Peer-review started: May 19, 2014
First decision: June 27, 2014
Revised: November 21, 2014
Accepted: December 3, 2014
Article in press: December 10, 2014
Published online: January 28, 2015
Processing time: 239 Days and 23.1 Hours
This article summarizes the clinical characteristics and imaging features of common gastrointestinal (GI) neoplasms in terms of conventional radiological imaging methods. Barium studies are readily available for displaying primary malignancies and are minimally or not at all invasive. A neoplasm may be manifested as various imaging findings, including mucosal disruption, soft mass, ulcer, submucosal invasion and lumen stenosis on barium studies. Benign tumors typically appear as smoothly marginated intramural masses. Malignant neoplasms most often appear as irregular infiltrative lesions on barium examination. Tumor extension to adjacent GI segments may be indistinct on barium images. Cross-sectional images such as computed tomography and magnetic resonance imaging may provide more accurate details of the adjacent organ invasion, omental or peritoneal spread.
Core tip: Gastrointestinal neoplasms are very common diseases. A neoplasm may be manifested as a wide spectrum of imaging findings. Barium studies are readily available for displaying primary malignancies in a short time and at low cost. Malignant neoplasms most often appear as irregular infiltrative lesions on barium examination. Cross-sectional imaging such as computed tomography or magnetic resonance imaging may provide more accurate details of the adjacent organ invasion, omental or peritoneal spread.
- Citation: Li YZ, Wu PH. Conventional radiological strategy of common gastrointestinal neoplasms. World J Radiol 2015; 7(1): 7-16
- URL: https://www.wjgnet.com/1949-8470/full/v7/i1/7.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i1.7
The techniques of gastrointestinal (GI) radiology have changed dramatically in the last three decades. The basic mission of modern GI radiology is earlier diagnosis, a better avenue to evidence-based treatment options, to predict tumor response to treatment and non-invasive follow-up[1].
Barium enema can provide valuable information. multislice computed tomography (CT) has proven to provide more valuable information for abdominal imaging. High-resolution magnetic resonance imaging (MRI) with its high soft tissue contrast has documented its clinical application in abdominal imaging. Positron emission tomography (PET) has excellent tissue penetration. However, apart from conventional imaging such as barium enema and multi-slice CT, the other options should increase their specificity and sensitivity using exogenous tracers or contrast agents.
The objective of this paper is to review the conventional radiological imaging of common GI neoplasms.
About 80% of esophageal neoplasms are malignant and more than 90% of these are squamous cell carcinomas (SCCs) or adenocarcinomas[2].
SCC is the most common malignant esophageal neoplasm worldwide. It arises from epithelial cells with stratified squamous differentiation, which develops from precursor lesions of intraepithelial neoplasia[3]. Alcohol and tobacco use are the most important risk factors for SCC of the esophagus. Most SCCs occur in the middle third of the esophagus, then the upper and lower third of the esophagus[4,5]. Pathologically, SCCs appear as a variety of gross morphological types, polypoid mass, flat or ulcerated lesions. About 65% of SCC patients are men and the peak age range is from 60 to 74 years[6]. Adenocarcinomas are the second common malignant tumor of the esophagus. The majority of esophageal adenocarcinomas develop from malignant degeneration of underlying Barrett epithelium, which are located in the distal esophagus, and the gastroesophageal junction, and these tumors have a tendency to invade the stomach[7]. About 85% of esophageal adenocarcinoma patients are men[2].
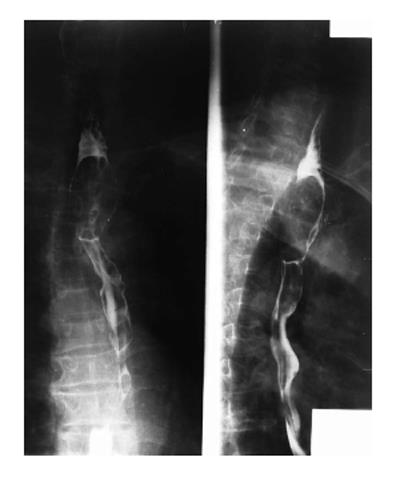
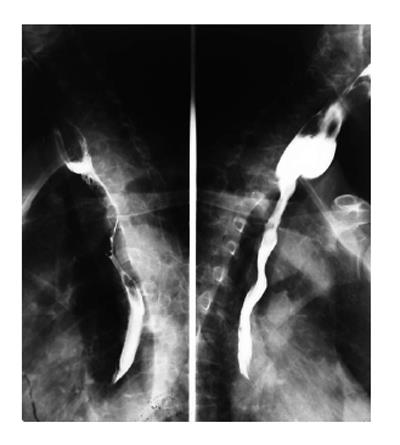
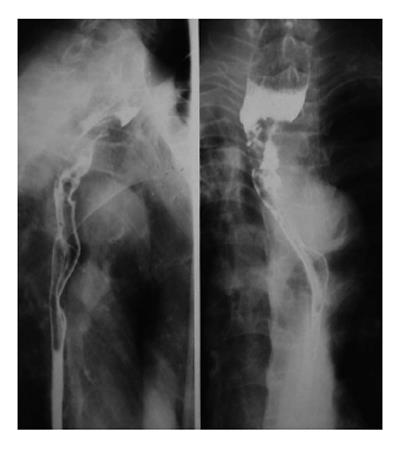
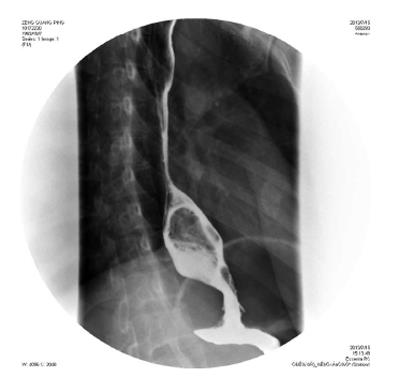
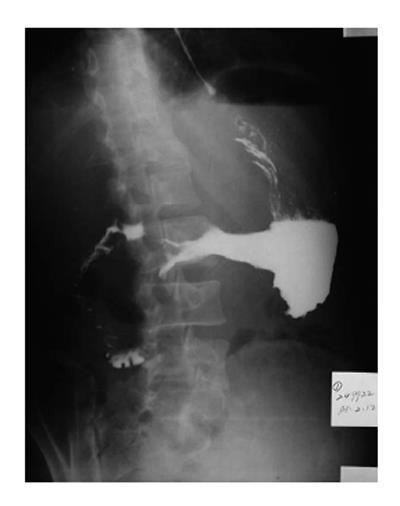
On barium esophagography, most SCCs show an infiltrative irregular luminal stricture, filling defect, with or without areas of ulceration (Figures 1-3).
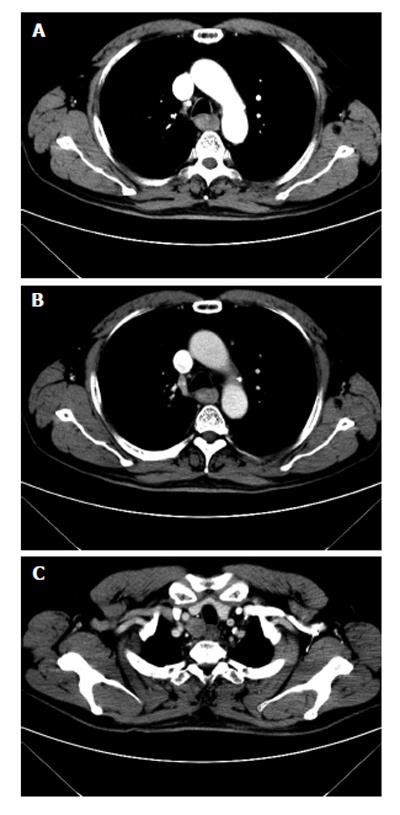
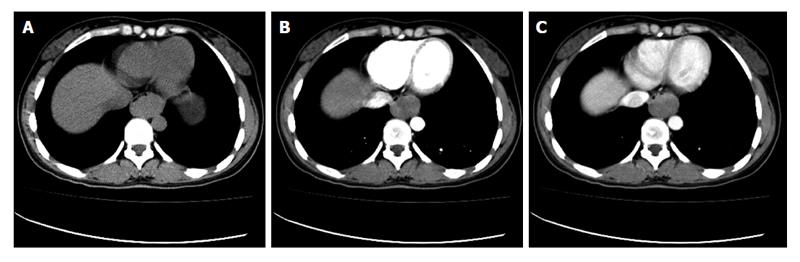
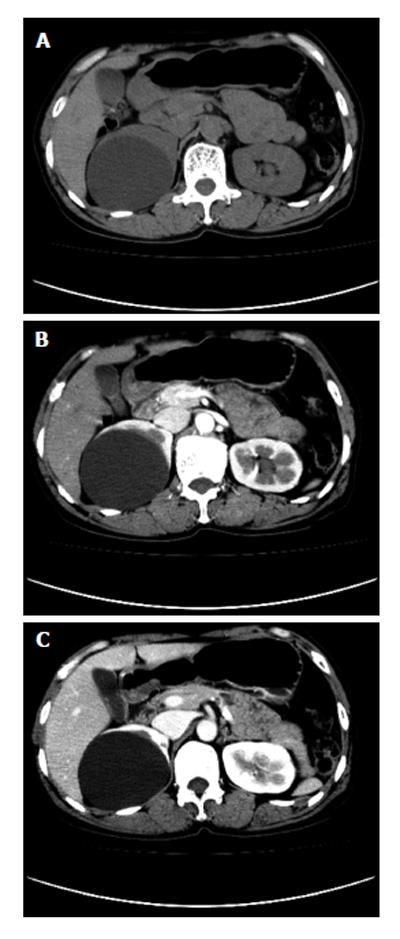
On CT, esophageal cancer shows a soft tissue mass or localized irregular esophageal wall thickening, with irregular luminal narrowing. The located wall thickening may be asymmetrical in the early stage and concentric thickening in the advanced stage. The lesions usually show moderate enhancement (Figure 4). CT plays very important roles in evaluating the primary tumor, mediastinal invasion, lymph node involvement, distant metastases, such as liver, lungs and bones, and the complications of esophageal obstruction.
On MRI, esophageal carcinoma reveals an irregular soft tissue mass with low T1 weighted signal intensity and intermediate T2 weighted signal intensity. MRI was reported to be comparable to CT in evaluating the tumor’s features, including local spread, distant metastases and lymph node involvement[8,9].
On 18F-fluorodeoxyglucose-PET, esophageal carcinoma and its metastases show avid uptake value. However, the regional lymph nodes may be obscured by the high uptake tissue of primary tumor[10].
Leiomyosarcoma is a relatively more common malignant tumor of esophagus than the others, apart from esophageal carcinoma. On esophagography, this tumor shows intramural mass with large exophytic parts, with or without calcification and tracking areas. On CT images, they reveal heterogeneous mass with exophytic edge[11]. On MRI, they appear as an intermediate signal intensity heterogeneous mass with exophytic edge on T1W images and slightly high signal intensity on T2W images.
The lesions usually show moderate arterial phase enhancement both on CT and MR images.
Benign tumors are usually small and without ulceration, peritumoral invasion or distant metastases and do not cause symptoms, so less than 1% of esophageal tumors gain clinical attention[12]. General imaging features of these tumors show a smooth intramural or intraluminal homogeneous mass, without necrosis or peritumoral spread.
Leiomyomas are the commonest benign esophageal neoplasm. They arise in the mature smooth muscle cells of the esophagus. Most patients with esophageal leiomyomas are asymptomatic, the rest may develop dysphagia or pain, depending on the lesion’s size and the amount of encroachment on the esophageal lumen. On barium examination, an intramural mass with a sharply defined, smooth or lobulated filling defect forms the typical findings[13] (Figure 5). On CT, esophageal leiomyomas show a sharply marginated homogeneous mass in the mid to lower third esophagus. These tumors show isoattenuating of slight hypoattenuating to muscle at nonenhanced CT and moderate enhancement, occasionally with coarse calcification (Figure 6). On MRI, slightly hyperintense at T2 weighted, slightly hypointense at T1 weighted images, and with moderate homogeneous enhancement without necrosis are shown[14].
Most primary gastric cancers are adenocarcinoma. Adenocarcinoma represents over 95% of malignant tumors of the stomach[15]. Common risk factors relating to the development of gastric adenocarcinoma include Helicobacter pylori infection[16], chronic gastritis, pernicious anemia and adenomatous polyps[17]. Gastric primary adenocarcinoma most often occurs in the gastric antrum, followed by the lesser curvature and cardia of the stomach.
Early gastric cancer: Radiologically, early gastric cancer is identified as a superficial lesion that is confined to the gastric mucosa and has not spread to the muscularis propria. It may feature as polypoid, superficial or depressed lesions[18]. The folds entering the center of the lesion are often irregular, nodular or club-shaped, or it may be manifested by a plaque-like elevation[19]. On CT scans, gastric cancer is shown as thickening of the gastric wall (Figure 7). The major benefits of CT scanning in patients with gastric cancer are preoperative staging, treatment planning, prognosis evaluation and recurrence detection.
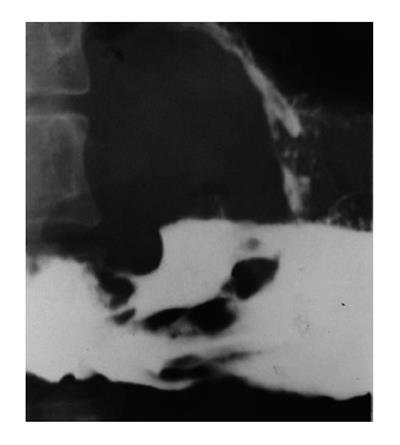
Advanced carcinoma: Advanced gastric carcinomas may be classified as polypoid, ulcerative or infiltrative lesions according to their gross morphological appearance. Imaging feature overlapping may occur in this classification[17]. Polypoid-type tumors usually appear as a large mass lesion. The surface of the lesion may be highly irregular. Large lesions may protrude into the lumen[20]. There may be a distinct angular demarcation or “shelf” at the tumor margins. When the lesion is located in the antrum, the tumor may obstruct the outlet of the stomach. Ulcerated-type carcinoma reveals an irregular ulcer crater. Radiating folds are irregular, converging at the edge of the ulcer crater[20] (Figure 8). Infiltrative lesions display a diffuse, predominantly submucosal infiltrative tumor, with irregular narrowing of the lumen and rigidity of the majority or the whole gastric wall due to the desmoplastic reaction, the so-called linitis plastica[21] (Figure 9).
In adults, the stomach is the most frequent organ of GI tract lymphomas. Non-Hodgkin’s lymphoma is more common than Hodgkin’s disease[22]. Much of this tumor spread is submucosal. The invasion of the gastric wall by the lymphoma is with relative flexibility. Findings of enlargement of the spleen and involvement of retrogastric and other regional lymph nodes are suggestive of lymphoma. On barium studies, gastric lymphoma reveals a nodular polypoid mass. The marked sign is thickened gastric folds[23,24]. Its findings may mimic other tumors, such as adenocarcinoma and leiomyosarcoma.
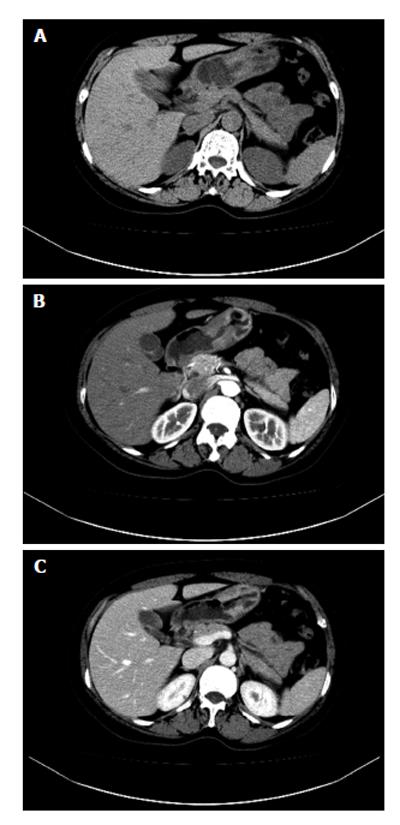
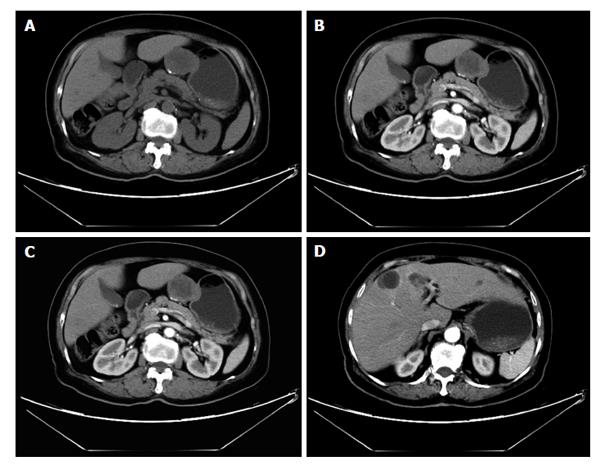
On CT, gastric lymphoma reveals a segmental or diffuse, smooth homogeneous, isoattenuated (minimal enhancement), wall thickening or mass (Figure 10). Lymphoma usually involves more than one site of the stomach. It seldom leads to gastric outlet obstruction, unlike gastric adenocarcinoma, because lymphoma is a “soft” tumor[25]. In contrast, gastric adenocarcinoma usually appears with more focal wall thickening, more enhancement, direct infiltration beyond the gastric wall, mural rigidity and luminal narrowing (linitis plastica), which may result in gastric outlet obstruction[26].
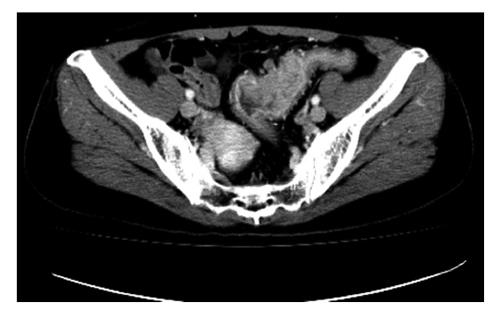
Gastrointestinal stromal tumors (GISTs) are the most frequent mesenchymal tumors of the GI tract which arise from the interstitial cells of Cajal. Previously, mesenchymal tumors of the GI tract were classified as leiomyosarcomas or leiomyomas[27,28]. However, more and more evidence has suggested that GISTs are a unique entity and so GISTs were separated from leiomyomas and leiomyosarcomas. GISTs are now defined as spindle cell, epithelioid and pleomorphic mesenchymal tumors of the GI tract. GISTs express the KIT protein (CD117, stem cell factor receptor) detected at immunohistochemistry[29-31]. This finding differentiates GISTs from leiomyomas, leiomyosarcomas, schwannomas and neurofibromas which do not express the KIT protein[32]. The prolonged survival of GIST patients and the progress in the recognition of GISTs have made imaging more and more important for diagnosis and monitoring the treatment outcome. CT is the preferred option of imaging modality for these purposes[33]. There are three important factors in determining the malignancy of GISTs: mitotic rate, tumor size and site[34].
On CT, the frequent imaging findings of GISTs are of a round, exophytic, well-circumscribed heterogeneously enhancing tumor appearing as a mass extrinsic to the wall of the stomach (Figure 11). Central fluid attenuation indicative of necrosis is common in larger lesions. Smaller lesions may be homogeneous in density. The liver is the most common site for metastasis[35]. CT may display spreading to the adjacent organ, ascites and omental or peritoneal infiltrate. Associated lymphadenopathy is more infrequent than gastric adenocarcinoma or lymphoma[28].
Primary neoplasms of the small intestine are rare. Primary tumors of the small intestine are less than 2% of primary GI tumors[36,37]. They may occur in association with genetic diseases and chronic intestinal inflammatory disorders. The frequent benign tumors of the small intestine are leiomyoma, lipoma, hamartoma and desmoid tumors. These tumors are usually asymptomatic. The frequent small intestinal primary malignancies are adenocarcinoma, leiomyosarcoma and lymphoma. These tumors may lead to intestinal obstruction, jaundice and bleeding. Extraintestinal tumors may lead to peritoneal metastasis[38].
Adenocarcinoma of the small intestine is frequent, approximately 30% to 50% of all malignant tumors in the small intestine[39,40]. Proximal duodenum and jejunum is the usual site of primary small intestinal adenocarcinoma, except in the setting of Crohn’s disease[38,41]. Predisposing risk factors include villous adenomas, adenomatous polyps, celiac disease and long-standing Crohn’s disease[42,43]. The most frequent clinical features of small intestinal adenocarcinomas are obstruction, overt or occult GI bleeding, weight loss and jaundice. Enteroclysis is a reliable sensitive diagnostic means for depicting intracavity small intestinal disease[44,45]. The presence of extracavity spread or metastases can be evaluated with CT scan; in some instances, magnetic resonance imaging, EUS or angiography may also be useful[38].
On barium examination, the general radiological appearances are filling defects, annular narrowing, polypoid and/or ulcerated masses, or a combination of these. Infiltrating adenocarcinomas are the most frequent type[46,47]. These are almost revealed as short, sharply demarcated, annular constricting lesions with mucosal disruption. Polypoid-type adenocarcinomas may be displayed as large, irregular, polypoid-filling defects. Ulceration is common in adenocarcinomas. Mixed radiological features such as infiltrating, polypoid and ulcerating lesions indicate an advanced lesion. CT is used for demonstrating the intestine, mesentery, lymph nodes and liver metastases in a single examination and for staging the tumor, as well as follow-up after the treatment.
Lymphoma is one of the most frequent tumors of the small intestine. Intestinal lymphomas are considered to be primary when the lesion is found in the small intestine and the clinical symptoms are related to intestinal invasion[48]. The majority of small intestine lymphomas are non-Hodgkin’s lymphomas (NHL)[49] coming from mucosa-associated lymphoid tissue. This tumors are mostly located in the ileum. The lymphoid follicles of the submucosa are the site of origin of lymphoma as these are more numerous in the ileum than in the jejunum[50].
On barium examination, the general radiological signs include luminal narrowing, cavitation, ulceration, valvulae conniventes thickening, discrete intraluminal filling defects and intramural masses[48]. The frequent finding of NHL is the lumen narrowing without intestinal obstruction. A discrete, broad-based mass are characteristic radiological features of lymphomas[48,51]. The valvulae conniventes thickening is a relatively less frequent finding of lymphoma[20]. Focal aneurysmal dilatation is highly suggestive of lymphoma[48].
CT and MRI can reveal the sites and extent of the tumor involvement, such as abdominal lymph nodes and solid viscera, and staging at a single examination. The lymphoma may be displayed as a homogeneous soft tissue mass with only a small intraluminal component. The tumors have only slight or moderate enhancement after the intravenous injection of contrast medium[50].
Leiomyosarcoma is the fourth most frequent primary small intestine tumor. It is equally distributed in the jejunum and ileum. Leiomyosarcomas are predominantly extraluminal and eccentric. Acute bleeding is the most common presenting symptom. Necrosis and hemorrhage are also frequent. Occasionally, less frequent signs may be seen: calcification, fistula formations and secondary infection[46,47].
The most common radiological feature is a large irregular exophytic mass with or without ulceration, cavitation or fistula formation[17]. There is marked enhancement of the solid component of the tumor following intravenous contrast medium[52]. Less frequently, leiomyosarcoma may be displayed as a large irregular cavity filled with barium[17].
Leiomyomas are the most common primary benign tumors in the small bowel. The jejunum, ileum and duodenum are the most frequent locations[53]. Leiomyomas are usually single, firm soft tissue masses with well-defined margins. It is reported that there are four different types: intraluminal, intramural, extraluminal and dumbbell shaped[54]. Microscopically, leiomyomas consist of bundles of well-differentiated smooth muscle with no evidence of mitosis. The absence of mitosis is a critical parameter in differentiating it from leiomyosarcoma[38]. Leiomyomas are shown as regular smooth intramural filling defects on barium studies or regular homogenous soft tissue mass on CT scans. There is moderate enhancement of the tumor following intravenous contrast medium.
Adenomas are the most frequent asymptomatic small intestinal benign tumors. Histologically, there are three types: villous, tubular and tubulovillous. Villous with atypia and/or large size increase the risk for malignancy[55]. Because of their potential for malignant transformation, these tumors should be resected or ablated endoscopically[38]. The general radiological findings show adenomas as round smooth intraluminal filling defects on barium examination or smooth well-defined regular soft tissue mass on CT scans and with moderate enhancement after intravenous contrast medium.
Colorectal carcinoma is an extremely common malignancy of the large bowel and in developed countries, it is one of the most frequent causes of death from cancer[56]. Other types of malignant tumors, such as primary lymphoma and leiomyosarcoma are relatively rare[17]. About 69% of carcinomas occur in the colon and only 31% of them are in the rectum and rectosigmoid junction[57,58]. Usually, colorectal carcinomas are not diagnosed until they are relatively advanced.
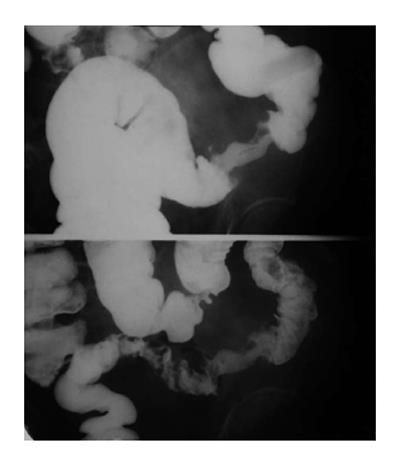
Barium enema examination has been proven safe and accurate for detecting colorectal tumors. Early carcinoma usually presents as a polypoid lesion. Polyps show as filling defects in the barium column or as soft tissue densities coated with barium within the air-filled lumen. Larger polypoid lesions generally reveal an irregular and/or ulcerated mucosal surface[17,59] (Figure 12). A flat ulcerating type of carcinoma is more frequent in the transverse and descending colon[60]. Infiltrating type of carcinoma is featured by thickened bowel wall due to infiltrated submucosal and muscular layers[61].
CT can be used for assessing the extension areas of the tumor, such as locoregional spreading and distant metastases[62]. On CT scanning, the polypoid type reveals a bulky mass with lobulated margins. An infiltrating type appears with an eccentric or concentric thickening wall. The density of the lesion increases markedly after injection of contrast medium[62] (Figure 13). CT may be used in assessing the extramural spread of tumor when extraintestinal infiltration is diagnosed in the presence of irregularity and dense stripes in the peri-intestinal adipose tissue. CT may also be used in assessment of the extension of any locoregional spread and distant metastases, such as spreading to the adjacent or distant organs (bladder, vagina and abdominal and pelvic muscles). The liver is the most commonly infiltrated organ by metastases from colorectal cancer and CT has more than 90% sensitivity in diagnosing lesions larger than 1 cm[63,64]. Complications of colorectal carcinoma include obstruction, perforation, pericolic abscess, ischemic colitis and intussusception.
Other malignances that invade the colon are rare. Primary lymphomas of the colon often involve the cecum and rectum and are non-Hodgkin in type. The tumors may appear as localized, large, extraluminal masses or constricting. Diffuse infiltration is the most frequent form of colonic lymphoma and is featured by nodules with intact mucosal surface and diffuse or segmental distribution[17,23,24]. The local complications of lymphoma include perforation, pneumatosis coli and intussusception.
A “polyp” is a raised mucosal lesion. This term does not imply any histological features. Metaplastic polyps are relatively more common in the rectum and less common in the colon. These have no malignant potential. Most adenomas are polyps. Adenomas are well-defined circumscribed areas of dysplastic epithelium. The overall pattern of the epithelium and stroma may be classified as tubular, villous or mixed. The significance of adenoma is its malignant potential. The imaging features created by a polyp depend on the angle at which it is viewed and its relationship to the barium pool. Several signs are described on the radiological features: (1) meniscus sign: en face view showing a clearly defined inner and outer margin of the meniscus; (2) increased density sign: on barium enema, polyps are intraluminal lesions coated with barium. The incident X-ray beam may pass through four layers of barium. These factors may increase the density. A localized area of “increased density” may also be drawn; and (3) filling defect: a polyp on the dependent wall creates a “shadow” (low density area) in the barium pool. This feature is helpful in differentiating that the lesion is intraluminal. If the polyp is on the non-dependent wall, it may be hidden by the dense barium pool. To confirm the relationship between the lesions and the intestinal wall, it is helpful to turn the patients from side to side with the table flat and view the lesions at different angles.
In general, the radiological features of a polyp may be noted as six “S’s”: its site, size, shape (regular/irregular), surface texture (smooth, lobulated, very nodular), symmetry of the base (smooth/irregular, indrawn) and singularly or otherwise[65]. Although polyposis syndrome is generally rare, it plays important roles in relation to cancer prevention, screening and genetic counseling.
Conventional radiographs can provide valuable information on detection, characterization, staging and prediction of treatment response of GI malignancies. However, barium imaging has some limited roles in staging GI tract tumors. Tumors spreading to adjacent GI segments and accompanying complications may not be disclosed by barium imaging. Cross-sectional imaging may provide more accurate details of the adjacent organ invasion, omental or peritoneal spread.
P- Reviewer: Chiu KW, Tsushima Y, Verma S S- Editor: Song XX L- Editor: Roemmele A E- Editor: Liu SQ
| 1. | Hoeffel C, Mulé S, Romaniuk B, Ladam-Marcus V, Bouché O, Marcus C. Advances in radiological imaging of gastrointestinal tumors. Crit Rev Oncol Hematol. 2009;69:153-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 2. | Lewis RB, Mehrotra AK, Rodriguez P, Levine MS. From the radiologic pathology archives: esophageal neoplasms: radiologic-pathologic correlation. Radiographics. 2013;33:1083-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 3. | Glickman JN. Section II: pathology and pathologic staging of esophageal cancer. Semin Thorac Cardiovasc Surg. 2003;15:167-179. [PubMed] |
| 4. | Levine MS, Laufer I, Yamada A. Tumors of the esophagus. In Laufer I, Levine MS (eds). Double contrast radiology of the gastrointestinal tract. 157-190. |
| 5. | Meyers MA. Carcinoma of the esophagus: Imaging, staging and management. In Meyers MA (ed). Neoplasms of the digestive tract: imaging, staging and management. Philadelphia: Lippincott-Raven 1998; 9-19. |
| 6. | Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998-2003. Int J Cancer. 2008;123:1422-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Bosch A, Frias Z, Caldwell WL. Adenocarcinoma of the esophagus. Cancer. 1979;43:1557-1561. [PubMed] |
| 8. | Riddell AM, Hillier J, Brown G, King DM, Wotherspoon AC, Thompson JN, Cunningham D, Allum WH. Potential of surface-coil MRI for staging of esophageal cancer. AJR Am J Roentgenol. 2006;187:1280-1287. [PubMed] |
| 9. | Takashima S, Takeuchi N, Shiozaki H, Kobayashi K, Morimoto S, Ikezoe J, Tomiyama N, Harada K, Shogen K, Kozuka T. Carcinoma of the esophagus: CT vs MR imaging in determining resectability. AJR Am J Roentgenol. 1991;156:297-302. [PubMed] |
| 10. | Bruzzi JF, Munden RF, Truong MT, Marom EM, Sabloff BS, Gladish GW, Iyer RB, Pan TS, Macapinlac HA, Erasmus JJ. PET/CT of esophageal cancer: its role in clinical management. Radiographics. 2007;27:1635-1652. [PubMed] |
| 11. | Levine MS, Buck JL, Pantongrag-Brown L, Buetow PC, Hallman JR, Sobin LH. Leiomyosarcoma of the esophagus: radiographic findings in 10 patients. AJR Am J Roentgenol. 1996;167:27-32. [PubMed] |
| 12. | Rice TW. Benign esophageal tumors: esophagoscopy and endoscopic esophageal ultrasound. Semin Thorac Cardiovasc Surg. 2003;15:20-26. [PubMed] |
| 13. | Levine MS. Benign tumors of the esophagus: radiologic evaluation. Semin Thorac Cardiovasc Surg. 2003;15:9-19. [PubMed] |
| 14. | Yang PS, Lee KS, Lee SJ, Kim TS, Choo IW, Shim YM, Kim K, Kim Y. Esophageal leiomyoma: radiologic findings in 12 patients. Korean J Radiol. 2001;2:132-137. [PubMed] |
| 15. | Fishman EK, Urban BA, Hruban RH. CT of the stomach: spectrum of disease. Radiographics. 1996;16:1035-1054. [PubMed] |
| 16. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [PubMed] |
| 17. | Gourtsoyiannis N, Grammatikakis J, Prassopoulos P. Role of conventional radiology in the diagnosis and staging of gastrointestinal tract neoplasms. Semin Surg Oncol. 2001;20:91-108. [PubMed] |
| 18. | Farley DR, Donohue JH. Early gastric cancer. Surg Clin North Am. 1992;72:401-421. [PubMed] |
| 19. | White RM, Levine MS, Enterline HT, Laufer I. Early gastric cancer. Recent experience. Radiology. 1985;155:25-27. [PubMed] |
| 20. | Levine MS, Megibow AJ. Carcinoma. In Gore RM, Levine MS, Laufer I (eds). Textbook of gastrointestinal radiology. Philadelphia: WB Saunders 1994; 660-683. |
| 21. | Levine MS, Kong V, Rubesin SE, Laufer I, Herlinger H. Scirrhous carcinoma of the stomach: radiologic and endoscopic diagnosis. Radiology. 1990;175:151-154. [PubMed] |
| 22. | Papadimitriou CS, Papacharalampous NX, Kittas C. Primary gastrointestinal malignant lymphomas. A morphologic and immunohistochemical study. Cancer. 1985;55:870-879. [PubMed] |
| 23. | Dodd GD. Lymphoma of the hollow abdominal viscera. Radiol Clin North Am. 1990;28:771-783. [PubMed] |
| 24. | Zornoza J, Dodd GD. Lymphoma of the gastrointestinal tract. Semin Roentgenol. 1980;15:272-287. [PubMed] |
| 25. | Horton KM, Fishman EK. Current role of CT in imaging of the stomach. Radiographics. 2003;23:75-87. [PubMed] |
| 26. | Gollub MJ. Imaging of gastrointestinal lymphoma. Radiol Clin North Am. 2008;46:287-312, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Clark RA, Alexander ES. Computed tomography of gastrointestinal leiomyosarcoma. Gastrointest Radiol. 1982;7:127-129. [PubMed] |
| 28. | Pannu HK, Hruban RH, Fishman EK. CT of gastric leiomyosarcoma: patterns of involvement. AJR Am J Roentgenol. 1999;173:369-373. [PubMed] |
| 29. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [PubMed] |
| 30. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] |
| 31. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [PubMed] |
| 32. | Hersh MR, Choi J, Garrett C, Clark R. Imaging gastrointestinal stromal tumors. Cancer Control. 2005;12:111-115. [PubMed] |
| 33. | Bano S, Puri SK, Upreti L, Chaudhary V, Sant HK, Gondal R. Gastrointestinal stromal tumors (GISTs): an imaging perspective. Jpn J Radiol. 2012;30:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478-483. [PubMed] |
| 35. | Burkill GJ, Badran M, Al-Muderis O, Meirion Thomas J, Judson IR, Fisher C, Moskovic EC. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology. 2003;226:527-532. [PubMed] |
| 36. | Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6-29. [PubMed] |
| 37. | Barclay TH, Schapira DV. Malignant tumors of the small intestine. Cancer. 1983;51:878-881. [PubMed] |
| 38. | Gill SS, Heuman DM, Mihas AA. Small intestinal neoplasms. J Clin Gastroenterol. 2001;33:267-282. [PubMed] |
| 39. | Brücher BL, Roder JD, Fink U, Stein HJ, Busch R, Siewert JR. Prognostic factors in resected primary small bowel tumors. Dig Surg. 1998;15:42-51. [PubMed] |
| 40. | Ojha A, Zacherl J, Scheuba C, Jakesz R, Wenzl E. Primary small bowel malignancies: single-center results of three decades. J Clin Gastroenterol. 2000;30:289-293. [PubMed] |
| 41. | Miller TL, Skucas J, Gudex D, Listinsky C. Bowel cancer characteristics in patients with regional enteritis. Gastrointest Radiol. 1987;12:45-52. [PubMed] |
| 42. | Swinson CM, Slavin G, Coles EC, Booth CC. Coeliac disease and malignancy. Lancet. 1983;1:111-115. [PubMed] |
| 43. | Collier PE, Turowski P, Diamond DL. Small intestinal adenocarcinoma complicating regional enteritis. Cancer. 1985;55:516-521. [PubMed] |
| 44. | Maglinte DD, Kelvin FM, O’Connor K, Lappas JC, Chernish SM. Current status of small bowel radiography. Abdom Imaging. 1996;21:247-257. [PubMed] |
| 45. | Bessette JR, Maglinte DD, Kelvin FM, Chernish SM. Primary malignant tumors in the small bowel: a comparison of the small-bowel enema and conventional follow-through examination. AJR Am J Roentgenol. 1989;153:741-744. [PubMed] |
| 46. | Gourtsoyiannis NC, Nolan DJ. Primary malignant neoplasms: “Imaging of small intestinal tumours”. Amsterdam: Elsevier 1997; 105-189. |
| 47. | Gourtsoyiannis N, Makó E. Imaging of primary small intestinal tumours by enteroclysis and CT with pathological correlation. Eur Radiol. 1997;7:625-642. [PubMed] |
| 48. | Gourtsoyiannis NC, Nolan DJ. Lymphoma of the small intestine: radiological appearances. Clin Radiol. 1988;39:639-645. [PubMed] |
| 49. | Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer. 1978;42:693-707. [PubMed] |
| 50. | Grainger RG, Allison DJ, Baert A. Diagnostic radiology: A text book of medical imaging: Third edition, Volume two. 995-996. |
| 51. | Sartoris DJ, Harell GS, Anderson MF, Zboralske FF. Small-bowel lymphoma and regional enteritis: radiographic similarities. Radiology. 1984;152:291-296. [PubMed] |
| 52. | Grainger RG, Allison DJ, Baert A. Diagnostic radiology: A text book of medical imaging: 3rd ed. Volume two. . |
| 53. | Blanchard DK, Budde JM, Hatch GF, Wertheimer-Hatch L, Hatch KF, Davis GB, Foster RS, Skandalakis JE. Tumors of the small intestine. World J Surg. 2000;24:421-429. [PubMed] |
| 54. | Starr GF, Dockerty MB. Leiomyomas and leiomyosarcomas of the small intestine. Cancer. 1955;8:101-111. [PubMed] |
| 55. | Bjork KJ, Davis CJ, Nagorney DM, Mucha P. Duodenal villous tumors. Arch Surg. 1990;125:961-965. [PubMed] |
| 56. | Padhani AR. Advances in imaging of colorectal cancer. Crit Rev Oncol Hematol. 1999;30:189-199. [PubMed] |
| 57. | Coates RJ, Greenberg RS, Liu MT, Correa P, Harlan LC, Reynolds P, Fenoglio-Preiser CM, Haynes MA, Hankey BF, Hunter CP. Anatomic site distribution of colon cancer by race and other colon cancer risk factors. Dis Colon Rectum. 1995;38:42-50. [PubMed] |
| 58. | Devesa SS, Chow WH. Variation in colorectal cancer incidence in the United States by subsite of origin. Cancer. 1993;71:3819-3826. [PubMed] |
| 59. | Laufer I. Double contrast enema: technical aspects. In Laufer I, Levine MS (eds). Double contrast gastrointestinal radiology. Philadelphia: WB Saunders 1992; 423-445. |
| 60. | Boland CR. Malignant tumors of the colon. In Yamada T (ed). Textbook of gastroenterology. Philadelphia: Lippincott-Raven 1995; 1967-2026. |
| 61. | Kelvin FM. Diagnosis of colorectal cancer by conventional radiology. In Meyers MA (ed). Neoplasms of the digestive tract: imaging, staging and management. Philadelphia: Lippincott-Raven 1998; 219-235. |
| 62. | Angelelli G, Ianora AA, Scardapane A, Pedote P, Memeo M, Rotondo A. Role of computerized tomography in the staging of gastrointestinal neoplasms. Semin Surg Oncol. 2001;20:109-121. [PubMed] |
| 63. | Leen E, Angerson WJ, Wotherspoon H, Moule B, Cook TG, McArdle CS. Detection of colorectal liver metastases: comparison of laparotomy, CT, US, and Doppler perfusion index and evaluation of postoperative follow-up results. Radiology. 1995;195:113-116. [PubMed] |
| 64. | Horton KM, Abrams RA, Fishman EK. Spiral CT of colon cancer: imaging features and role in management. Radiographics. 2000;20:419-430. [PubMed] |
| 65. | Grainger RG, Allison DJ, Baert A. Diagnostic radiology: A text book of medical imaging: 3rd ed. Volume two. 1016-1021. |