INTRODUCTION
Ischemic heart disease (IHD) is the leading cause of morbidity and mortality in developed countries[1]. LVEF can provide valuable diagnostic, prognostic and therapeutic information[2,3]. LVEF, LV volume and mass are independent cardiac predictors of morbidity and mortality in patients with IHD[2-4]. LVEF is an important parameter which is needed to make clinical decisions to guide medical or surgical therapy, and assess prognosis and outcome[5]. Various noninvasive and invasive techniques have evolved over time to measure LVEF and cardiac volumes such as echocardiography[6,7], radionuclide ventriculography[8], cardiac MRI[9,10], and contrast ventriculography (CVG) in patients undergoing invasive cardiac catheterization. Echocardiography is the most commonly used technique to measure ventricular dimensions and LVEF in clinical practice, due to its ease, cost, portability, reproducibility, noninvasive nature and lack of radiation or contrast exposure. In patients undergoing cardiac computed tomographic angiography (CTA) to study CAD, it is now feasible to measure LV volume and LVEF using the same data set without the need for additional contrast or radiation exposure. Single detector row helical computed tomography (CT)[11] has been used to measure LVEF. However, this technique has limitations in studying coronary anatomy. Electron beam CT (EBCT)[12,13], with high temporal resolution of 50 milliseconds seems to give good measurement of LVEF, but the superiority of MDCT over EBCT in the detection of coronary stenosis in clinical practice has resulted in MDCT being the preferred imaging modality amongst cardiac CTA to detect coronary artery stenosis due to its ability for retrospective gating and higher spatial resolution despite lower temporal resolution. MDCT has been used to measure LVEF[14,15] and has been shown to be in good agreement with other techniques such as echocardiography, CVG, radionuclide techniques and MRI.
FEASIBILITY OF MDCT TO MEASURE LV VOLUMES AND LVEF
Rapid developments in both the hardware and software in MDCT technology have led to an increase in the use of this technology to detect CAD. The 16 slice CT scanner made it feasible to complete the cardiac scan with 1 breath hold time. However, it was the development of the 64 slice scanner that made it possible to obtain sub-millimeter slice thickness with a high level of spatial resolution in the X, Y and Z axis along with a significant improvement in temporal resolution. Three-dimensional isometric data sets (voxel) of nearly 0.5 mm each are now possible with the 64 slice or higher scanners due to its ability to post-process and reconstruct images in any plane without image distortion. Electrocardiographic gating during image acquisition and the ability to perform retrospective gating allows acquisition of three-dimensional volumetric data in relation to time reference and cardiac cycle, making it a truly four-dimensional data set. The development of 64 to 312 slice scanners have made it possible to complete the scan not only in one full breath, but also within 1-2 cardiac cycles, making it less prone to registration artifacts due to arrhythmias or breathing.
Developments in software technology have made it possible to post-process and create multi-planar reconstructions from these large numbers of original axial images in a very time efficient manner. This has made it possible to obtain reliable coronary anatomy imaging in most cases. Although limited by both spatial and temporal resolution compared to invasive coronary angiography, this technique of noninvasive coronary angiography by MDCT has come as close as possible to defining coronary anatomy and stenosis without the need for invasive cardiac catheterization in many cases. Hence, MDCT is being increasingly used in the evaluation of chest pain to detect CAD in appropriate subsets of patients.
Excellent visualization of bypass grafts and the 3-dimensional relationship between anomalous coronary artery origin and course have made this the test of choice for evaluation of bypass grafts and coronary anomalies. The study of pulmonary venous anatomy prior to pulmonary vein isolation ablation procedures and the integration of MDCT images in the electrophysiology laboratory help expedite the ablation procedure. Similarly, detailed analysis of the cardiac venous anatomy and identification of the lateral marginal vein are helpful in the implantation of CRT-D.
Noninvasive coronary angiography is the most common indication for cardiac CTA[16-18]. The same data set is now available to measure LV volumes and LVEF using retrospective gating and identify the end-systolic and end-diastolic frames. Typically, 8 phases of cardiac cycles are analyzed for coronary angiography, and this is usually sufficient for LVEF measurement as well. Additional phase analysis can be performed if needed. Reconstruction of the images at 0%, 12.5%, 25%, 37.5%, 50%, 62.5%, 75% and 87.5% phases of the cardiac cycle are automatically post-processed. In the manual technique, the short axis images of the LV cavity (multi-planar reconstruction) are arranged from the 0% to 87.5% phases and usually the 0% phase correlates with the end diastolic phase and the 37.5% phase correlates with the end-systolic phase. Using these multiphase reconstructions, the software can be used to play a cine loop of the LV systolic function.
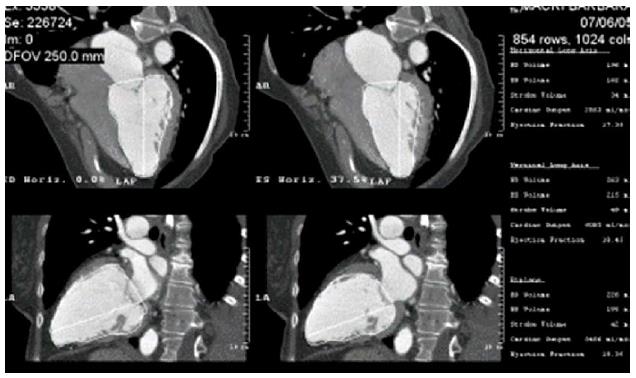
In the semi-manual method, LV cavity reconstruction is carried out by multi-planar reconstruction in the 4 chamber and 2 chamber views. Using the ellipsoid volumetric calculation by tracing the endocardial border, LV volumes are measured both in end-diastole (LVEDV) and end-systole (LVESV) in 4 chamber, 2 chamber and biplane views. LVEF is measured as LVEDV - LVESV/LVEDV (Figure 1).
Figure 1 Multi-planar reconstruction of left ventricular cavity in 4 chamber and 2 chamber views and semi-automated calculation of left ventricular volume in both end-systole and end-diastole in biplane and 4 and 2 chamber views by the area length method.
Patient with cardiomyopathy and a very low left ventricular ejection fraction of 0.20.
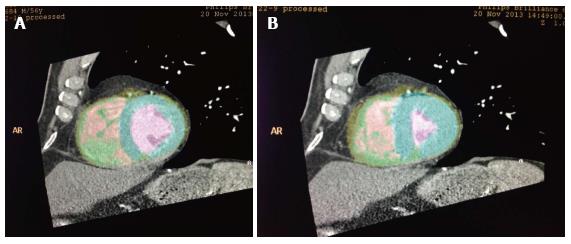
Contrast opacification of the LV is excellent during coronary CTA and this allows for good endocardial separation from the contrast filled LV cavity. The development of newer software which identifies the contrast density separation of the LV cavity from the myocardium has allowed semi-automated to fully automated measurements of LV volume and LVEF. One such technique allows an automated recognition and calculation of LVEDV and LVESV (Figure 2).
Figure 2 Automatic recognition of left ventricular cavity by automated software to calculate left ventricular volumes in end-diastole (A) and end-systole (B) to calculate left ventricular ejection fraction.
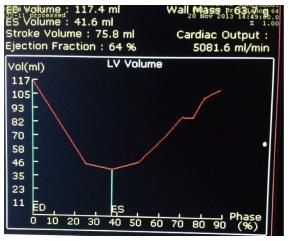
Different vendors have different software to calculate the LV volumes and LVEF. In the example shown in Figure 2, there is automatic endocardial edge detection and the software calculates the LVEDV, LVESV and LVEF as shown in Figure 3. The time-volume curve also shows the quality of the data obtained and good data are characterized by a smooth change in the LVEDV to LVESV and then back to the LVEDV as shown in Figure 3.
Figure 3 Time-volume curve display of left ventricular volume over different phases of the cardiac cycle (R-R interval) and calculation of left ventricular ejection fraction is displayed automatically.
Please note the smooth normal curve without registration artifact.
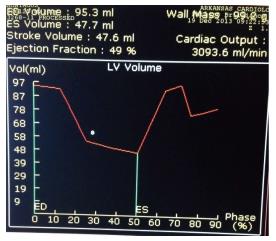
Figure 4 shows some compromise in the time volume data.
Figure 4 Shows some compromise in the time volume data as shown by the lack of smooth transition of the volume curve, and this likely represents some registration artifact towards the later part of diastole due to arrhythmias such as atrial fibrillation or frequent PVCs.
This can lead to errors in the calculation of Left ventricular ejection fraction (LVEF). A quick look at the analysis of this time volume curve data is helpful to assess the quality of the data obtained for LVEF assessment and its limitations.
Radiation exposure is a major concern during cardiac computed tomographic angiography, but it can be substantially diminished by dose modulation. Dose modulation is a technique which minimizes radiation exposure during cardiac computed tomographic angiography by prospective gating at phases which are usually not important for analysis of coronary anatomy. Since most of the coronary anatomy is analyzed around 75% phase (the phase where coronary arteries appear to have the least motion), radiation exposure can be substantially diminished by more than 50% by decreasing the current (milliAmperes) of radiation exposure at phases away from the 75% phase. Despite the use of dose modulation, left ventricular contrast opacification is adequate for endocardial definition even during systole and does not seem to compromise the capacity to measure ventricular volumes both in end-diastole and end-systole, and left ventricular ejection fraction.
COMPARISON OF THE TECHNIQUES OF LV VOLUME MEASUREMENT AND LVEF BY VARIOUS METHODS
MDCT using retrospective gating allows for LV volume and LVEF measurements, which appear to have a good correlation with cardiac MRI, currently accepted as the gold standard[14,15,19-22]. LVEF measurement is possible utilizing different noninvasive and invasive techniques such as echocardiography, radionuclide techniques, cardiac MRI, and CVG. Echocardiography is the most commonly used technique to measure LVEF. Echocardiography may have technical, acoustic and operator limitations[23]. It is also subject to alteration in ventricular geometry[6,7]. Nuclear imaging using a SPECT gating also has limitations due to restrictions in both the spatial resolution and the definition of endocardial borders within the myocardium[8,24]. However, for the purpose of LVEF measurement, the estimation of LV endocardial contour is done by radioactive count, and anatomical resolution is not always so important. Prospective gating EBCT has advantages in measuring LVEF because of high temporal resolution, but has limited spatial resolution and is inferior in defining coronary anatomy compared to MDCT. Although cardiac MRI appears to be more accurate in measuring left ventricular volume and LVEF, the technique has limitations in defining coronary anatomy compared to MDCT. This modality also takes a much longer time, is more expensive, and is not feasible in some patients who have implanted devices, non-compatible with MRI. Invasive contrast ventriculography (CVG) has limitations due to the invasive nature of the test. In addition, calculation of the left ventricular volume is based on the assumption of the shape of the ventricle, and this may not be accurate in patients with an altered left ventricular geometry. MDCT is frequently used as a noninvasive coronary angiographic tool to evaluate suspected symptomatic CAD patients. Simultaneous measurement of LVEF using the same data provides a unique opportunity and can add incremental value to the test.
LV volume measurements are possible using various techniques. Current automated software allows LV volume measurement by MDCT based on short axis image reformations as in echocardiography and cardiac MRI. For calculation of LV volume measurement and LVEF, identification of the end-diastolic and end-systolic phases is needed. This is made possible by retrospective gating and post-processing of the images at various phases. In our experience, phase reconstructions at 12.5% phase apart seem to suffice, but reconstructions at every 5% phase of the R-R interval can also be done, but will be more time-consuming. Short axis images at the mid ventricular level are obtained in a semi-automated technique. In the current automated software, end-systolic and end-diastolic short axis images are identified automatically as noted in Figure 2. LV volumes and LVEF are then measured automatically as shown in Figure 3.
The LV volume and EF can be measured by different methods depending on the technique used. The area length method is a technique used primarily in invasive left ventriculography and in echocardiography, as an ellipsoid model is used to measure the LV volume. This technique can also be used in some semi-automated techniques in MDCT using volume measurements in four chamber, two chamber and biplane views. The Simpson method is also commonly used in cardiac MRI, EBCT, MDCT and echocardiography.
The automated technique in MDCT, as shown in Figure 2, employs a threshold-based region growing algorithm. This allows identification of the cardiac chambers and their volume based on the separation of myocardium from LV cavity based on the separation of contrast and tissue signal density. LV volume measurements based on this method do not depend on geometric assumptions and are more accurate than area length method. Since cardiac CTA by MDCT is carried out primarily for coronary angiography, the current technique, volume and timing of contrast injection along with saline bolus allows for good opacification of the LV. The use of 64 slice or higher MDCT with rapid scan times provide superior results. The automated technique seems to work well even with dose modulation without any limitations of the detection of the contrast edge. Once the LVEDV and LVESV are measured, LVEF is calculated as discussed earlier. The time-volume curve allows the display of LV volume change over the different phases which provide additional qualitative information about the study. It can identify limitations due to arrhythmias, poor contrast opacification or obesity.
ACCURACY OF MDCT IN MEASURING LVEF
In our study of 52 patients, we compared the 16 row detector MDCT with TTE to measure LV volume and LVEF and found MDCT to be a useful tool for measuring LVEF[25]. Biplane measurements by these two techniques correlated better for LV volumes and LVEF, but MDCT gave higher values compared to TTE and this has also been shown in other studies using 64 slice[26,27]. Many other subsequent studies have found MDCT to be a useful tool for measuring LVEF when compared with TTE.
The feasibility of accurate assessment of LVEF and volume has been shown using a single heartbeat 320-row MDCT detector[28]. Similarly in a comparison of 128 slice CT compared to echocardiography, MDCT provided comparable results to echocardiography for LVEF and LV volumes, although LV volume was overestimated by MDCT compared to echocardiography[29]. A recent study using a head to head comparison of LVEF measurement with 64-slice MDCT, biplane LV CVG and both 2D and 3D TTE found 64-slice MDCT to be more accurate than LV CVG and TTE. This study used cardiac MRI as the reference standard for measuring LVEF[30]. However, it should be noted that gadolinium-enhanced cardiac MRI carries a risk of nephrogenic systemic fibrosis in patients with renal failure, and is, thus a limiting factor in this patient population, just as contrast-induced nephropathy would be a concern with MDCT in some patients.
Semi-automatic software to measure LV volume by MDCT has been found to have good reproducibility for LVEF measurement, but it is important to understand the limitations of MDCT in semi-automatic and automatic measurement of LVEF[31].
In a comparison of SPECT versus MDCT to measure LVEF in 292 patients, MDCT gave significantly higher LVEF compared to SPECT and the values may not be interchangeable between different methods of measurement[32]. In another small study of 15 patients, MDCT compared well with biplane LV CVG for measuring LVEF[33]. There appears to be a minor systematic overestimation of LVEDV and LVESV, and underestimation of EF of 2.1% by MDCT compared to cardiac MRI[34]. LVEF measurement in a wide spectrum of LV dysfunction requires further validation compared to other techniques such as EBCT[35]. Studies comparing LVEF measurement by simultaneous multimodality images such as echocardiography, LV CVG and SPECT are limited[36].
A recent systematic review and meta-analysis of 27 eligible studies concluded that MDCT can measure LVEF accurately compared to MRI and TTE[37]. Twelve studies compared MDCT with MRI and 15 studies compared MDCT with TTE to measure LVEF in this meta-analysis, and MDCT appears to be a useful tool for measuring LVEF in patients undergoing coronary CTA. Simultaneous measurement of LVEF at the time of coronary CTA by MDCT seems to provide additional incremental prognostic value[38].
LIMITATIONS OF MDCT IN LVEF MEASUREMENT AND TECHNIQUES TO IMPROVE THESE LIMITATIONS
It should be noted that the current limitations of MDCT do not favor the use of MDCT for the sole purpose of LVEF measurement. This is due to a myriad of reasons, including radiation and contrast exposure, cost, risks of iodine allergy and potential renal failure. The risk of contrast induced-nephropathy is increased in patients with preexisting renal dysfunction, diabetes, heart failure, increased age and other comorbid conditions. The high volume of contrast administered for cardiac CTA may also increase the risk of contrast-induced nephropathy in high risk patients. Pretest risk assessment and good hydration are important in all patients undergoing a contrast study such as cardiac CTA, and for that reason many patients may not be candidates for cardiac CTA for fear of contrast-induced nephropathy and its consequences. The technique has limitations in patients with obesity, renal failure, arrhythmias and difficult breath hold time On the other hand, simultaneous measurements of LVEF in patients undergoing cardiac CTA for noninvasive coronary angiography are feasible, reproducible, and fairly accurate compared to other modalities.
High temporal and spatial resolution is needed for accurate measurement of LVEF[39]. MDCT has good spatial resolution, but has a limited temporal resolution of 125-250 milliseconds compared to EBCT[40] or MRI and can cause motion artifacts[41]. Image quality in patients with higher heart rate may be of poor quality due to limited temporal resolution and may compromise the accuracy[42,43].
MDCT with temporal resolution of 20 milliseconds would be desirable to avoid motion artifacts, but is not yet feasible with current technology[44]. In the early studies using 4 row MDCT, LVEF measurement was underestimated due to poor temporal resolution, and overestimation of LVESV was found as compared to LV CVG and MRI[45,46].
An increase in temporal resolution is a desirable goal to improve the quality of MDCT, and two strategies have been utilized so far. First, gantry rotation time is shortened with the new scanners[16,47,48] and secondly, more gantry rotations allowing more R-R intervals for image reconstruction are available using multi-segmental image reconstruction algorithms[49,50]. Multiple cardiac cycles are used to create image reconstruction in this method, and thus may improve temporal resolution to less than 100 milliseconds. However, significant variations in the R-R cycle could be a limitation in the multi-segmental image reconstruction due to non-uniformity of ventricular contraction.
Rapid gantry rotations of up to 0.33 s per rotation attained with newer MDCTs can also improve the temporal resolution[51]. Dual source CT can also increase the temporal resolution to 83 milliseconds in single segmental reconstructions[52].
Lower heart rates are needed to obtain better images by MDCT to evaluate coronary anatomy, and consequently beta-blockers are frequently used to slow the heart rate during image acquisition. This introduces the effect of heart rate change and negative inotropic effects on LVEF measurement[42]. Dual-source CT is less dependent on the heart rate and may improve LVEF measurement. The patients with arrhythmia such as atrial fibrillation and frequent premature ventricular complexes may produce significant registration artifacts and may introduce error in the calculation of LVEF. However, with the use of recent higher slice MDCT, this should be less of a concern as most of the data acquisition can be completed within one or two cardiac cycles.
Techniques to reduce radiation exposure are possible using higher detector rows and faster rotation times. Reduced tube current during unnecessary cardiac phase (dose modulation) helps reduce radiation[53]. Since most of the coronary anatomy analysis is done in late diastole close to 75% phase, this normally does not compromise analysis of the coronary anatomy. The degree of contrast density separation of the LV cavity from the myocardium is adequate even with dose modulation in systole for the purpose of LVESV calculation and should not compromise LVEF measurement. Analysis of the quality of data and the LV time volume curve may be helpful in assessing the quality of the study.
LVEF measurement by MDCT is based on a volumetric data set. LVEF measurement should be less susceptible to error in patients with LV enlargement or deformity. LVEF measurement by MDCT correlates well with MRI in patients with LV dysfunction or LV dilation[54].
Cardiac MRI is considered the gold standard for the measurement of LV volume, LVEF and regional wall motion assessment. Lack of radiation and contrast exposure, along with higher temporal resolution are advantageous. However, MDCT requires a short breath hold time, and can be performed even in patients with pacemakers and implanted defibrillators. In contrast to MDCT using single breath hold image acquisition, cardiac MRI needs multiple short breath holds for cine MRI. Both techniques are susceptible to arrhythmias with image degradation. In addition, MDCT is superior to cardiac MRI for coronary imaging due to a higher spatial resolution, and it is in this group of patients that LVEF measurement can be performed to provide additional clinical information. Processing time may also be a limiting factor in some cases, but now with the use of automated software, the LVEF calculation is faster and likely to improve further.
CONCLUSION
LVEF measurement at the time of cardiac CTA for the study of coronary anatomy using MDCT seems reasonable given the feasibility, reproducibility, and accuracy of the data. This information can be obtained at the time of coronary imaging without the need for additional radiation or contrast exposure. Developments in hardware, software and work stations, along with the availability of automated techniques to measure LVESV and LVEDV have made this technique time efficient. The use of MDCT for the sole purpose of LVEF measurement is not reasonable at this time given the radiation exposure, contrast exposure and cost. Instead, this should be used as a complimentary technique to measure LVEF in patients undergoing cardiac CTA for noninvasive coronary angiography.
P- Reviewer: Lai S, Nagamachi S S- Editor: Wen LL L- Editor: Webster JR E- Editor: Lu YJ