Published online Jul 28, 2014. doi: 10.4329/wjr.v6.i7.502
Revised: May 28, 2014
Accepted: June 14, 2014
Published online: July 28, 2014
Processing time: 190 Days and 16.4 Hours
AIM: To analyse and summarize all the articles related to positron emission tomography and Takotsubo cardiomyopathy (TTC).
METHODS: We performed a systematic review of the existing literature on positron emission tomography/nuclear imaging and Takotsubo cardiomyopathy using PUBMED database. We combined search terms such as “takotsubo”, “takotsubo syndrome”, “myocardial positron emission tomography”, “positron emission tomography”. All case reports were excluded. The list included only four articles which were reviewed by two independent investigators. It was not possible to undertake a formal meta-analysis because of the heterogeneity of the studies; therefore, we made a narrative synthesis of the collected data.
RESULTS: Nuclear medicine techniques can be useful employed in the differential diagnosis of TTC from an acute coronary syndrome (ACS). In fact, transient left ventricular (LV) apical ballooning is a syndrome frequently misdiagnosed as an ACS and can mimic symptoms of myocardial infarction with ST-T segments changes on electrocardiography (ECG), a limited release of myocardial enzyme, mainly reported after sudden emotional or physical stress, and an akinesis or dyskinesis of the left ventricle apex which are completely reversible in a few weeks. In the studies included in this review, nuclear medicine techniques have demonstrated a discrepancy between normal perfusion and a reduced glucose utilization in TTC, commonly known as “inverse flow metabolism mismatch”. This suggests that apical ballooning represents a transient metabolic disorder on the cellular level, rather than a structural contractile disease of the myocardium, due to a transient decrease of glucose metabolism that might be related to a coronary microcirculation impairment followed by prolonged myocardial stunning.
CONCLUSION: Nuclear medicine techniques can be usefully used for the diagnosis of TTC and can increase our knowledge of the pathophysiological mechanisms of TTC.
Core tip: Takotsubo cardiomyopathy is a cardiac syndrome with symptoms similar to acute myocardial infarction (MI) including chest pain and electrocardiographic changes, in absence of coronary artery stenosis. This syndrome is characterized by reversible wall-motion abnormalities involving apical and midportion of left ventricle. In the acute phase it is clinically indistinguishable from acute MI but, recently, myocardial positron emission tomography have demonstrated to delineate this syndrome from acute coronary artery disease, also offering a new pathophysiological explanation for this particular syndrome. This clinical review aimed to summarise the most significant experiences on the use of myocardial positron emission tomography in Takotsubo cardiomyopathy.
- Citation: Testa M, Feola M. Usefulness of myocardial positron emission tomography/nuclear imaging in Takotsubo cardiomyopathy. World J Radiol 2014; 6(7): 502-506
- URL: https://www.wjgnet.com/1949-8470/full/v6/i7/502.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i7.502
Transient left ventricular (LV) apical ballooning is a syndrome frequently misdiagnosed as an acute coronary syndrome related to occlusive epicardial coronary artery disease (CAD)[1,2]. It is characterised by a rare form of transient LV dysfunction and dilatation accompanied by chest pain following emotional or physical stress, ischemic electrocardiographic changes, minimal release of cardiac enzymes, wall-motion abnormalities involving the apical and mid-portion of the left ventricle and absence of angiographically detectable coronary artery disease (CAD)[3]. Physiopathology of this particular heart disease, also called Takotsubo cardiomyopathy (TTC), is still unexplained although several mechanisms have been proposed, such as multivessel coronary vasospasm, abnormalities in coronary microvascular function, inflammatory process and catecholamine-mediated cardiotoxicity[4-9].
Recently, nuclear medicine techniques for diagnosis and improvement in pathophysiological explanation of TTC have been used, showing interesting results both for diagnosis and for speculative clinical research. This review aimed to summarise the most significant experiences on the use of myocardial positron emission tomography (PET) in TTC.
We performed a systematic review of the existing literature on this topic using PUBMED database, combining search terms such as “takotsubo”, “takotsubo syndrome”, “myocardial positron emission tomography”, “positron emission tomography”. Case reports were excluded. The list included only four articles which were reviewed by two investigators. A formal meta-analysis was not done because of the heterogeneity of studies, therefore, we undertook a narrative synthesis of the collected data.
Four articles[10-13] were included in our clinical review (see Table 1); one is from Japan, one from Italy, one from France and the last from Austria. The main characteristics of the studies included in this review are reported in Table 1. All publications were written in English. All of them were prospective studies, but just in three of them a period of follow-up was planned (ranged from 30 to 180 d). The studies ranged from 3 to 18 patients; a total of 49 patients were included in this review.
| No of patients (females) | Mean age | LVEF acute phase | Diagnostic techniques used for TTC | MRI (n) | SPECT (n) | FDG-PET (n) | MIBG | Time of follow-up (d) | LVEF follow-up | MRI follow-up (n) | SPECT follow-up (n) | FDG-PET follow-up (n) | MIBG follow-up (n) | |
| Yoshida et al[10] | 15 (12) | 72 | 47.7% ± 6.6% | ECG/CAG/EchoCG | - | 201Tl (10) | 8 | - | 90 | - | - | 1 | - | - |
| Feola et al[11] | 3 (3) | 75, 3 | 40.7% ± 5.1% | ECG/CAG/EchoCG | 3 | N13 (3) | 3 | - | 90 | 54% | 3 | 3 | 3 | - |
| Cimarelli et al[12] | 18 (13) | 67 | 35% ± 8% | ECG/CAG/EchoCG | - | 99mTc (7) or 201Tl (4) | 15 | 8 | 30-180 | 58% ± 6% | - | - | 6 | 2 |
| Rendl et al[13] | 13 (NR) | NR | 52% ± 13% | CAG/EchoCG | - | 99mTc (13) | 13 | - | 60 | 62% ± 13% | - | NR | 11 | - |
| TOTAL (n) | 49 | 3 | 37 | 39 | 8 | 3 | 4 | 20 | 2 |
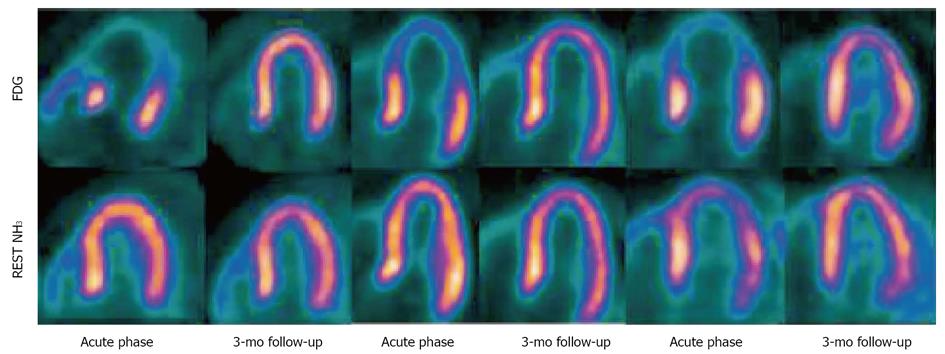
A total of 37 patients underwent a myocardial perfusion scintigraphy in the acute phase of TTC [14 patients with Thallium-201 (201Tl), 3 with nitrogen 13-ammonia (N13) and 20 with 99mTc-tetrofosmin (99mTc) used as radiotracer]. The interval between onset of acute symptoms and single photon emission computed tomography (SPECT) or PET scan imaging varied from 1 to 15 d; in two studies this interval was not clearly reported. In three out of fourteen patients, an early perfusion image demonstrated a decreased 201Tl uptake in a small area of the apex detected with a semiquantitative method. The hypoperfused apex region did not correlate with an angiographic obstructive epicardial coronary stenosis. Normal myocardial perfusion was observed in the other cases studied with 201Tl or 99mTc. Cimarelli et al[12] found, at the analysis of left ventricle motion, apical akinesis or severe hypokinesis in TTC and severe mid ventricular hypokinesis with preserved apical and basal function in patients with a particular variant of TTC, called “mid-ventricular ballooning syndrome”. The quantitative perfusion analysis using the ammonia tracer in PET scan in patients admitted for TTC showed a slight reduction in tracer uptake after adenosine in the apex and apical segments of the left ventricle that normalized at rest. Myocardial blood flow (MBF) and coronary flow reserve (CFR) were evaluated by Feola et al[11] in three female patients obtaining a significant reduction in MBF at rest in the apical segments in comparison to the mid-ventricular and basal LV segments. CFR also reduced in apical and, in 2 cases out of 3, in mid-ventricular segments in comparison to the basal LV segments (Figure 1).
A follow-up SPECT was provided in four patients three months after the acute onset of TTC. In one of them, studied with 201Tl radiotracer, apical abnormality returned to normal. In the other three patients, studied with N13 and stress/rest perfusion scan using adenosine, both MBF and CFR reduction observed in the acute phase recovered, demonstrating as the impairment of MBF and, above all, CFR seemed to be transient and localized in TTC.
The same population studied by Feola et al[11] was also analysed with cardiac magnetic resonance imaging (MRI) that confirmed, in the acute phase, the segmental disturb of LV contraction (hypo/akinesia in apex and periapical segments) determining an important systolic dysfunction. In none areas a delayed enhancement after gadolinium injection either in the acute phase or at 3 mo’ follow up phase emerged, suggesting that the damage in the dysfunctional areas was transient and did not include necrotic tissue.
Myocardial PET using fluorine 18 fluorodeoxyglucose (FDG) was totally used in 39 patients with acute TTC as an indicator of myocardial metabolism. Yoshida et al[10] studied 8 TTC patients, obtaining in 7 out of 8 patients a pattern of severe and diffuse reduced 18F-FDG uptake in left ventricle. Of these seven patients, six exhibited a LV dysfunction at echocardiography examination. Moreover, they observed that the extension of the metabolic defect was much larger and more severe than the perfusion defect with 201Tl. In the other three studies[11-13], most of patients (20/24, 83.3%) showed a severe reduction of metabolic activity in the apex and periapical segments in the acute phase of TTC. This reduction of FDG uptake in the dysfunctional areas recovered at follow-up, following the normalization of left ventricle function. In fact, considering the entire population of TTC studied with PET, FDG-PET was repeated in 20 patients, in a follow-up period variable from 30 to 180 d: in 18/20 cases a complete recovery of FDG uptake emerged, confirming the hypothesis of a complete reversibility of the FDG impairment. The severe reduction of FDG uptake in the dysfunctional myocardial segments has not been completely understood and might be related to a partial volume effect in enlarged, impaired areas or to a specific metabolic impairment of the glucose utilization.
Cimarelli et al[12] observed in 8 TTC patients, underwent myocardial 123I-mIBG SPECT, a severe reduction/absence of mIBG uptake in the dysfunctional segments of apical or mid-ventricular regions. Four out of eight patients repeated 123I-mIBG SPECT six months after acute symptoms, demonstrating an improvement of tracer uptake in apical segments. Furthermore, analysing the 4 patients underwent myocardial SPECT, 123I-mIBG SPECT and 18F-FDG PET, they concluded that dysfunctional but normally perfused LV segments were characterized by severe decrease of 123I-mIBG and 18F-FDG uptake. Moreover, the distribution of 123I-mIBG and 18F-FDG uptake defects was largely overlapping. These data added an interesting element in the pathophysiology of TTC syndrome, highlighting as the dysfunctioning segments presented a pattern of severe denervation and metabolic glucose uptake that ameliorated and even normalized in the follow-up following the improvement of myocardial function.
TCC is a severe, reversible form of left ventricular dysfunction in patients with normal coronary angiography. Because the onset of this syndrome is very similar to that of an ACS, it is really important to differentiate one from the other, also considering the different treatment that should be undertaken.
TTC is often preceded by emotional or physical stress and this have suggested the hypothesis of catecholamine-mediated multivessel epicardial spasm, microvascular coronary spasm[2] or possible direct catecholamine-mediated myocyte injury[6] as possible pathophysiological mechanisms. The multivessel spasm may be responsible for a self-limited ischemic event able to generate stunned myocardium but not long enough to cause myocardial necrosis[7].
Nuclear medicine techniques have been successfully employed in the differential diagnosis of TTC from ACS and have demonstrated a discrepancy between normal perfusion and reduced 18F-FDG uptake in TTC[8,9]. This association is commonly known as “inverse metabolic-perfusion mismatch” and represented a transient metabolic disorder at the cellular level, demonstrated by evidence of tissue’s impairment metabolism in the dysfunctioning left ventricle with preserved MBF at rest[10-13]. Moreover, PET imaging, using the quantitative analysis, confirmed the light impairment of MBF in dysfunctioning LV segments in the acute phase together with a clearly reduction of CFR after vasodilator stimuli. These MBF modifications recovered completely in the follow-up, probably justifying the favourable clinical prognosis in those patients. Further studies and a larger patient population are needed to confirm these findings.
Takotsubo cardiomyopathy (TTC) is a severe, reversible form of left ventricular dysfunction that can mimic symptoms similar to acute myocardial infarction (MI) in absence of coronary artery stenosis. Because the onset of this syndrome is very similar to that of an acute coronary syndrome (ACS), it is really important to differentiate one from the other, also considering the different treatment that should be undertaken.
Recently, nuclear medicine techniques, such as myocardial positron emission tomography (PET), have demonstrated to delineate this syndrome from acute coronary artery disease, also offering a new pathophysiological explanation for this particular syndrome. This clinical review aimed to summarise the most significant experiences on use of myocardial positron emission tomography in TTC.
Recent reports on use of PET in TTC have highlighted a discrepancy between normal myocardial perfusion and reduced glucose utilization in this particular syndrome, commonly known as “inverse flow metabolism mismatch”. This suggests that TTC represents a transient metabolic disorder on the cellular level, rather than a structural contractile disease of the myocardium, due to a transient decrease of glucose metabolism that might be related to a coronary microcirculation impairment. In an other study this interesting finding has been associated to a severe denervation of the myocardium.
Nuclear medicine techniques have been successfully employed in the differential diagnosis of TTC from ACS and have offered a new pathophysiological explanation for this particular syndrome, useful in improving the knowledge of this poorly understood syndrome.
Takotsubo cardiomyopathy: syndrome, mainly reported after sudden emotional or physical stress, characterized by chest pain, changes on electrocardiography, limited release of myocardial enzyme and reversible wall-motion abnormalities involving apical and midportion of left ventricle. PET: a nuclear medicine, functional imaging technique that can use biologically active molecules (e.g., glucose); their uptake, showed in the tissue, indicate the presence of metabolic activity.
In this article the authors aimed to summarize the results of four articles on the use of myocardial PET in Takotsubo cardiomiopathy. It is a good work.
P- Reviewer: Biondi-Zoccai G, Hosoda T, Imbriaco M, Patanè S S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1020] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 2. | Abe Y, Kondo M, Matsuoka R, Araki M, Dohyama K, Tanio H. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol. 2003;41:737-742. [PubMed] |
| 3. | Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases]. J Cardiol. 1991;21:203-214. [PubMed] |
| 4. | Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 990] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 5. | Nef HM, Möllmann H, Elsässer A. Tako-tsubo cardiomyopathy (apical ballooning). Heart. 2007;93:1309-1315. [PubMed] |
| 6. | Mann DL, Kent RL, Parsons B, Cooper G. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992;85:790-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 562] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 7. | Tsubokawa A, Lee JD, Shimizu H, Nakano A, Uzui H, Takeuchi M, Tsuchida T, Yonekura Y, Ishii Y, Ueda T. Recovery of perfusion, glucose utilization and fatty acid utilization in stunned myocardium. J Nucl Med. 1997;38:1835-1837. [PubMed] |
| 8. | Bybee KA, Murphy J, Prasad A, Wright RS, Lerman A, Rihal CS, Chareonthaitawee P. Acute impairment of regional myocardial glucose uptake in the apical ballooning (takotsubo) syndrome. J Nucl Cardiol. 2006;13:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Obunai K, Misra D, Van Tosh A, Bergmann SR. Metabolic evidence of myocardial stunning in takotsubo cardiomyopathy: a positron emission tomography study. J Nucl Cardiol. 2005;12:742-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Yoshida T, Hibino T, Kako N, Murai S, Oguri M, Kato K, Yajima K, Ohte N, Yokoi K, Kimura G. A pathophysiologic study of tako-tsubo cardiomyopathy with F-18 fluorodeoxyglucose positron emission tomography. Eur Heart J. 2007;28:2598-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Feola M, Chauvie S, Rosso GL, Biggi A, Ribichini F, Bobbio M. Reversible impairment of coronary flow reserve in takotsubo cardiomyopathy: a myocardial PET study. J Nucl Cardiol. 2008;15:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Cimarelli S, Sauer F, Morel O, Ohlmann P, Constantinesco A, Imperiale A. Transient left ventricular dysfunction syndrome: patho-physiological bases through nuclear medicine imaging. Int J Cardiol. 2010;144:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Rendl G, Rettenbacher L, Keinrath P, Altenberger J, Schuler J, Heigert M, Pichler M, Pirich C. Different pattern of regional metabolic abnormalities in Takotsubo cardiomyopathy as evidenced by F-18 FDG PET-CT. Wien Klin Wochenschr. 2010;122:184-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |