accountability Act. Study-specific written consent was obtained from all subjects. Patients were recruited from the Urogynecology clinic for this single-institution study, in which prospectively collected data from consecutive women who met the enrollment criteria and participated in the study were analyzed in a retrospective fashion. A target accrual of 20 patients with SUI and 10 volunteers was established for this pilot study. A total of 31 women were recruited. The inclusion criteria were as follows: (1) SUI documented on UDs (for patients with SUI) or no clinical symptoms of SUI (for volunteers); (2) study-specific informed consent signed prior to study entry; (3) no contraindications to MRI. Subject exclusion criteria were as follows: (1) unable to give valid informed consent; (2) unable to undergo MR, e.g., patients with contra-indications to MR imaging; (3) latex allergy; (4) extraurethral incontinence and bladder abnormalities causing incontinence; (5) greater than grade II prolapse on clinical exam; (6) pregnancy and (7) metallic implant(s) that might compromise quality of MRI.
MRI was performed on 1.5 T magnet (Signa Excite, GE Medical Systems, Waukesha, WI). For the assessment of the urethra and integrity of the urethral support structures, we applied the high-resolution endocavitary MRI, such as intraurethral MRI, endovaginal or endorectal MRI. For the functional imaging of the urethral support, we performed dynamic MRI with the pelvic phased array coil.
Intraurethral MRI: The 14F endourethral coil (SurgiVision, Inc., Gaithersburg, MD) was inserted under sterile conditions into the urethra. Both patient and volunteer groups underwent intraurethral MRI. All patients tolerated the procedure well and there were no complications from the coil placement. Patients were not pre-medicated with antibiotics. The imaging protocol included ultra-high resolution T2-weighted sequences in the axial and coronal planes (TR/TE 4000-6000 ms/90-120 ms, slice/spacing 2.5-3 mm/0-1 mm, FOV 6-10 cm centered on the urethra, matrix 256 × 256 ZIP interpolated to 512, NEX 6-8).
Endovaginal or endorectal MRI: The MRInnervu coil (Medrad, Indianola, PA) was placed in the vagina or rectum, depending on the patient’s or volunteer’s preference. The imaging protocol included T2-weighted fast spin echo (FSE) acquisition in three-plane with the field of view 10-14 cm and anatomical coverage between the symphysis pubis and coccyx. Imaging parameters were as follows: TR/TE 4000-6000 ms/90-120 ms, slice/space 3 mm/0-1 mm, matrix 256 × 192, NEX 3-4. The axial images were acquired in the oblique plane, perpendicular to the urethral axis. The frequency direction was AP to avoid endorectal/vaginal coil motion artifact over the urethra.
Pelvic MRI: Larger field of view imaging (20-30 cm) with the pelvic phased array coil was performed with the following parameters for the T2-weighted FSE images in axial and sagittal planes: TR/TE 4000-6000 ms/90-120 ms, slice/space 4-6 mm/0-1 mm, matrix 512 × 512, NEX 1-2. The dynamic pelvic floor imaging was performed in sagittal plane during rest and maximal strain with single shot fast spin echo (SSFSE) sequence TR/TE 15000/70 ms, slice/space 6 mm/2 mm, matrix 512 × 256, NEX 0.5; each image was acquired in 2 s, 10 images per sequence. At least two dynamic sequences were performed at maximal strain. Patients were asked to empty their bladder prior to the MRI, at the time of the dynamic evaluation they had their bladder at least half-full.
We assessed the following parameters in both the patient and the volunteer groups: (1) urethral angle; (2) bladder neck descent; (3) status of the periurethral ligaments; (4) vaginal shape; (5) urethral sphincter integrity, length and muscle thickness at mid urethra; (6) bladder neck funneling; (7) status of the puborectalis muscle (PRM); and (8) pubo-vaginal distance (PVD).
The urethral angle was defined as an angle between the patient body axis and the axis of the urethra, assessed at rest and during maximal strain. UH was diagnosed if the angle changed over 30 degrees between the rest and strain (as per definition of hypermobility)[11]. The bladder neck descent was measured as a distance (cm) between its position at rest and strain in reference to the pubococcygeal line (PCL, a line drawn from the inferior margin of the pubic bone to the last coccygeal joint).
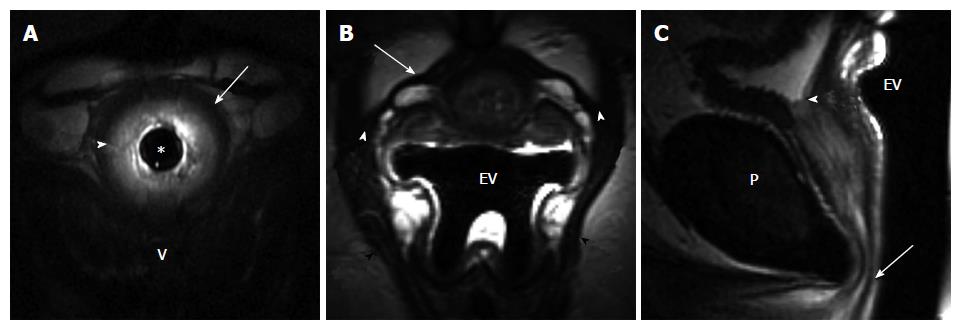
We assessed the integrity of the periurethral ligament that was seen in all patients. The periurethral ligament is the hypointense linear structure extending from PRM attachment on both sides of the pelvis and running in front of the urethra. The ligament status (intact/symmetric vs disrupted), as well as the site of disruption was evaluated. The ligament was judged as intact when the attachments were maintained and the ligament had a taut appearance and normal course. The ligament was deemed disrupted when there was a wavy/laxed appearance to the ligament, discontinuity of the ligament was present, or the attachment at PRM was lost.
The normal vaginal shape, assessed as an H-shaped contour on axial images, was deemed as a sign of the normal vaginolevator attachments. The loss of the H-shape vaginal morphology on axial images was interpreted as the presence of abnormal vaginolevator attachments (paravaginal defect) reflecting the loss of vaginal support. Laterality of the paravaginal defect was assessed. The PVD was measured as a distance between the posterior margin of the pubis and the anterior margin of the vagina at the mid urethra level (mid urethra defined at 50% of the sphincter length from the internal meatus).
The urethral sphincter length was measured at rest on the coronal and sagittal views, and a mean from both measurements was obtained as the total sphincter length. In addition to the total sphincter length, we assessed the functional length of the sphincter above the pelvic floor level (above the inferior pubic margin) and expressed it as a percentage of the total sphincter length. We evaluated the urethral sphincter muscle status for focal defects or signal changes within the sphincter. We measured the sphincter muscle thickness at the mid urethra level, for the anterior, lateral, and posterior urethral walls, separately for the striated and smooth muscle layers and for the total wall thickness.
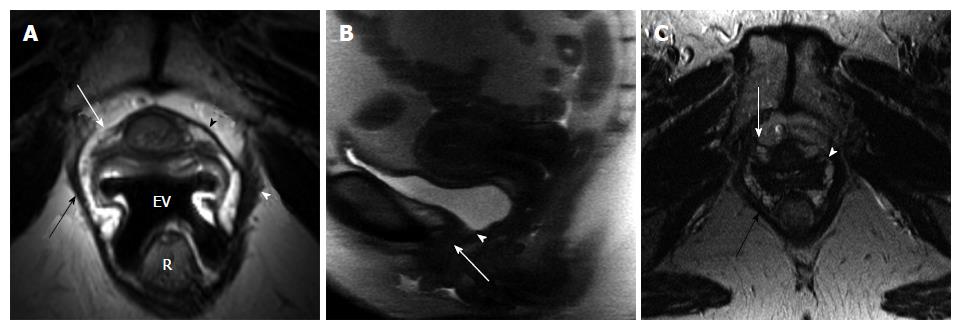
We assessed the presence or absence of the bladder neck funneling, defined as an opening of the internal meatus of the urethral sphincter at rest or during strain.
The status of the PRM was evaluated at the mid urethra level. The muscle thickness (measured at mid length of the muscle), its length, and an angle between the PRM and an obturator internus muscle were assessed.
The anonymized MRI scans were reviewed on a PACS workstation (eFilm Workstation, Merge Healthcare, Milwaukee, WI) by two investigators (KJM with 10 years of experience in GU MRI and RG with 20 years of experience in urogynecology) blinded to the subject’s status as a patient with SUI vs continent volunteer. The MRI parameters, as above, were assessed by consensus as to the presence of the finding, symmetricity and/or severity. The review was performed at least 1 year post-imaging date, to avoid a memory bias as to the pelvic anatomy of the subjects. Data were entered into the Excel (Microsoft, Bellevue, WA) spreadsheet for subsequent transfer to the STATA 9.0 (StataCorp LP, College Station, TX).
The urodynamics exam was performed on a UD-2000 MMS (Medical Measurement System) using Millar Micro-tip 8F catheter transducers following a standard protocol: The patient empties her bladder and a post-void residual is measured. With the patient in the sitting position at 45°, and after the sensors have been zeroed to atmospheric pressure before insertion, a dual sensor catheter is inserted in the urethra with the proximal sensor in the bladder and the distal sensor positioned at the area of maximal urethral closure pressure (MUCP). A single sensor catheter is also inserted intravaginally to indirectly record intra-abdominal pressure. The detrusor pressure is monitored continually as an automatically subtracted pressure. An infusion of sterile water is instilled at a rate of 60 mL/mn. Volume at first desire, strong desire and urge to urinate are recorded in standard fashion. After 250 mL have been instilled in the bladder a Valsalva leak point pressure (VLPP) is obtained. It is defined as the pressure increase leading to leakage in the absence of detrusor contraction. The subtracted nature of the pressure eliminates the effect of different pressures obtained with different types of catheters. Usually, the patient is asked to bear down maximally to determine the presence of incontinence and then incrementally to determine the lowest pressure leading to incontinence. In the absence of leakage with maximal Valsalva generated pressure, the patient was asked to cough maximally and incrementally for the same purpose. For this study group, a VLPP was obtained. The Q-tip test is obtained in the following manner. With the patient lying on the chair leveled with the horizontal, a sterile cotton-tipped swab is lubricated and placed in the urethra to the level of the urethro-vesical junction. With the patient at rest, the angle of the distal end of the swab is measured relative to the horizontal and recorded. The patient then is instructed to bear down to maximal Valsalva effort and the angle is measured again. The difference in the two angles is recorded as the Q-tip test angle.
Urodynamics parameters were assessed in the patient study group as follows: (1) urethral mobility angle on Q-tip test; (2) VLPP measured at 250 cc bladder volume; and (3) MUCP. The UH type of SUI was defined with the Q-tip test angle over 30 degrees, and VLPP pressure over 60 cm H2O. The ISD incontinence was defined with MUCP pressure below 20 cm H2O, and VLPP pressure less or equal to 60 cm H2O. Out of 21 incontinent patients, 18 had a complete UDs exam, 3 had incomplete exam that did not include the MUCP measurement. The volunteer group did not undergo the UDs exam as volunteer women had no clinical symptoms of urinary incontinence and therefore were not subjected to the invasive UDs workup.
As a primary analysis, we considered the statistical associations between the MRI and clinical data with the binary outcome of incontinence (present/absent). Continuous variables were assessed using the two sample t-test with a two-sided alpha of 0.05. We used the χ2 test and, when appropriate, the Fisher’s exact test, for categorical and dichotomous data. As a secondary analysis, we looked at only the subset of women with the diagnosis of SUI. These analyses included an assessment of the correlation between the UDs variables of MUCP and VLPP with the MRI data, as well as an investigation of the MRI variables that discriminated between women with a UDs diagnosis of SUI due to pure UH vs a diagnosis of SUI with an ISD component (pure ISD or mixed UH/ISD). In the former analyses, we used the Pearson’s correlation coefficient along with scatter plots and linear regression analyses. In the latter case, multivariate logistic regression models were created on the binary outcome of SUI with pure UH vs SUI with an ISD component using MRI and other clinical variables that at least trended toward statistical significance (e.g., P value < 0.1) in the univariate analyses. ROC analysis was then performed in order to assess the discriminative power of the MRI variables in the logistic regression models, and to obtain estimates of sensitivities, specificities, positive predictive values, and negative predictive values using the UDs diagnosis as the reference standard. All statistical analyses were performed using STATA version 9.0 (StataCorp LP, College Station, TX) by a biomedical statistician.
Thirty one women recruited for this study were considered in the analysis, 21 with SUI and 10 volunteer controls. The characteristics of participants according to continent vs incontinent status are listed in Table 1.
| Characteristics | Continent volunteersn = 10 | Incontinent patientsn = 21 | P value |
| 1Age (yr) | 45.1 (13.6) | 54.4 (11.8) | 0.0610 |
| 2Race | |||
| White | 5 (50) | 14 (67) | |
| Black | 5 (50) | 6 (29) | |
| Other | - | 1 (4) | |
| 1BMI index | 29.98 (7.0) | 29.95 (6.6) | 0.9915 |
| 1Parity, n births | 1.5 (1.2) | 1.9 (1.2) | 0.3329 |
| 2Vaginal, n | 11 (65) | 39 (90) | |
| 2C-section, n | 4 (24) | 2 (5) | |
| 2Nullipara, n | 2 (12) | 2 (5) | |
| 2OB Trauma, n | 0 (0) | 11 (52) | < 0.0014 |
| 2Hormones, n | 0 (0) | 5 (24) | |
| 2Hysterectomy, n | 2 (2) | 8 (38) | |
| 3Urethra wall thickness | |||
| Ant total, mm | 0.47 (0.43-0.50) | 0.59 (0.33-0.84) | 0.5161 |
| Ant striated muscle, mm | 0.17 (0.13-0.2) | 0.21 (0.12-0.29) | 0.5467 |
| Ant smooth muscle, mm | 0.30 (0.3-0.3) | 0.38 (0.20-0.55) | 0.5055 |
| Post total, mm | 0.40 (0.36-0.43) | 0.39 (0.36-0.42) | 0.7713 |
| Post striated muscle, mm | 0.11 (0.08-0.13) | 0.09 (0.08-0.11) | 0.3180 |
| Post smooth muscle, mm | 0.29 (0.26-0.31) | 0.29 (0.27-0.32) | 0.7952 |
| 3Urethral sphincter length | |||
| Total, cm | 3.67 (3.45-3.88) | 3.77 (3.47-4.06) | 0.6432 |
| Length, suprapubic, cm | 3.03 (2.59-3.46) | 2.95 (2.65-3.24) | 0.7361 |
| 3Urethra mobility angle | 53 (19.6-86.6) | 65 (50.1-80.2) | 0.4116 |
| 3Bladder neck descent | |||
| Total, cm | 1.98 (0.73-3.23) | 2.56 (1.96-3.15) | 0.3158 |
| Rest, above PCL, cm | 1.42 (0.43-2.41) | 1.51 (1.16-1.85) | 0.8195 |
| Strain, below PCL, cm | 0.56 (0.99-2.12) | 1.05 (0.46-1.63) | 0.4272 |
| 2Funneling, n | 1 (10) | 11 (52) | 0.184 |
| 3Retropubic distance to urethra, mm | 5.2 (4.19-6.20) | 5.0 (4.16-5.92) | 0.8259 |
| 3Pubo-vaginal distance | |||
| Right, cm | 1.81 (1.59-2.02) | 1.59 (1.47-1.70) | 0.0372 |
| Left, cm | 1.87 (1.72-2.01) | 1.67 (1.53-1.79) | 0.0475 |
| 2Normal vaginal shape, n | 7 (70) | 8 (40) | 0.128 |
| 3Puborectalis muscle thickness | |||
| Right, mm | 3.4 (2.6-4.1) | 3.8 (1.8-3.0) | 0.4903 |
| Left, mm | 3.9 (2.9-4.9) | 4.7 (3.7-5.5) | 0.2748 |
| 1Puborectalis muscle length | |||
| Right, cm | 1.81 (0.30) | 1.59 (0.24) | 0.037 |
| Left, cm | 1.87 (0.21) | 1.67 (0.28) | 0.048 |
| 3Puborectalis angle | |||
| Right, ° | 21 (17-24) | 25 (21-28) | 0.0781 |
| Left, ° | 23 (19-27) | 24 (19-28) | 0.7323 |
| 2Periurethral ligament status | |||
| Intact/symmetric, n | 7 (70) | 4 (20) | 0.0154 |
| Disrupted, n | 3 (30) | 16 (80) |
When differences in clinical variables by incontinent status were considered, a history of obstetrical (OB) trauma was the only variable found to be statistically significant. Eleven of the 21 women with incontinence (52%) had a history of trauma during child birth (episiotomy, forceps delivery, perineal laceration) as compared to none of the control volunteer women (P < 0.001, Fisher’s exact test). Incontinent women tended to be older with a mean (SD) age of 54.4 (11.8) years vs 45.1 (13.6) years for controls (P = 0.0610, t-test). There was no difference between the continent volunteers and incontinent patients in body habitus as assessed by the body mass index (BMI). Among the MRI variables, PVD and periurethral ligament disruption were found to be significantly associated with incontinence status (Table 1). Seven of the 10 control patients (70%) had intact, symmetric periurethral ligament, as opposed to only 4 of the 20 incontinent (20%) women (P = 0.015, Fisher’s exact test) (Figures 1 and 2). When these variables were considered in a multivariable logistic regression model, we found that only periurethral ligament status was statistically associated with incontinence. Having intact periurethral ligament reduced the odds of incontinence by 87% as compared to those who had periurethral disruption [Adjusted odds ratio 95%CI: 0.13 (0.018, 0.961), P = 0.046].
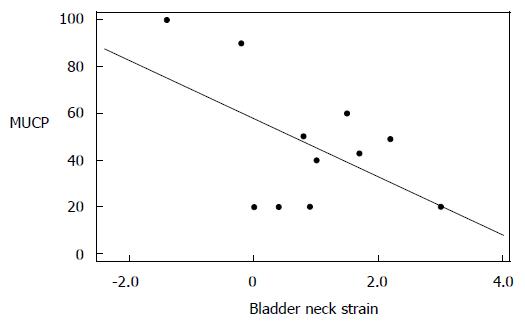
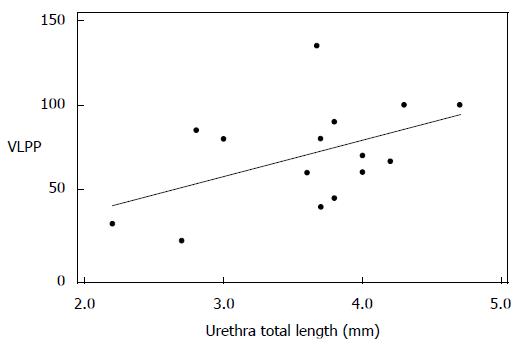
Among the subset of incontinent patients, we found an inverse correlation between bladder neck descent and MUCP that trended towards statistical significance (Pearson’s correlation = -0.537, P = 0.089). When we looked at VLPP, only total urethral sphincter length trended to significance, giving a Pearson’s correlation coefficient of 0.478 (P = 0.072) (Figures 3 and 4).
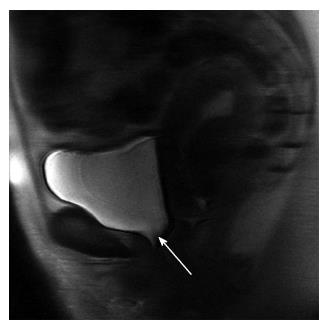
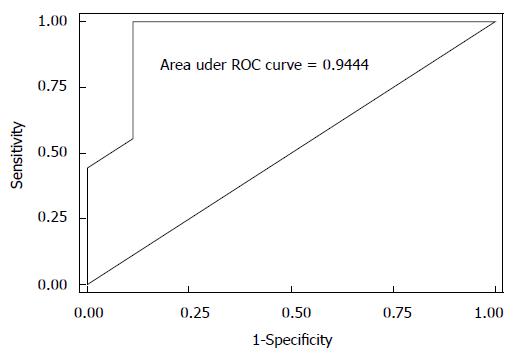
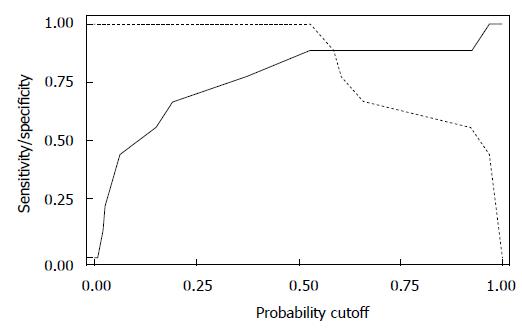
When we looked at the relationship of UDs diagnosis in patients who had complete UDs (SUI due to pure UH in 9 patients vs SUI with ISD component in 9 patients) and the MRI data, both bladder neck funneling (absent vs present) (Figure 5) and the functional suprapubic urethra sphincter length were found to be significantly associated with UDs diagnosis. In the univariate logistic model, being positive for funneling reduced the odds of pure UH diagnosis by almost 95% as compared to no funneling [OR (95%CI) = 0.036 (0.003, 0.484), P = 0.012]. In contrast, increasing the suprapubic urethral sphincter length was highly associated with an UDs diagnosis of UH [OR (95%CI) = 12.95 (1.17, 143.18), P = 0.037]. Both MRI variables considered in the multivariable logistic regression resulted in a highly predictive model for UDs diagnosis (area under the ROC = 0.944); Figure 6 shows the ROC curve for this model, and Table 2 gives the corresponding predictive statistics and 95%CIs. The prediction statistics for this model were quite good, giving a 100% sensitivity and 88.9% specificity (Figure 7). Probability of UH diagnosis, alternative propensity scores, and covariate and outcome values are listed in Table 3. The predicted probability of UH at 0.529 cutoff was associated with suprapubic urethral sphincter length above 3.0 cm.
| Predicted probability of urethral hypermobility | Score | FunnelingY = 1,n = 0 | Suprapubicurethral sphincterlength (cm) | UH = 1ISD/mixed = 0 |
| 0.008 | -4.77 | 1 | 1.8 | 0 |
| 0.020 | -3.84 | 1 | 2.1 | 0 |
| 0.027 | -3.53 | 1 | 2.2 | 0 |
| 0.027 | -3.53 | 1 | 2.2 | 0 |
| 0.065 | -2.6 | 1 | 2.5 | 0 |
| 0.149 | -1.67 | 1 | 2.8 | 0 |
| 0.193 | -1.36 | 1 | 2.9 | 0 |
| 0.377 | -0.43 | 1 | 3.2 | 0 |
| 0.529 | 0.19 | 1 | 3.4 | 1 |
| 0.587 | 0.38 | 0 | 2.3 | 1 |
| 0.605 | 0.5 | 1 | 3.5 | 1 |
| 0.659 | 0.69 | 0 | 2.4 | 1 |
| 0.925 | 2.55 | 0 | 3.0 | 1 |
| 0.925 | 2.55 | 0 | 3.0 | 0 |
| 0.969 | 3.48 | 0 | 3.3 | 1 |
| 0.969 | 3.48 | 0 | 3.3 | 1 |
| 0.969 | 3.48 | 0 | 3.3 | 1 |
| 0.996 | 5.65 | 0 | 4.0 | 1 |
MRI allows the visualization of the urethra and its supporting structures with great detail, especially with a multi-coil MRI technique[15,16], and also permits the evaluation of urethral mobility and bladder neck competence during strain and Valsalva. The importance of the integrity of the urethral attachments to the maintenance of continence is supported by our findings that the visualization of the periurethral ligament and assessment of its status leads to the prediction of incontinence. The majority of continent control patients (70%) in our study had intact, symmetric periurethral ligament, as opposed to only 20% of incontinent women. Having intact, symmetric periurethral ligament reduced the odds of incontinence by 87% as compared to those who had periurethral disruption. Similar results of distorted periurethral ligaments were found in 56% of the patients with SUI vs 13% of the women who were continent in the study by Kim et al[12], and also in a recent study by Tasali et al[10] where there was a significantly higher pubourethral ligament distortion and larger vesicourethral angle in women with SUI. In our study the PVD was also found to be significantly associated with incontinence status. Incontinent women had a shorter PVD than the control volunteers. Previous studies demonstrated that the volume of paravaginal fascia (connective tissue that contained venous plexus anterior to vagina) was reduced in patients with stress incontinence compared to reference continent subjects[13], therefore our finding of shortening of the distance between the anterior vaginal wall and pubic wall in incontinent women may indirectly relate to diminishing volume of paravaginal tissue noted by deSouza et al[13]. However, in the study by Tasali et al[10], authors documented lack of association between the dimension of the retropubic space and the SUI. Notably, in our study there was no difference in BMI between the incontinent patients and continent volunteers, therefore our results should not be influenced by the contribution of retropubic fat pad to the PVD in our study sample.
Contrary to findings by Kim et al[12], Tasali et al[10], and Morgan et al[17], but in agreement with results from the study by Pontbriand-Drolet et al[18], we did not find a statistically significant difference between the urethral sphincter striated muscle thickness in women with SUI vs that in the continent group. In our study, we performed precise measurements of the sphincter on images acquired with endourethral coil, both groups were comparable in body habitus and the mean age difference for incontinent patients and continent volunteers was less than a decade. It has been demonstrated that with aging, there is a decrease in the relative volume of urethral striated muscle and blood vessels[19].
Urethral mobility is tested clinically with the Q test[16]. Clinical Q test without visualization of urethral attachment defects may not be a reliable test, as continent women may also demonstrate hypermobility of the urethra. In our study groups there was an overlap between urethral mobility angles for continent women (mean 53 degrees) and incontinent women (mean 65 degrees) when assessed based on MRI. MRI is able to demonstrate not only the presence of hypermobility, but also other associated findings. UH is often accompanied by moderate to severe bladder descent with anterior bulging of the vagina. Bladder neck descent can be quantified and its competence/coaptation can be assessed on MRI along with hypermobility. We found an inverse correlation between bladder neck strain and MUCP that supports the hypothesis that, with increased inferior translation of the bladder neck due to loosening of the bladder neck attachment, there is decreasing urethral closure pressure caused by loss of coaptation of the sphincter as it descends. We also noted a correlation approaching significance between the increasing total urethral sphincter length and increasing VLPP; the longer the sphincter, the higher the leak point pressure.
On MRI, urethral and bladder descent are assessed in reference to the level of the pelvic floor which can be defined by the PCL[20-23]. Yang et al[21] demonstrated that the normal vertical distance from PCL to the bladder base at strain should be no more than 1 cm below the line. In our study group, bladder neck descended to mean distance 0.56 cm below PCL in continent women during strain and 1.1 cm below PCL for incontinent women (P = 0.43).
Other findings associated with UH that can be detected on MRI are distortion of urethral support ligaments, either partial or complete. Partial defects include laxity, fluttering or focal attenuation of ligaments. Complete disruption shows a discontinuity of ligamentous fibers[6,12]; mostly affected and reproducibly visualized on imaging is PEL and pubourethral ligaments[10]. Findings are frequently accompanied by the abnormal vaginal configuration (loss of normal H-shape vaginal contour, or dropping vaginal fornix), best seen on axial images, and widening of the para-vaginal attachments. In our study group, normal vaginal shape was maintained in 70% of continent volunteers and 40% of incontinent women.
The levator ani muscle signal and integrity can be well evaluated on MR images, on axial and coronal T2-weighted images. Levator ani should be symmetric without defects or fraying. Abnormal signal in the levator muscle, when compared to the obturator internus, and thinning can be observed in patients with stress incontinence and can be a result of fatty infiltration and atrophy as well as direct muscle injury[24]. The normal thickness of the PRM is 5-6 mm[25]. Interruption of the muscle fibers, lateral deviation of the muscle that is frequently associated with vaginal shape distortion on the affected side, can be observed. In our study group however, there was no difference between the thickness of PRM or the puborectalis angle between incontinent and continent women.
In patients with SUI, there are some imaging findings that are more likely to be associated with ISD, such as a short urethra, urethral muscle thinning, or bladder neck weakness demonstrated by funneling. Our results in incontinent women demonstrate that when the total urethral sphincter is shorter than 3.0 cm, or when the segment of urethral sphincter above the pelvic floor level is less than 3.0 cm, this decreased functional length of the urethra can lead to incontinence with urethral sphincter weakening associated with ISD on UDs. Also, funneling at the bladder neck, which is the opening of the urethrovesical junction at rest or during strain, can be seen on MRI in patients with SUI. An open bladder neck and proximal urethra was shown to indicate an ISD[26], however funneling can be also found in some postmenopausal continent women. In our study, funneling was seen in 52% of incontinent women and only in 1 (10%) continent volunteer. In a recent study by Pontbriand-Drolet et al[18] women with SUI symptoms were more likely to exhibit bladder neck funneling and a larger posterior urethrovesical angle at rest than both continent and mixed urinary incontinence women.
When we looked at the relationship of UDs diagnosis (SUI with pure UH vs SUI with ISD component) and the MRI data, both bladder neck funneling and the suprapubic urethral sphincter length were found to be predictive of UDs diagnosis; being positive for funneling on MRI reduced the odds of pure UH diagnosis on UDs by almost 95% as compared to no funneling. Our results are consistent with previous reports showing that funneling predicts ISD, as it results from the weakness of the proximal sphincter[26]. In contrast, increasing suprapubic urethral sphincter length was highly associated with a UDs diagnosis of pure UH, as the dysfunction of the sphincter results from the inferior translation of the urethra that is poorly supported rather than from shortening or intrinsic weakness of the sphincter itself. Both variables considered in the multivariable logistic regression analyses resulted in a highly predictive model for UDs diagnosis (area under the ROC = 0.944). The prediction statistics for this model was good, giving a 100% sensitivity and 88.9% specificity. However, due to a small size of the sample, the confidence intervals are large. A larger cohort study is needed to address these findings. Some patients may demonstrate imaging findings of both UH and ISD, as the ISD with bladder neck funneling may represent a secondary deficiency related to abnormal proximal urethral wall traction and shearing that can over time overcome the urethral coaptation such as in chronic hypermobility[27,28].
A limitation of our study is a relatively small sample size for incontinent group as only 18 incontinent women had a complete UDs exam to be included in the logistic regression model. Our study group patients were nearly-matched by age to within a decade. They were matched by BMI. However, there was a significant difference in the incidence of OB trauma, with 90% of incontinent patients reporting vaginal deliveries and 52% at least one incident of episiotomy, perineal laceration, or forceps delivery. No history of OB trauma was reported in the control group. This finding indirectly may relate to a known risk of pelvic floor injury during vaginal delivery as a cause of SUI. However, since the study sample was small, we were unable to control for individual types of injury to directly correlate the MRI findings with the severity of pelvic floor trauma. Another potential limitation of our study is imaging in the supine position, which is not a physiological position when patients experience SUI. However, we performed dynamic pelvic floor strain imaging to allow assessment of the changes in the urethra that take place during increases of intra-abdominal pressures. Our study group was imaged with pelvic MRI that included three different imaging components, with intraurethral imaging and dynamic pelvic floor imaging performed in all patients, and endovaginal or endorectal imaging being performed based on patients’ preference. This could have resulted in some measurements inconsistencies where endovaginal coil placement may have caused vaginal shape distortion.
In conclusion, our study demonstrated that there are specific morphological defects in women with SUI detectable on MRI that can be evaluated to differentiate incontinence related to the pure UH vs incontinence that has a component of ISD. Currently, MRI is usually considered in the diagnostic work-up of women who failed prior surgeries for incontinence or who have severe and complex pelvic organ prolapse. Further studies are needed to address the effects of aging, parity, pelvic floor injury, and hormonal status on SUI, in the context of specific anatomical defects related to SUI that can be observed and quantified on MR images, in order to evaluate the role of MRI in the assessment and treatment planning of women with SUI.
Anatomical defects related to stress urinary incontinence (SUI) can be observed and quantified with magnetic resonance imaging (MRI) and may contribute to diagnostic work-up and treatment planning of women with SUI.
To date the role of MRI in the specific diagnosis of SUI caused by urethral hypermobility (UH) and/or intrinsic sphincter deficiency (ISD) has not been documented.
The results from this pilot study suggest that two MRI parameters, the bladder neck funneling and length of the suprapubic urethral sphincter on MRI can predict the UH and ISD types of incontinence when urodynamics results are used as a reference standard.
MRI may play a critical role in assessing the contribution of hypermobility and sphincteric dysfunction to the stress urinary incontinence in women when considering treatment options. Further studies are warranted to assess the potential added value of MRI to management strategy selection.
The authors performed an interesting pilot study aimed at the identification of MRI parameters differentiating UH and intrinsic sphincteric deficiency in women with SUI.
P- Reviewer: Francesco C, Tsai HH S- Editor: Qiu S L- Editor: A E- Editor: Jiao XK
| 1. | Abrams P, Blaivas JG, Stanton SL, Andersen JT. The standardisation of terminology of lower urinary tract function. The International Continence Society Committee on Standardisation of Terminology. Scand J Urol Nephrol Suppl. 1988;114:5-19. [PubMed] |
| 2. | Blaivas JG, Romanzi LJ, Heritz DM. Urinary incontinence: pathophysiology, evaluation, treatment overview, and nonsurgical management. Campbell’s Urology. Philadelphia: WB Saunders Co 1997; 1007-1043. |
| 3. | McGuire EJ, Fitzpatrick CC, Wan J, Bloom D, Sanvordenker J, Ritchey M, Gormley EA. Clinical assessment of urethral sphincter function. J Urol. 1993;150:1452-1454. [PubMed] |
| 4. | Haliloglu B, Karateke A, Coksuer H, Peker H, Cam C. The role of urethral hypermobility and intrinsic sphincteric deficiency on the outcome of transobturator tape procedure: a prospective study with 2-year follow-up. Int Urogynecol J. 2010;21:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Sand PK, Bowen LW, Panganiban R, Ostergard DR. The low pressure urethra as a factor in failed retropubic urethropexy. Obstet Gynecol. 1987;69:399-402. [PubMed] |
| 6. | Shah SM, Gaunay GS. Treatment options for intrinsic sphincter deficiency. Nat Rev Urol. 2012;9:638-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Macura KJ, Genadry RR. Female urinary incontinence: pathophysiology, methods of evaluation and role of MR imaging. Abdom Imaging. 2008;33:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | el-Sayed RF, Morsy MM, el-Mashed SM, Abdel-Azim MS. Anatomy of the urethral supporting ligaments defined by dissection, histology, and MRI of female cadavers and MRI of healthy nulliparous women. AJR Am J Roentgenol. 2007;189:1145-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Macura KJ, Genadry RR, Bluemke DA. MR imaging of the female urethra and supporting ligaments in assessment of urinary incontinence: spectrum of abnormalities. Radiographics. 2006;26:1135-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Tasali N, Cubuk R, Sinanoğlu O, Sahin K, Saydam B. MRI in stress urinary incontinence: endovaginal MRI with an intracavitary coil and dynamic pelvic MRI. Urol J. 2012;9:397-404. [PubMed] |
| 11. | Stoker J, Rociu E, Bosch JL, Messelink EJ, van der Hulst VP, Groenendijk AG, Eijkemans MJ, Laméris JS. High-resolution endovaginal MR imaging in stress urinary incontinence. Eur Radiol. 2003;13:2031-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Kim JK, Kim YJ, Choo MS, Cho KS. The urethra and its supporting structures in women with stress urinary incontinence: MR imaging using an endovaginal coil. AJR Am J Roentgenol. 2003;180:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | deSouza NM, Daniels OJ, Williams AD, Gilderdale DJ, Abel PD. Female urinary genuine stress incontinence: anatomic considerations at MR imaging of the paravaginal fascia and urethra initial observations. Radiology. 2002;225:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Constantinou CE. Dynamics of female pelvic floor function using urodynamics, ultrasound and Magnetic Resonance Imaging (MRI). Eur J Obstet Gynecol Reprod Biol. 2009;144 Suppl 1:S159-S165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Bergman A, McCarthy TA, Ballard CA, Yanai J. Role of the Q-tip test in evaluating stress urinary incontinence. J Reprod Med. 1987;32:273-275. [PubMed] |
| 16. | Macura KJ, Genadry R, Borman TL, Mostwin JL, Lardo AC, Bluemke DA. Evaluation of the female urethra with intraurethral magnetic resonance imaging. J Magn Reson Imaging. 2004;20:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Morgan DM, Umek W, Guire K, Morgan HK, Garabrant A, DeLancey JO. Urethral sphincter morphology and function with and without stress incontinence. J Urol. 2009;182:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Pontbriand-Drolet S, Tang A, Madill SJ, Tannenbaum C, Lemieux MC, Corcos J, Dumoulin C. Differences in pelvic floor morphology between continent, stress urinary incontinent, and mixed urinary incontinent elderly women: An MRI study. Neurourol Urodyn. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Carlile A, Davies I, Rigby A, Brocklehurst JC. Age changes in the human female urethra: a morphometric study. J Urol. 1988;139:532-535. [PubMed] |
| 20. | Borghesi G, Simonetti R, Goldman SM, Szejnfeld J, Srougi M, Ortiz V, Bruschini H. Magnetic resonance imaging urodynamics. Technique development and preliminary results. Int Braz J Urol. 2006;32:336-341; discussion 341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Yang A, Mostwin JL, Rosenshein NB, Zerhouni EA. Pelvic floor descent in women: dynamic evaluation with fast MR imaging and cinematic display. Radiology. 1991;179:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 211] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Gufler H, DeGregorio G, Allmann KH, Kundt G, Dohnicht S. Comparison of cystourethrography and dynamic MRI in bladder neck descent. J Comput Assist Tomogr. 2000;24:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Goh V, Halligan S, Kaplan G, Healy JC, Bartram CI. Dynamic MR imaging of the pelvic floor in asymptomatic subjects. AJR Am J Roentgenol. 2000;174:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Tunn R, Paris S, Fischer W, Hamm B, Kuchinke J. Static magnetic resonance imaging of the pelvic floor muscle morphology in women with stress urinary incontinence and pelvic prolapse. Neurourol Urodyn. 1998;17:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Singh K, Reid WM, Berger LA. Magnetic resonance imaging of normal levator ani anatomy and function. Obstet Gynecol. 2002;99:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Mostwin JL. Urinary incontinence. J Urol. 1995;153:352-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Mostwin JL, Genadry R, Saunders R, Yang A. Stress incontinence observed with real time sonography and dynamic fastscan magnetic resonance imaging--insights into pathophysiology. Scand J Urol Nephrol Suppl. 2001;94-99; discussion 106-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Huang WC, Yang JM. Bladder neck funneling on ultrasound cystourethrography in primary stress urinary incontinence: a sign associated with urethral hypermobility and intrinsic sphincter deficiency. Urology. 2003;61:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |