Published online Aug 28, 2014. doi: 10.4329/wjr.v6.i8.619
Revised: June 24, 2014
Accepted: July 17, 2014
Published online: August 28, 2014
Processing time: 122 Days and 0.5 Hours
AIM: To reveal angiographic findings to predict the result of balloon test occlusion (BTO).
METHODS: The cerebral angiograms of 42 consecutive patients who underwent cerebral angiography including both the Matas and Allcock maneuvers and BTO were retrospectively analyzed. Visualization of the anterior cerebral artery (ACA) and the middle cerebral artery (MCA) by the cross flow on the tested side during the Matas or Allcock maneuver was graded on a 5-point scale. Circle of Willis (COW) anatomy with respect to the presence/absence of a collateral path to reach the tested internal carotid artery (ICA) was classified into four categories. A univariate logistic analysis was used to analyze the associations between each angiographic finding and the BTO result. Sensitivity, specificity, accuracy, positive predictive value, and negative predictive value for each finding were calculated.
RESULTS: Five patients (12%) were BTO-positive and the remaining 37 patients (88%) were BTO-negative. Visualizations of the ACA and MCA as well as the COW anatomy were significantly associated with the BTO result (P = 0.0051 for ACA, P = 0.0002 for MCA, and P < 0.0001 for COW anatomy). In particular, good MCA visualization and the presence of an anterior connection (collateral path to the tested ICA from the contralateral ICA via the anterior communicating artery) in the COW were highly predictive for negative BTO (negative predictive value = 100% for both).
CONCLUSION: A BTO result may be predicted by angiographic findings including ACA/MCA visualization and COW anatomy.
Core tip: Balloon test occlusion (BTO) is widely performed to assess the ischemic tolerance among preoperative patients. It is safe and useful, however, it sometimes carries the risk of thrombosis, dissection and infarction. Our study revealed that visualizations of the anterior cerebral artery and middle cerebral artery (MCA) as well as the circle of Willis (COW) anatomy were significantly associated with the BTO result. In particular, good MCA visualization and/or the presence of an anterior connection [collateral path to the tested internal carotid artery (ICA) from the contralateral ICA via the anterior communicating artery] in the COW were highly predictive for negative BTO.
- Citation: Kikuchi K, Yoshiura T, Hiwatashi A, Togao O, Yamashita K, Honda H. Balloon test occlusion of internal carotid artery: Angiographic findings predictive of results. World J Radiol 2014; 6(8): 619-624
- URL: https://www.wjgnet.com/1949-8470/full/v6/i8/619.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i8.619
The treatment of skull base lesions such as tumors, aneurysms, and traumatic lesions may require internal carotid artery (ICA) sacrifice[1,2]. Pretherapeutic knowledge of carotid artery dependence is essential in these patients, since vascular bypass or alternative surgical approaches may be necessary in those unable to tolerate ICA sacrifice. Balloon test occlusion (BTO) is an angiographic test to evaluate ischemic tolerance after permanent occlusion of an ICA[1,2]. During BTO, the cerebral hemisphere ipsilateral to the tested ICA may be perfused by collateral blood flow, depending on the development of collateral pathways including the circle of Willis (COW). Although BTO is widely accepted, it sometimes carries the risk of thrombosis, dissection and infarction[2,3]. We hypothesized that the BTO results can be predicted by angiographic findings. Therefore the purpose of the present study was to test whether angiographic findings can be used to predict BTO results.
Kyushu University Institutional Review Board for Clinical Research approved this retrospective study. Between 1996 and 2011, BTO was performed in 77 patients at Kyushu University Hospital. Among them, those who underwent both the Matas maneuver (angiography of the non-tested ICA during manual carotid compression on the tested side) and the Allcock maneuver (angiography of the vertebral artery during manual carotid compression on the tested side) were selected. The cerebral angiograms of 42 consecutive patients (17 male and 25 female patients; mean age, 54.2 years; median, 57 years; range 25-70 years) were thus included in this retrospective analysis. Of the 42 BTO procedures, the right ICA was tested in 23 patients, and the left ICA was tested in 19 patients. The diagnosis at the time of treatment included 13 ICA aneurysms and 29 tumors (18 meningiomas, 3 schwannomas, 2 pituitary adenomas, 2 cervical lymph node metastatic tumors, 2 thyroid tumors, 1 chondrosarcoma and 1 hemangiopericytoma).
A 5 Fr or 5.5 Fr femoral introducer was inserted in the right femoral artery to perform the cerebral angiography using a digital subtraction angiography (DSA) unit (DFP-200A; Toshiba Medical Systems, Tokyo, Japan and AXIOM Artis zee Biplane system; Siemens AG, Healthcare Sector, Forchheim, German) with a 5 Fr diagnostic catheter (TERUMO Clinical Supply, Gifu, Japan and Hanaco Medical, Tokyo). After the femoral punctures, 3000 IU of heparin was systematically administered intra-arterially by bolus, followed by an additional intra-arterial infusion of 1000 IU/h. For each patient, both Matas and Allcock maneuvers as well as a complete biplane DSA study including bilateral common and ICAs and vertebrobasilar system were performed. Angiogram of external carotid artery was added when necessary. A total of 8 to 10 mL of iodinate contrast [iopamidol 300 mgI/mL (Iopamiron™, Bayer, Osaka, Japan)] was injected at 4 to 5 mL/s for the ICA angiography, and 10 to 12 mL at 5 to 6 mL/s was used for the vertebral artery (VA) angiography.
The cervical portion of the tested ICA was occluded up to 20 min using a 5 Fr balloon catheter (balloon size 8 mm; head hunter/BHW type, MOIYAN balloon catheter; Miyano Medical Corp., Kobe, Japan). The balloon was carefully inflated under fluoroscopic observation. Complete occlusion was confirmed by an angiogram through the balloon catheter. The patient then underwent a continuous neurologic evaluation throughout the examination. The neurologic evaluations were performed by clinical neurospecialists who maintained continuous verbal dialogues with the patient and were continuously evaluating the patient’s muscle strength, sensation, cognition, and cranial nerve function during the testing period. Electroencephalographic (EEG) monitoring was performed in 36 patients. The balloon was immediately deflated when any neurologic deficit or EEG abnormality was detected. In such cases, the BTO was judged to be positive. The BTO was considered negative when the patient tolerated the 20-min occlusion.
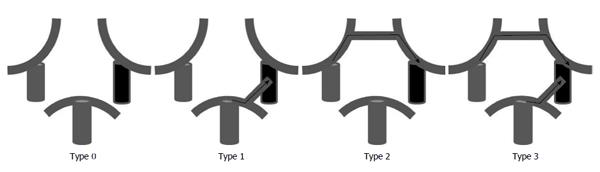
Two board-certified radiologists (Kazufumi Kikuchi and Osamu Togao) reviewed all angiographic images obtained during the Matas and Allcock maneuvers in a consensus reading, and they evaluated the visualization of the anterior cerebral artery (ACA) and the middle cerebral artery (MCA) on the tested side by the cross flow. Visualization of the ACA and the MCA was graded on a 5-point scale according to the established anatomical segment of each artery (A1-4 for the ACA and M1-4 for the MCA[4]). It was graded as A0/M0 when no visualization of each artery was noted. The best arterial visualization during the Matas and Allcock maneuver was chosen as the representative of each artery (ACA and MCA). Subsequently, the Grading was classified as either poor (Grades 0-2) or good (Grades 3-4). The same two board-certified radiologists evaluated the COW anatomy. The COW anatomy was classified into four types (Types 0-3) according to the presence/absence of an anterior connection (connection between the right and left ICAs via the anterior communicating artery) and the presence/absence of a posterior connection (connection between the tested ICA and basilar artery) (Figure 1). Subsequently, the COW anatomy was classified into poorly-developed (either Type 0 or 1) and well-developed (either Type 2 or 3).
We performed a univariate logistic analysis to determine the associations between each angiographic finding and the BTO result (positive vs negative). We calculated the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of ACA/MCA visualization by cross flow (poor vs good) and COW anatomy (poorly developed vs well-developed) for predicting the BTO result. For all analyses, P < 0.05 was considered significant.
Five patients (12%) were BTO-positive and the remaining 37 patients (88%) were BTO-negative. The BTO-induced abnormalities were hyposthenia (n = 3), aphasia (n = 3), loss of consciousness (n = 2), slow brain waves (n = 2) and facial paralysis (n = 1). In all patients, the neurological deficits disappeared immediately after balloon deflation.
Tables 1 and 2 summarize the visualization of the ACA and MCA, respectively. Among the four patients with poor ACA visualization, three (75%) were BTO-positive, whereas among the 38 cases with good ACA visualization, only two (5%) were BTO-positive (Table 1). Among the nine patients with poor MCA visualization, 5 (56%) were BTO-positive, whereas none (0%) of the 37 patients with good MCA visualization were BTO-positive. For both the ACA and the MCA, poor visualization by cross flow was significantly associated with a positive BTO result (P = 0.0051; 95%CI: 0.13-7.61 for ACA, P = 0.0002; 95%CI: 0.07-14.19 for MCA, respectively). Visualization of cross flow to the ACA (either poor or good) predicted BTO results with a sensitivity of 60.0%, a specificity of 97.3%, an accuracy of 92.9%, a positive predictive value (PPV) of 75.0%, and a negative predictive value (NPV) of 94.7%. Similarly, visualization of cross flow to the MCA showed a sensitivity of 100%, a specificity of 89.2%, an accuracy of 90.5%, a PPV of 55.6%, and an NPV of 100%.
| ACA visualization | BTO-positive (%) | BTO-negative (%) | Total (%) | |
| Poor | Grade 0 | 2 (5) | 0 (0) | 2 (5) |
| Grade 1 | 0 (0) | 0 (0) | 0 (0) | |
| Grade 2 | 1 (2) | 1 (2) | 2 (5) | |
| Good | Grade 3 | 0 (0) | 5 (12) | 5 (12) |
| Grade 4 | 2 (5) | 31 (74) | 33 (78) | |
| Total | 5 (12) | 37 (88) | 42 (100) | |
| MCA visualization | BTO-positive (%) | BTO-negative (%) | Total (%) | |
| Poor | Grade 0 | 3 (7) | 1 (2) | 4 (9) |
| Grade 1 | 1 (2) | 0 (0) | 1 (2) | |
| Grade 2 | 1 (2) | 3 (7) | 4 (9) | |
| Good | Grade 3 | 0 (0) | 12 (29) | 12 (29) |
| Grade 4 | 0 (0) | 21 (50) | 21 (50) | |
| Total | 5 (12) | 37 (88) | 42 (100) | |
Table 3 shows the comparison of COW anatomy and BTO results. Both (100%) of the two patients with Type 0 COW anatomy had a positive BTO outcome. Among the six patients with Type 1 anatomy, 3 (50%) were BTO-positive. Among the 34 patients with well-developed COW (either Type 2 or Type 3 anatomy), none (0%) was BTO-positive. The logistic analysis showed that poorly-developed COW was significantly associated with positive BTO result (P < 0.0001; 95%CI: 0.31-3.21).
| COW anatomy | BTO-positive (%) | BTO-negative (%) | Total (%) | |
| Poorly- | Type 0 | 2 (5) | 0 (0) | 2 (5) |
| developed | Type 1 | 3 (7) | 3 (7) | 6 (14) |
| Well- | Type 2 | 0 (0) | 4 (10) | 4 (10) |
| developed | Type 3 | 0 (0) | 30 (71) | 30 (71) |
| Total | 5 (12) | 37 (88) | 42 (100) | |
When the COW anatomy was classified into poorly-developed and well-developed, the sensitivity, specificity, accuracy, PPV, and NPV were 100%, 91.9%, 92.9%, 62.5%, and 100%, respectively.
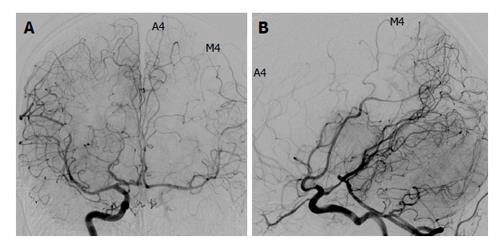
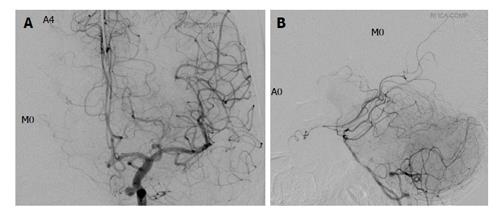
Figures 2 and 3 show representative patients with negative and positive BTO, respectively.
Therapeutic occlusion of the ICA remains an important procedure[5-11]. In such cases, temporary BTO is imperative to evaluate ischemic risks before complete blockage is performed[11-14]. The introduction of clinical BTO of the ICA was associated with significant reduction in post-occlusion morbidity; a review of the literature comprising 516 patients demonstrated that the use of BTO of the ICA reduced the morbidity of permanent ICA occlusion from 26% to 13%[15]. However, complications due to BTO have been reported, and the incidence of neurological deficits during BTO ranges from 3.2% (3) to 3.7% (2). The risk of complication would rise when the wall of the ICA is under a pathological condition such as severe atheromatous disease[16] or vulnerability due to Ehlers-Danlos syndrome[17].
Our present results demonstrate that for both the ACA and the MCA, poor visualization by cross flow was significantly associated with a positive BTO result. In particular, MCA visualization showed a high sensitivity (100%) and NPV (100%). Poor development of the COW was significantly associated with a positive BTO result. Similarly to MCA visualization, COW anatomy showed a high sensitivity (100%) and NPV (100%). Both of the two patients with Type 0 COW anatomy were BTO-positive. Among the patients with Type 1 anatomy (only a posterior connection is present), 50% (3/6) were BTO-positive, whereas none of those with Type 2 (only an anterior connection is present) anatomy (0/4) were BTO-positive. This finding indicates that the anterior circulation has a greater effect on the risk of ischemic compared to the posterior circulation, as indicated in other studies[18-21].
Our results showed that good MCA cross-flow visualization and well-developed COW anatomy (i.e., the presence of the anterior connection) were highly predictive of a negative BTO result. In this study, angiographic findings during the Matas and Allcock maneuvers were evaluated. However, it is apparent that the predictive value of these two findings (good MCA cross-flow visualization and well-developed COW anatomy) would be applicable to angiograms obtained without carotid compression. When either of these two predictive findings is present, avoidance of BTO may be considered, especially in patients at a high risk for complications associated with BTO. Conversely, poor visualization of ACA/MCA and poorly-developed COW anatomy were frequently associated with a positive BTO result (Tables 1-3). BTO should be performed carefully in patients with those angiographic findings.
This study has several limitations. First, the number of patients is limited. Moreover, due to the retrospective nature, patient selection biases may be present. There may be technical variability in manual carotid compression. Incomplete compression might have resulted in poor cross flow visualization. We compared the angiographic findings with BTO results, but not directly with ischemic events following permanent ICA occlusion. In the majority of our patients, subsequent permanent occlusion was not performed. Neurological evaluation and EEG monitoring were used to detect ischemia during the BTO. We did not use perfusion imaging techniques during the BTO, which would provide precise information about localized ischemia that may not be detected by neurological assessment.
In conclusion, angiographic findings during Matas and Allcock maneuvers including the ACA/MCA visualization by cross flow and the COW anatomy were significantly correlated with the BTO result. Good visualization of the MCA by cross flow and the presence of the anterior connection in the COW are predictive for negative BTO.
Balloon test occlusion (BTO) is an angiographic test to evaluate ischemic tolerance after permanent occlusion of an internal carotid artery (ICA). BTO is widely accepted, it sometimes carries the risk of thrombosis, dissection and infarction. The authors hypothesized that the BTO results can be predicted by angiographic findings.
The data revealed that visualizations of the anterior cerebral artery (ACA) and middle cerebral artery (MCA) as well as the circle of Willis (COW) anatomy were significantly associated with the BTO result. In particular, good MCA visualization and the presence of an anterior connection (collateral path to the tested ICA from the contralateral ICA via the anterior communicating artery) in the COW were highly predictive for negative BTO.
This study represents a BTO result may be predicted by angiographic findings including ACA/MCA visualization and COW anatomy.
The results may be useful for avoiding the risk of complication when the wall of the ICA is under a pathological condition such as severe atheromatous disease or vulnerability due to Ehlers-Danlos syndrome.
The authors have performed a good study, the manuscript is interesting.
P- Reviewer: Kettering K, Paraskevas KI S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Erba SM, Horton JA, Latchaw RE, Yonas H, Sekhar L, Schramm V, Pentheny S. Balloon test occlusion of the internal carotid artery with stable xenon/CT cerebral blood flow imaging. AJNR Am J Neuroradiol. 1988;9:533-538. [PubMed] |
| 2. | Tarr RW, Jungreis CA, Horton JA, Pentheny S, Sekhar LN, Sen C, Janecka IP, Yonas H. Complications of preoperative balloon test occlusion of the internal carotid arteries: experience in 300 cases. Skull Base Surg. 1991;1:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Mathis JM, Barr JD, Jungreis CA, Yonas H, Sekhar LN, Vincent D, Pentheny SL, Horton JA. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol. 1995;16:749-754. [PubMed] |
| 4. | Osborn AG. Diagnostic cerebral angiography. 1998;. |
| 5. | Adams GL, Madison M, Remley K, Gapany M. Preoperative permanent balloon occlusion of internal carotid artery in patients with advanced head and neck squamous cell carcinoma. Laryngoscope. 1999;109:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Nayak UK, Donald PJ, Stevens D. Internal carotid artery resection for invasion of malignant tumors. Arch Otolaryngol Head Neck Surg. 1995;121:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Sanna M, Piazza P, Ditrapani G, Agarwal M. Management of the internal carotid artery in tumors of the lateral skull base: preoperative permanent balloon occlusion without reconstruction. Otol Neurotol. 2004;25:998-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Larson JJ, Tew JM, Tomsick TA, van Loveren HR. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery. 1995;36:26-30; discussion 30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Fox AJ, Viñuela F, Pelz DM, Peerless SJ, Ferguson GG, Drake CG, Debrun G. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg. 1987;66:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 279] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Field M, Jungreis CA, Chengelis N, Kromer H, Kirby L, Yonas H. Symptomatic cavernous sinus aneurysms: management and outcome after carotid occlusion and selective cerebral revascularization. AJNR Am J Neuroradiol. 2003;24:1200-1207. [PubMed] |
| 11. | Lubicz B, Gauvrit JY, Leclerc X, Lejeune JP, Pruvo JP. Giant aneurysms of the internal carotid artery: endovascular treatment and long-term follow-up. Neuroradiology. 2003;45:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Brunberg JA, Frey KA, Horton JA, Deveikis JP, Ross DA, Koeppe RA. [15O]H2O positron emission tomography determination of cerebral blood flow during balloon test occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 1994;15:725-732. [PubMed] |
| 13. | Michel E, Liu H, Remley KB, Martin AJ, Madison MT, Kucharczyk J, Truwit CL. Perfusion MR neuroimaging in patients undergoing balloon test occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 2001;22:1590-1596. [PubMed] |
| 14. | van der Schaaf IC, Brilstra EH, Buskens E, Rinkel GJ. Endovascular treatment of aneurysms in the cavernous sinus: a systematic review on balloon occlusion of the parent vessel and embolization with coils. Stroke. 2002;33:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Linskey ME, Jungreis CA, Yonas H, Hirsch WL, Sekhar LN, Horton JA, Janosky JE. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994;15:829-843. [PubMed] |
| 16. | Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke. 1990;21:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 281] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | North KN, Whiteman DA, Pepin MG, Byers PH. Cerebrovascular complications in Ehlers-Danlos syndrome type IV. Ann Neurol. 1995;38:960-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 100] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Mount LA, Taveras JM. Arteriographic demonstration of the collateral circulation of the cerebral hemispheres. AMA Arch Neurol Psychiatry. 1957;78:235-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Kluytmans M, van der Grond J, van Everdingen KJ, Klijn CJ, Kappelle LJ, Viergever MA. Cerebral hemodynamics in relation to patterns of collateral flow. Stroke. 1999;30:1432-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Doblar DD, Plyushcheva NV, Jordan W, McDowell H. Predicting the effect of carotid artery occlusion during carotid endarterectomy: comparing transcranial doppler measurements and cerebral angiography. Stroke. 1998;29:2038-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Liebeskind DS, Flint AC, Budzik RF, Xiang B, Smith WS, Duckwiler GR, Nogueira RG; for the MERCI and Multi-MERCI Investigators. Carotid I's, L's and T's: collaterals shape the outcome of intracranial carotid occlusion in acute ischemic stroke. J Neurointerv Surg. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |