Published online Sep 28, 2014. doi: 10.4329/wjr.v6.i9.716
Revised: July 13, 2014
Accepted: July 27, 2014
Published online: September 28, 2014
Processing time: 259 Days and 11.3 Hours
Recognition and characterization of central nervous system infections poses a formidable challenge to the neuro-radiologist. Imaging plays a vital role, the lesions typically being relatively inaccessible to tisue sampling. The results of an accurate diagnosis are endlessly rewarding, given the availability of excellent pharmacological regimen. The availability of numerous magnetic resonance (MR) sequences which provide functional and molecular information is a powerful tool in the hands of the radiologist. However, the plethora of sequences and the possibilities on each sequence is also intimidating, and often confusing as well as time consuming. While a large number of reviews have already described in detail the possible imaging findings in each infection, we intend to classify infections based on their imaging characteristics. In this review we describe an algorithm for first classifying the imaging findings into patterns based on basic MR sequences (T1, T2 and enhancement pattern with Gadolinium), and then sub-classify them based on more advanced molecular and functional sequences (Diffusion, Perfusion, Susceptibility imaging, MR Spectroscopy). This patterned approach is intended as a guide to radiologists in-training and in-practice for quickly narrowing their list of differentials when faced with a clinical challenge. The entire content of the article has also been summarised in the form of flow-charts for the purpose of quick reference.
Core tip: The plethora of magnetic resonance sequences available with the radiologist today provides a wealth of information about anatomical, pathological, physiological, functional and molecular aspects of the brain. While this provides an opportunity to transform patient management, the vast number of possibilities can be bewildering, particularly for the radiologist in-training. It is often easy to get lost in the details while forgetting the larger picture. In this article we first classify the infections into broad imaging patterns, and subsequently sub-classify them based on more advanced sequences (molecular and functional imaging). The flow-charts in the article are intended as a source of quick reference to the radiologist when faced with a clinical challenge.
- Citation: Rangarajan K, Das CJ, Kumar A, Gupta AK. MRI in central nervous system infections: A simplified patterned approach. World J Radiol 2014; 6(9): 716-725
- URL: https://www.wjgnet.com/1949-8470/full/v6/i9/716.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i9.716
Central nervous system (CNS) infections are a significant cause of mortality and morbidity world-wide. This is particularly true owing to its association with conditions of immunological compromise and the increasing incidence of human immunodeficiency virus (HIV) infection is further adding to the problem[1]. Today with the availability of excellent antimicrobials, many of these disorders are potentially treatable, making early recognition imperative. Like in other disorders of the CNS, non-invasive imaging based diagnosis is the key as possibility of a tissue diagnosis by means of fine needle aspiration cytology (FNAC) or biopsy is difficult. Early diagnosis will also help to minimize long term complications related to the disease and its treatment.
The primary imaging modality, like in most CNS disorders is magnetic resonance imaging (MRI)[2]. Coming to an exact etiological agent on the basis of conventional MRI sequences with Gadolinium enhancement is always difficult due to overlapping imaging characteristics. With the possibility of molecular and functional imaging with newer MRI techniques however, the radiologist today is better equipped to handle this dilemma. Though the use of such multiple MRI sequences adds lots of information to narrow the differential possibilities, this vast information is difficult to recall when faced with a clinical problem.
The purpose of this review is to provide a rational MRI approach to narrow the list of differentials, to quickly classify and characterize CNS infections. The flow-charts presented in this review guides the radiologist to first recognize the pattern of findings on routine MRI sequences and subsequently narrow the differential diagnosis based on the addition of other MR parameters such as diffusion weighted imaging (DWI) and MR spectroscopy (MRS).
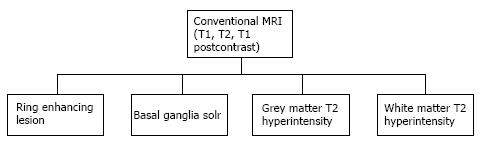
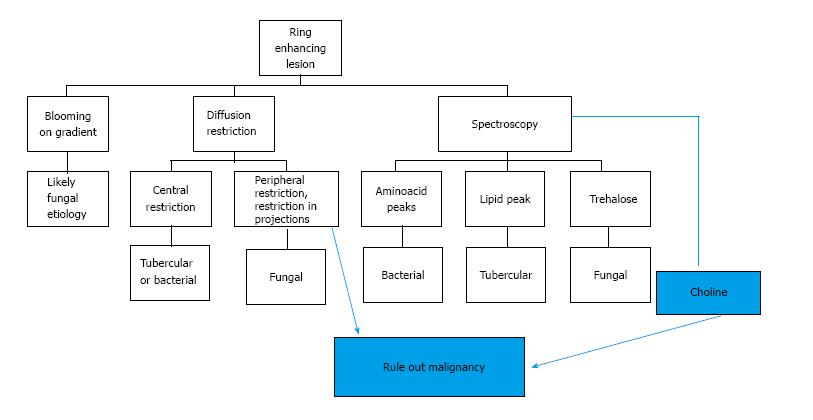
Most infections in the CNS may be classified in one of the following categories based on their T1, T2 and contrast enhancement characteristics (Figure 1) as follows (an image demonstrating a typical lesion in each category has been provided in Figures 2-5): Ring enhancing lesions (Figure 2), Basal ganglia space occupying lesions (Figure 3), Grey matter hyperintensities (Figure 4), White matter hyperintensities (Figure 5).
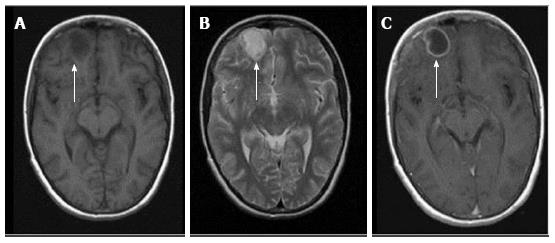
Peripheral ring-like enhancement is a common finding in CNS imaging. Ring enhancing lesions on conventional MRI sequences have a long list of differentials ranging from infectious processes to high grade necrotic neoplasm. Glioblastoma multiforme represents the most important condition. Abscesses are usually associated with a thin smooth rim, in contrast to the nodular irregular rim seen in Glioblastoma multiforme. Satellite lesions are commonly seen in abscesses, unlike necrotic neoplasm[1]. Recent work by some investigators has suggested a role for susceptibility weighted imaging (SWI) in this differentiation. They found that a smooth, complete rim of susceptibility is seen in abscesses in contrast to incomplete irregular rims seen in necrotic neoplasm[3].
All mature abscesses whether bacterial, fungal or pyogenic are hypointense on T1, hyperintense on T2 and show ring enhancement following intravenous Gadolinium injection. Further differentiation of abscesses for possible etiological cause may be made as follows.
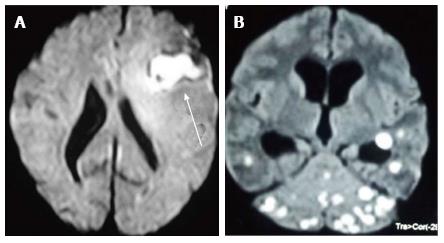
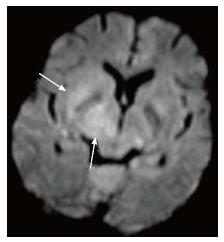
DWI explores the molecular characteristic of diffusivity of particles within a region. It is based on the application of two gradients at a set interval of time, in such a way that only a molecule that experiences both gradients at the same position (does not exhibit motion between the two gradients) produces signal. Therefore regions of the brain that show “restricted diffusion” are hyperintense on DWI. This restricted diffusion appears as hyperintense area on DWI and needs to be corroborated with computer generated apparent diffusion coefficient (ADC) maps which show corresponding hypointense area. This corroboration rules out T2-shine through effect. Bacterial as well as tubercular abscesses show central diffusion restriction[4,5] due to highly viscous necrotic tissue within (Figure 6). Fungal abscesses show intracavitary projections. The wall of abscess and the projections may demonstrate diffusion restriction[5,6], though no restriction is seen in the abscess core (Figure 7).
DWI plays an important role in the differentiation of these abscesses from necrotic neoplasms, which usually demonstrate high ADC values within the core[7].
Spectroscopy provides information about metabolic alterations within a voxel by exploiting changes in the microenvironment produced by unique chemical characteristics of specific metabolites. Thus using this technique it is possible to infer the presence of a microorganism, based on the expected products of the microorganisms metabolism reflected in the metabolic signature.
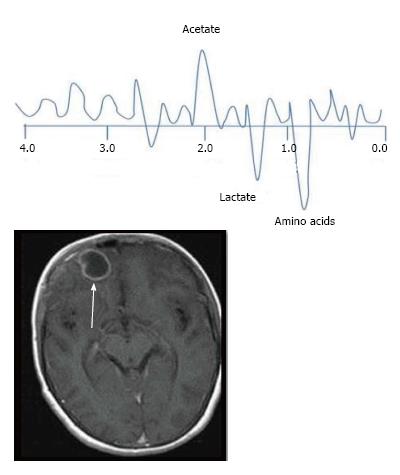
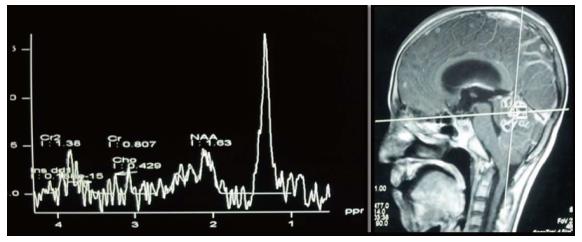
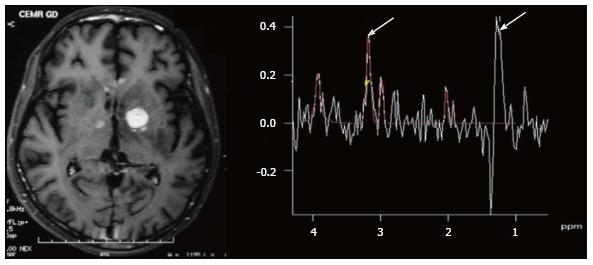
The characteristic of the spectrum in bacterial abscesses is the presence of amino acid peak at 0.9 parts per million (ppm) [inverted peak at an time of echo (TE) 136 ms] representing valine, leucine and isoleucine (Figure 8)[2]. The detection of succinate (2.4 ppm) and acetate (1.92 ppm) is proposed to indicate anaerobic organisms[2].
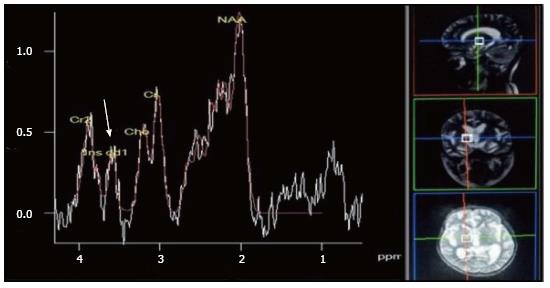
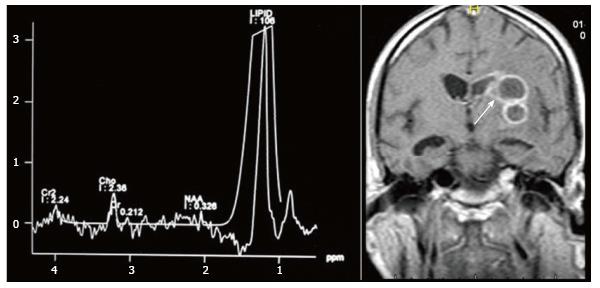
Tuberculosis is characterised by lipid peaks at 0.9, 1.3, 2.0 and 2.8 ppm. The presence of lipids in the absence of other amino acids, lactate and succinate is strongly suggestive of tubercular abscess (Figure 9)[5,8]. 0.9, 1.3, 2.0, 2.8, and 3.7 peaks correspond to specific chemical groups within metabolites found in these infections- 0.9 corresponds to a terminal methyl group, 1.3 to a methylene group, 2.0 and 2.8 to specific groups in fatty acyl chains and 3.7 to phosphoserine.
While lactate, acetate and succinate can all be seen in fungal abscesses, the presence of multiple signals between 3.6 and 3.8 ppm (representing Trehalose) has been seen in some forms of fungal abscesses (Figure 10)[5]. The absence of choline peak on MRS provides important supportive evidence for an infective etiology as compared to neoplasms which show increased choline[7,9].
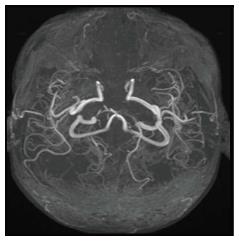
Tubercular vasculitis results in extensive infarction due to inflammation of vessels coursing through the basal exudates (Figure 11)[10]. Vascular involvement with formation of aneurysms is seen in fungal infections. These aneurysms are seen as irregular dilatation of vessel wall on MRA.
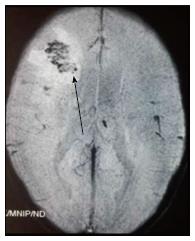
The principle underlying SWI is the alteration of local magnetic field by substances that show paramagnetic properties. This is a gradient sequence, so molecules in the vicinity of a paramagnetic substance dephase rapidly, thus do not contribute to signal production. Voxels containing molecules with different magnetic susceptibilities are imaged when they are exactly out-of-phase, such that the whole voxel appears to be of low signal intensity[2]. This is seen as an area of “blooming”. The presence of haemorrhage is often a clue to underlying fungal cause of infection (Figure 12) due to its angioinvasive nature [11].
The presence of a complete, smooth hypointense rim on SWI favours a diagnosis of an abscess against a necrotic neoplasm. A brief summary of approach to a peripherally enhancing lesion is presented as a flowchart (Figure 13).
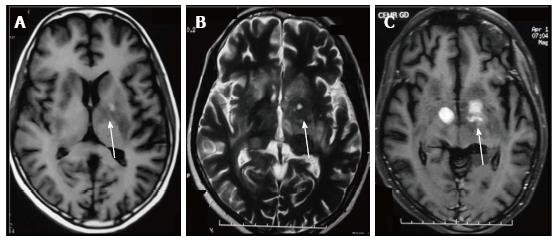
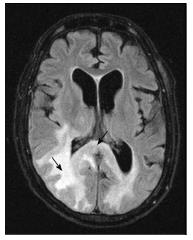
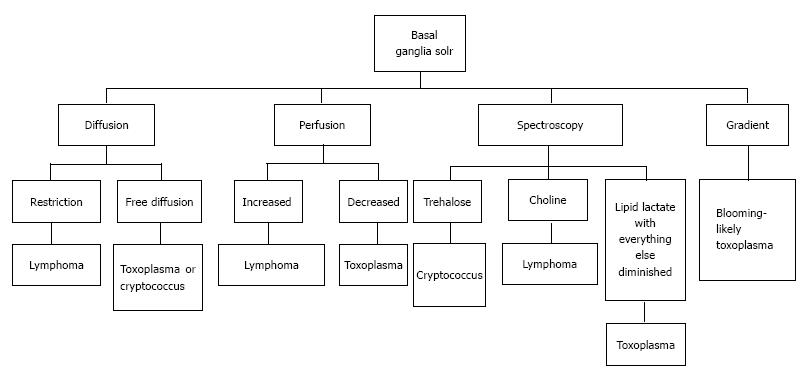
A number of CNS lesions are seen characteristically involving the region of basal ganglia. The differential diagnosis in this category includes cryptococcosis, toxoplasmosis and primary CNS lymphoma. Cryptococcosis usually does not show enhancement after gadolinium injection.Further characterisation often requires additional sequences.
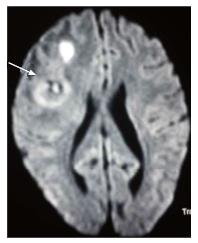
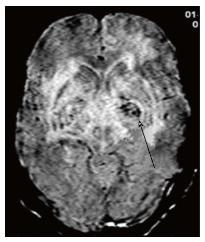
Toxoplasmosis does not usually show significant restriction of diffusion, though a wide range of ADC value have been encountered[2,12]. A proposed explanation is the lack of viscous contents within these lesions. Peripheral areas may show hyperintensity due to the presence of haemorrhage[13]. Lymphoma which usually are highly cellular, shows restricted diffusion[2,12], helping differentiation from toxoplasmosis (Figure 14). The paucity of intercellular spaces results in a decreased diffusivity of water molecules within these lesions. Cryptococcosis is also known to show restricted diffusion within the pseudocysts[2] owing to the viscosity of gelatinous material. Cryptococcomas have been shown to exhibit peripheral diffusion restriction akin to a necrotic brain tumour[14], though often they may not show any restricted diffusion[12] (Figure 15).
Presence of peak between 3.6 to 3.8 ppm (trehalose) has been observed in cryptococcosis (which is a fungus)[5] (Figure 10). Toxoplasma lesions (Figure 16) show markedly elevated lipid and lactate with diminished levels of all other metabolites[2]. Lymphoma (Figure 17) shows mild to moderate increase in lipid and lactate with markedly elevated choline peak[2].
Toxoplasmosis shows normal or decreased cerebral blood volume (CBV). Primary CNS lymphoma on the other hand shows elevated CBV[12].
Presence of hemorrhage (blooming on gradient echo sequences) points towards toxoplasmosis, as lymphoma rarely show hemorrhage before treatment[12,15] (Figure 18). A brief summary of the approach to space occupying lesions in the basal ganglia is presented as a flowchart in Figure 19.
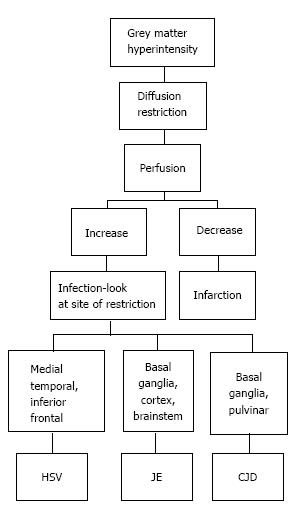
T2/ FLAIR hyperintensity involving the grey matter may be seen in encephalitis as well as infarction. This differentiation is aided by diffusion and perfusion sequences (Table 1).
| Diffusion | Perfusion | |
| Infection | Restricted | Increased |
| Infarction | Restricted | Decreased |
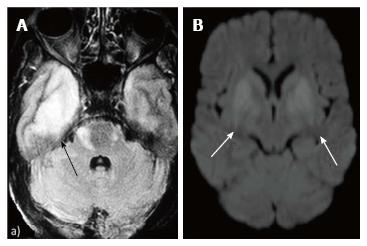
Reduced diffusion with increased perfusion points to an infective etiology. Reduced diffusion with decreased perfusion characterizes ischemic events. The further characterization of infection is typically based on characteristic neuro-anatomic location of the lesion on T2/FLAIR/ DWI (Figure 20)[2,16]. Prion disease can also show similar imaging manifestation. The imaging appearance of these encephalitis may be fairly non-specific. The dengue virus has been reported to show imaging features similar to Japanese encephalitis[17] in regions where this virus is common. Cytomegalovirus can also show non-specific manifestations, though the predominant involvement of grey matter (more than white matter) is an important differentiating feature[1]. The presence of a pencil-thin rim of enhancement in the subependymal region is considered to be a specific finding[1]. Approach to lesions presenting with hyperintensity predominantly involving the Grey matteris presented as a flowchart in Figure 21.
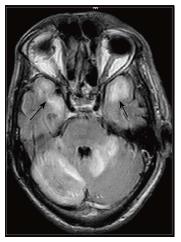
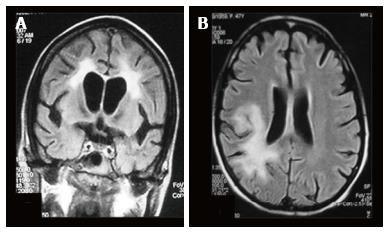
The major diagnostic considerations are HIV encephalopathy (HIVE) and progressive multifocal leukoencephalopathy (PML) (Figure 22). Though both entities have characteristic imaging features on conventional MR sequences (Table 2), they may be difficult to differentiate due to overlapping features.
| T1 | T2 | T1 + contrast | |
| HIV Encephalopathy | Isointense | Bilaterally symmetrical periventricular white matter hyperintensities | No enhancement |
| PML | Hypointense | Asymmetrical lesions involving subcortical and periventricular white matter | Faint peripheral areas of enhancement may sometimes be seen |
Magnetization transfer (MT) reduction is seen in both PML and HIVE. In PML, it is due to demyelination whereas in HIVE it is primarily related to gliosis. Thus larger reduction in MT has been observed in PML as compared to HIVE[18]. The major role of MT sequence in this setting is in early detection of disease.
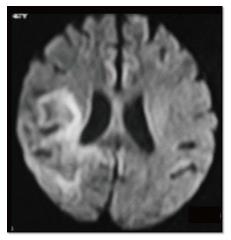
Fractional anisotropy is seen to be reduced in HIVE and PML before the morphologic changes in conventional sequences[19]. Reduced diffusion is seen in the periphery and free diffusion in the centre of PML lesions[12] (Figure 23).
Reduction of N-acetylaspartate is seen in HIVE even before the onset of symptoms. Raised choline and myoinositol is also seen in the spectra. A summary of suggested approach to these white matter lesions is presented as a flowchart in Figure 24.
Imaging features of CNS infections constitute a complex myriad. Their classification based on conventional MRI sequences, may provide a quick guide to narrowing the differential diagnosis followed by further sub-differentiation into single etiology using advanced MRI sequences and techniques.
P- Reviewer: Asensi VC, Mueller WC, Radenovic L S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Aiken AH. Central nervous system infection. Neuroimaging Clin N Am. 2010;20:557-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Whiteman ML, Bowen BC, Post MJ, Bell MD. Intracranial infections. In Scott W Atlas, editor. Magnetic Resonance Imaging of Brain and Spine. 3rd ed. Philadelphia: Lippincott Williams and Wilkins 2002; 1099-177. |
| 3. | Toh CH, Wei KC, Chang CN, Hsu PW, Wong HF, Ng SH, Castillo M, Lin CP. Differentiation of pyogenic brain abscesses from necrotic glioblastomas with use of susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012;33:1534-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Desprechins B, Stadnik T, Koerts G, Shabana W, Breucq C, Osteaux M. Use of diffusion-weighted MR imaging in differential diagnosis between intracerebral necrotic tumors and cerebral abscesses. AJNR Am J Neuroradiol. 1999;20:1252-1257. [PubMed] |
| 5. | Luthra G, Parihar A, Nath K, Jaiswal S, Prasad KN, Husain N, Husain M, Singh S, Behari S, Gupta RK. Comparative evaluation of fungal, tubercular, and pyogenic brain abscesses with conventional and diffusion MR imaging and proton MR spectroscopy. AJNR Am J Neuroradiol. 2007;28:1332-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Gaviani P, Schwartz RB, Hedley-Whyte ET, Ligon KL, Robicsek A, Schaefer P, Henson JW. Diffusion-weighted imaging of fungal cerebral infection. AJNR Am J Neuroradiol. 2005;26:1115-1121. [PubMed] |
| 7. | Lai PH, Ho JT, Chen WL, Hsu SS, Wang JS, Pan HB, Yang CF. Brain abscess and necrotic brain tumor: discrimination with proton MR spectroscopy and diffusion-weighted imaging. AJNR Am J Neuroradiol. 2002;23:1369-1377. [PubMed] |
| 8. | Gupta RK, Roy R, Dev R, Husain M, Poptani H, Pandey R, Kishore J, Bhaduri AP. Finger printing of Mycobacterium tuberculosis in patients with intracranial tuberculomas by using in vivo, ex vivo, and in vitro magnetic resonance spectroscopy. Magn Reson Med. 1996;36:829-833. [PubMed] |
| 9. | Lai PH, Weng HH, Chen CY, Hsu SS, Ding S, Ko CW, Fu JH, Liang HL, Chen KH. In vivo differentiation of aerobic brain abscesses and necrotic glioblastomas multiforme using proton MR spectroscopic imaging. AJNR Am J Neuroradiol. 2008;29:1511-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Trivedi R, Saksena S, Gupta RK. Magnetic resonance imaging in central nervous system tuberculosis. Indian J Radiol Imaging. 2009;19:256-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Jain KK, Mittal SK, Kumar S, Gupta RK. Imaging features of central nervous system fungal infections. Neurol India. 2007;55:241-250. [PubMed] |
| 12. | Smith AB, Smirniotopoulos JG, Rushing EJ. From the archives of the AFIP: central nervous system infections associated with human immunodeficiency virus infection: radiologic-pathologic correlation. Radiographics. 2008;28:2033-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Lee GT, Antelo F, Mlikotic AA. Best cases from the AFIP: cerebral toxoplasmosis. Radiographics. 2009;29:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Ho TL, Lee HJ, Lee KW, Chen WL. Diffusion-weighted and conventional magnetic resonance imaging in cerebral cryptococcoma. Acta Radiol. 2005;46:411-414. [PubMed] |
| 15. | Trenkwalder P, Trenkwalder C, Feiden W, Vogl TJ, Einhäupl KM, Lydtin H. Toxoplasmosis with early intracerebral hemorrhage in a patient with the acquired immunodeficiency syndrome. Neurology. 1992;42:436-438. [PubMed] |
| 16. | Ukisu R, Kushihashi T, Tanaka E, Baba M, Usui N, Fujisawa H, Takenaka H. Diffusion-weighted MR imaging of early-stage Creutzfeldt-Jakob disease: typical and atypical manifestations. Radiographics. 2006;26 Suppl 1:S191-S204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Borawake K, Prayag P, Wagh A, Dole S. Dengue encephalitis. Indian J Crit Care Med. 2011;15:190-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Ernst T, Chang L, Witt M, Walot I, Aronow H, Leonido-Yee M, Singer E. Progressive multifocal leukoencephalopathy and human immunodeficiency virus-associated white matter lesions in AIDS: magnetization transfer MR imaging. Radiology. 1999;210:539-543. [PubMed] |
| 19. | Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res. 2001;106:15-24. [PubMed] |