INTRODUCTION
Acute aortic syndrome (AAS) is a term used to describe a constellation of emergency aortic conditions requiring prompt diagnosis and treatment. These inter-related conditions include aortic dissection (AD), intramural haematoma (IMH) and penetrating atherosclerotic ulcer (PAU)[1]. In addition, unstable thoracic aortic aneurysm at high risk for rupture may also be considered an AAS. This syndrome has an estimated incidence of (2-3.5)/100000 per year[2]. The most common risk factors are hypertension and genetic conditions of the connective tissue such as Marfan’s syndrome. The clinical presentations of the various entities of AAS are essentially indistinguishable from each other with severe chest or abdominal pain being the most common symptom. Clinical suspicion of AAS should herald prompt diagnostic imaging as mortality from AAS increases by approximately 1%-2% per hour[3]. MDCT typically with electrocardiographic gating (ECG-gated MDCT) is the technique of choice for diagnosis of AAS due to the rapid acquisition times and ready availability in the emergency department[4]. Other diagnostic imaging modalities include conventional and transoesophageal echocardiography and magnetic resonance imaging (MRI), that are usually reserved for problem solving or if there is contraindication to the use of iodinated contrast[1,2,5]. This review will focus on MDCT in the diagnosis of AAS. MDCT protocols will be discussed along with the discriminating and overlapping radiological diagnostic features of AAS.
MDCT PROTOCOL FOR AAS
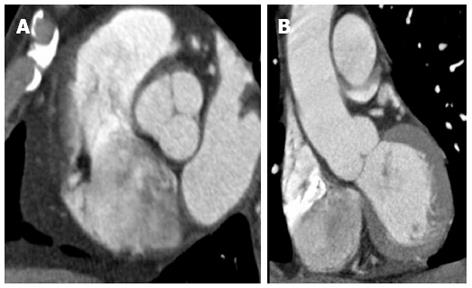
MDCT angiography with electrocardiographic (ECG) gating enables the evaluation of AAS with reduced pulsation artefacts (Figure 1) in the ascending aorta compared to non-gated MDCT angiography[6]. The ECG-gating can either be performed prospectively (scanning performed at a specified segment of the cardiac cycle), or retrospectively (scanning performed throughout the cardiac cycle but data gathered in a specified segment of the cardiac cycle is selected retrospectively for generating images). A retrospective acquisition allows increased scope for correction of artefacts from dysrhythmias or motion, but comes at the cost of increased radiation exposure[1,6]. ECG-gating with motion-free images allows for improved assessment of the ascending aorta, aortic annulus, sinuses of Valsalva and coronary arteries[6,7]. There is also improved depiction of the site of primary intimal tear, extent of the intimomedial flap and involvement of the aortic branches. Studies have also shown a reduction in radiation dose of as much as 45%-50% using ECG-gated high pitch CT compared to non-ECG-synchronised standard-pitch CT[8,9]. Although ECG-gating is preferred, its absence rarely precludes the diagnosis of clinically significant acute aortic conditions if an experienced reader is interpreting the non-gated CT aortogram.
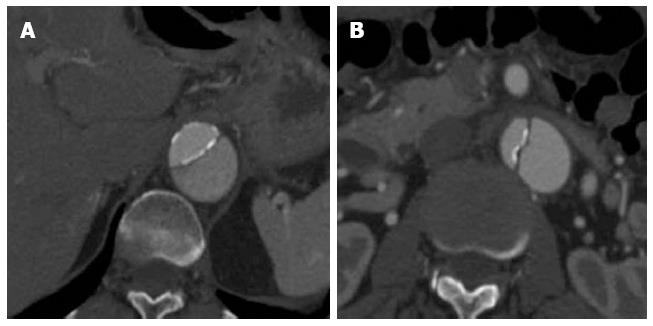
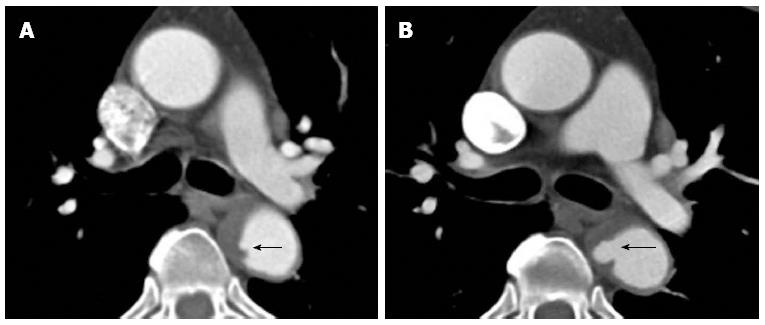
Figure 1 Cardiac-gated computed tomography aortogram in a 55-year-old with chest pain and suspected acute aortic syndrome.
A: Axial oblique; B: Coronal reconstructions. No dissection or aneurysm was detected. Retrospective reconstruction at 78% of the cardiac cycle allowed for accurate evaluation of the aortic root and valve cusps in both axial oblique and coronal reconstructions with no pulsation artefacts.
The details of the scan protocol vary according to the scanner system being used. As an illustration we have described here the protocol of ECG-gated CT angiography on a 64-section helical CT system (Somatom Sensation, Siemens, Erlangen, Germany) performed in our department. Z-axis coverage is determined on a planning topogram from the root of the neck to the common femoral artery bifurcation. A 100 mL bolus injection of intravenous contrast (Iohexol 300 mg/mL, Nycomed) is injected at a rate of 3-4 mL a second. Bolus tracking is used to trigger scanning when the attenuation of the descending aorta reaches 150 HU. Retrospective gating is typically used with images reconstructed at 78% of the R-R interval. The section thickness is 0.6 mm with 3 mm reconstructions in the axial, coronal and sagittal oblique planes sent to the PACS for reporting. Additional post processing is performed by the reporting radiologist when clinically indicated for three-dimensional reconstructions to obtain volume rendered images, maximum intensity projections and shaded surface display. Delayed phase images are useful in selected cases of suspected aortic rupture or in cases of dissection to determine the opacification of both the lumina. Although a contrast enhanced CT angiogram is the standard of care, a non-enhanced CT that precedes the angiogram is useful in AAS to evaluate for IMH, which can progress to frank dissection. NECT can also be useful to assess for secondary signs of aortic rupture such as hyperdense, haemorrhagic pericardial, pleural or mediastinal fluid collections. In patients with known allergy to iodinated contrast medium or at high risk of contrast induced nephropathy, the preliminary information obtained through a non-enhanced CT scan may sometimes suffice to make the further clinical decision.
AD
The most common pathology in AAS is AD that begins as a tear or ulcer in the aortic intima allowing blood to penetrate and disrupt the aortic media[2]. Haemorrhage may also occur de novo within the media due to rupture of the vasa vasorum leading to dissection. Irrespective of the initiating cause AD involves separation of the aortic layers and formation of a false lumen[10]. The false lumen is separated from the true lumen by an intimomedial flap (Figures 2-7). The dissection may then propagate in an antegrade and/or less likely retrograde fashion with a potential to fenestrate back into the aortic lumen or rupture out through the adventitia with life threatening consequences[11]. Dissection can extend into aortic branches and when it involves major visceral arteries, it can lead to catastrophic consequences such as a cerebrovascular event, bowel ischemia, acute renal failure, limb gangrene etc.
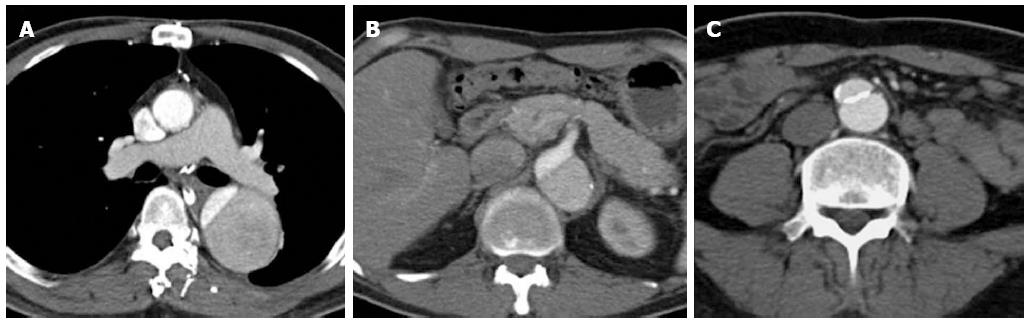
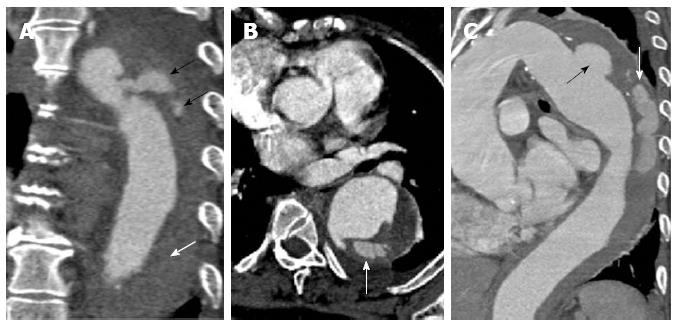
Figure 2 A 47-year-old gentleman with Stanford type B aortic dissection.
The dissection flap is flat in all the computed tomography angiographic images. The ascending aorta is not involved and the true lumen is smaller in calibre and shows early and more intense enhancement than the false lumen at the level of the right pulmonary artery (A). The small calibre true lumen gives rise to the coeliac axis (B) and is outlined by atherosclerotic calcifications (C).
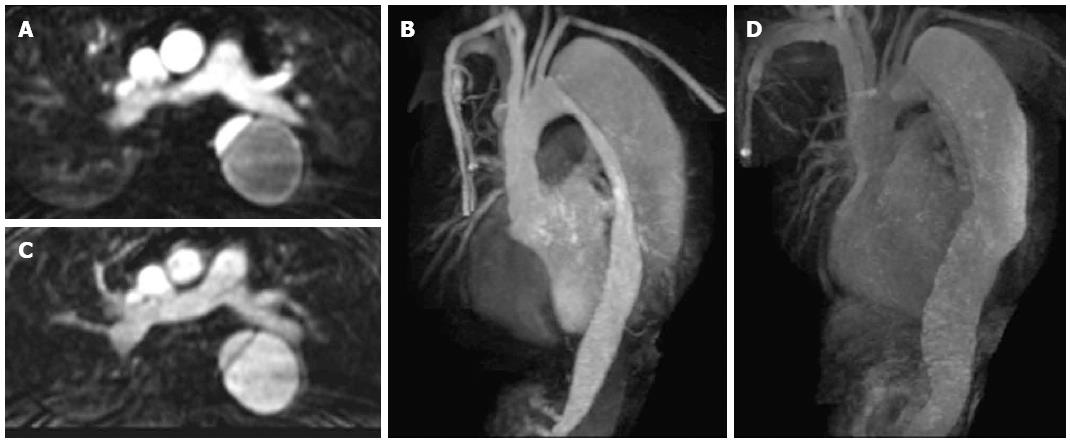
Figure 3 Axial contrast enhanced magnetic resonance aortogram of the same patient as in Figure 2 with type B aortic dissection.
In the arterial phase image (A: Axial; B: Coronal 3D reconstruction; 30 s post injection) the true lumen is of small calibre and shows early intense contrast enhancement compared to the larger false lumen. In the second delayed phase (70 s post injection) the enhancement between the lumens becomes more similar (C: Axial; D: Coronal 3D reconstruction).
Figure 4 A 35-year-old gentleman with Marfan’s syndrome and a type B aortic dissection.
Axial images (A and B) from a computed tomography aortogram reveals an intimomedial flap (arrows) with strands of incompletely sheared aortic media or “cobwebs” seen in the descending thoracic aorta (dashed arrows).
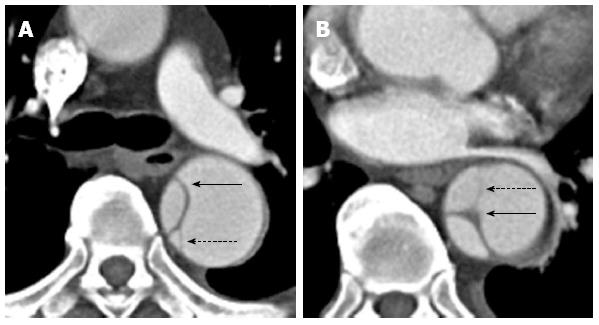
Figure 5 A 59-year-old lady with a type B aortic dissection.
Axial images from a computed tomography aortogram show atherosclerotic calcifications outlining the true lumen at the lower thoracic aorta (A). The true lumen is of smaller calibre and shows early and more intense enhancement than the false lumen at this level. More inferiorly at the level of the left renal vein (B), eccentric intimomedial flap calcification (note the calcification along the true luminal aspect of the flap) is exquisitely demonstrated.
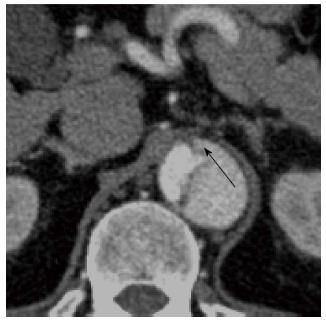
Figure 6 A 45-year-old with a type B aortic dissection.
An axial image from a computed tomography aortogram at the upper abdominal aorta shows the “beak” sign (arrow): note the acute angle between the dissection flap and the outer wall of the larger calibre false lumen. The “beak” or space formed by the acute angle is filled with high-attenuation contrast-enhanced blood in this case but it may stay unopacified when filled with clots.
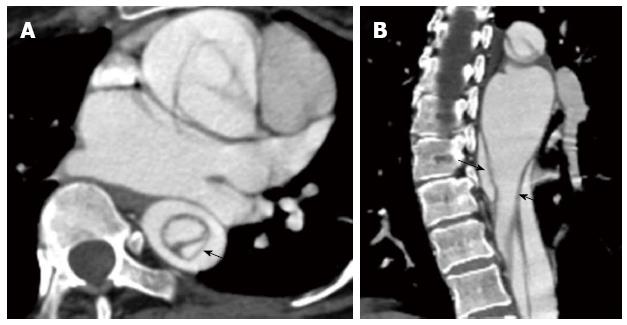
Figure 7 A 37-year-old gentleman with Marfan’s syndrome and a type A aortic dissection.
The axial image at the level of the left atrium shows an intimointimal intussusception type dissection in the descending thoracic aorta with the true lumen surrounded by the false lumen (A and B; intimomedial flap highlighted by the arrows).
Risk factors for dissection include hypertension, smoking, trauma (typically road traffic accidents), vascular inflammation (e.g., Takayasu’s arteritis) or infection (e.g., syphilis) and genetic connective tissue disorders (e.g., Marfan’s and Ehlers-Danlos syndromes). Propagation of the blood within the media to form a dissection requires pre-existing medial degeneration or cystic medial necrosis, which leads to a weakened aortic wall and represents the end point of many of the risk factors listed above[2,10].
Two anatomical classification systems exist for AD: De Bakey and Stanford. The Stanford classification is most commonly used as it has a direct bearing on the subsequent therapy. Stanford type A dissections involve the ascending aorta (proximal to the origin of the brachiocephalic artery origin) with or without aortic arch or descending aorta (distal to the left subclavian artery origin) involvement[2]. Type A dissections are typically treated as a surgical emergency. Mortality is estimated at 20% in the first 24 h without immediate surgical management and approximately 40% in the first week[12,13]. Complications of type A dissection include aortic regurgitation, aortic rupture, tamponade and compromise of the arch branches or coronary arteries leading to myocardial infarction. Type B constitutes all those dissections that do not involve the ascending aorta. It more often involves the descending aorta and is typically treated medically with anti-hypertensive medications. Without adequate management, uncomplicated type B dissections have an estimated 10% mortality at 1 mo in comparison to 50% for type A dissections[2,12]. Type B dissections can be complicated by branch disruption and end organ malperfusion, progression to type A dissection and rupture. The role of endovascular repair is well established in Stanford type B lesions while in select situations it may be performed even in type A lesions[14]. The aim of endovascular intervention is to occlude the intimal tear allowing for false lumen thrombosis and regression, typically by placing an aortic endograft. End-organ ischaemia and malperfusion may also be improved by placement of branch stents and the use of aortic fenestrations to relieve compression of the true by a distended false lumen[1,14,15].
MDCT angiography has a sensitivity and specificity of close to 100% for diagnosis of acute AD[16,17]. Cardiac synchronisation should be performed to limit pulsation artefacts in the ascending aorta, which on non-gated MDCT are often the cause of false-positive findings of a thoracic dissection. In AD, the role of MDCT angiography can be summarized as to identify[1,14,18]: (1) Sites of primary entry and re-entry; (2) Intimomedial flap, false and true lumen morphology along with the presence of calcifications and thrombus; (3) Extent of the dissection and involvement of the ascending and descending aorta; (4) Evidence of rupture; (5) Involvement of the aortic valve, coronary and aortic arch branches; (6) Abdominal aortic branch patency and evidence of end-organ malperfusion; and (7) Morphology and diameter of the aorta along with the patency, size and tortuosity of the iliac and femoral arteries (useful for endovascular treatment planning).
The intimomedial flap forms a double-barrelled aorta and separates the true from the false lumen[4]. This is seen in approximately 70% of cases on MDCT angiography and with three dimensional reconstructs the complex, often spiralling nature of the dissection can be seen in better detail[19]. Other appearances include a circumferential intimomedial flap due to complete dissection of the intima. The true lumen takes on a cylindrical or filiform shape and this may result in an intimointimal intussusception producing a “windsock” appearance (Figure 7). Differentiation between the false and true lumen is useful for endovascular treatment planning as an endograft should be placed within the true lumen[1,15].
The true lumen can usually be identified by tracing back or forth from an uninvolved portion of the aorta; this may be difficult if the aortic root is involved proximally or the dissection extends into the iliac vessels distally[1,20]. In these cases the most useful imaging signs include a false lumen that is larger in calibre than the true lumen and the “beak”sign[20]. In most cases of acute dissections the false lumen is larger in calibre than the true lumen. This is likely due to sustained systolic pressure in the false lumen exceeding that of the true lumen leading to compression. The “beak” sign (Figure 6) is only seen in the false lumen and is often present in most patients with dissection. It is usually noted both in acute and chronic dissection and is defined as the presence of an acute angle between the dissection flap and the outer wall of the false lumen; the space formed by the acute angle could be filled with high-attenuation material (contrast-enhanced blood) or low-attenuation material (hematoma)[21,22]. Other less common and less reliable signs for differentiation between the true and false lumen have been documented in the literature. The false lumen may contain thrombus and fine ribbons of low attenuation (“cobwebs”), which likely represent strands of incompletely sheared aortic media (Figure 4)[22]. Differential contrast enhancement between the true and false lumen is not unusual with the true lumen often showing early opacification with contrast in the arterial phase, while the false lumen starts opacifying later in the portovenous and delayed phase (Figure 3)[23]. The surface of the dissection flap that is calcified generally involves the intimal surface of the true lumen; this is described as eccentric flap calcification. The side of the flap subtending the false lumen will have soft tissue attenuation (Figure 5). In acute dissections the lumen with outer wall calcification tends to be the true lumen. Along with eccentric flap calcification this is due to the presence of atherosclerotic calcified plaque in the aortic intima of the true lumen (Figures 2 and 5). A less useful sign is the curvature of the intimomedial dissection flap. Researchers have found equal incidence of curvature of the flap towards or away from the false lumen. However, in chronic dissections the dissection flap is more likely to be flat. This is likely due to interval healing and development of fibrosis and thickening leading to reduced flap mobility[19,24].
A thrombosed dissection and an aneurysm with mural thrombus may have a similar appearance. The following features will aid the differentiation: (1) Mural thrombus would not have a spiralling pattern like a dissection; (2) Mural thrombus tends to have an irregular surface rather than the smooth surface of the dissection flap; and (3) in an aneurysm with mural thrombus, the calcium will be along the outer wall while in the thrombosed false lumen it would be along the flap. A thrombosed dissection can be indistinguishable from intramural hematoma; however the differentiation is of only academic interest since management stays the same in both conditions.
AORTIC IMH
Aortic IMH is considered a variant of dissection and is characterised by bleeding of the vasa vasorum in the aortic media without intimal tear (Figure 8). It accounted for 5.7% of AAS in the International Registry of Acute Aortic Dissection (IRAD)[12,25]. The vasa vasorum may spontaneously rupture or haemorrhage may occur due to an intimal defect/PAU[1]. IMH may progress to aneurysmal dilatation and rupture (20%-45%), provoke a secondary intimal tear that can progress to dissection (28%-47%), or may regress (10%)[26]. The most common site of IMH is in the descending aorta (approximately 2/3rds) and risk factors are similar to those for dissection with hypertension being the most prevalent one[2,25]. The clinical presentation of IMH is also similar to aortic dissection; chest pain is the usual symptom in ascending aortic IMH while back pain accompanies descending aortic IMH[27]. IMH is classified along the lines of dissection into Stanford type A and B categories[1]. Stanford type A IMH is typically treated surgically, with 30 d mortality of 14% vs 36% for those treated medically[28]. In contrast, type B IMH is initially treated medically with antihypertensive medications with a 30-d mortality of 8%[27]. However, close CT follow-up is recommended in these patients as aneurysmal dilatation or progression to frank dissection of the aorta will require emergency open surgical or endovascular repair[1,27].
Figure 8 A 61-year-old lady with severe chest pain, breathlessness and a prior history of atrial flutter.
Computed tomography aortogram in the emergency department shows hyperdense, eccentric wall thickening of the aortic wall consistent with an intramural haematoma (A, white arrow). A concurrent contrast enhanced axial image at the same level shows no leakage of contrast into this thickened aortic wall (B). The intramural haematoma shows partial resolution at 1 mo (C) and complete resolution at 6 mo (D).
On MDCT, IMH is seen as a hyperdense, crescent shaped region within the aortic wall on non-enhanced CT[1,4,29]. No enhancement is seen and by definition no intimomedial flap or tear should be visualized[30]. Unlike a dissection that spirals down, IMH tends to have constant circumferential relationship with the aortic lumen. With the higher resolution of modern imaging technologies, small projections or ulcerations can sometimes be seen communicating between the aortic lumen and the IMH[4,31]. Studies based on non-cardiac gated CT showed a sensitivity of greater than 96% for the detection of IMH using both unenhanced and contrast enhanced CT[1,32].
PAU
This entity is a manifestation of advanced, severe atherosclerotic disease that leads to disruption of the aortic intima with extension of blood into the aortic media (Figure 9)[1,2,4]. This is in contrast to the underlying pathological process in AD, which usually results from disease of the media without any underlying atherosclerotic intimal plaque[33,34]. Disruption of the media by the deep ulceration may lead to vasa vasorum haemorrhage and IMH producing acute chest or back pain with risk of progression to aortic dissection[25,35]. PAU can also penetrate beyond the aortic media leading to focal outpouching of the adventitia, producing a pseudoaneurysm with risk of frank rupture[4,29]. Saccular aortic aneurysms may represent the end point of such PAU[36].
Figure 9 Computed tomography aortogram in a 75-year-old man shows an intramural hematoma (note the eccentric wall thickening in the descending aorta) with intimal surface defect (A: arrow).
At one year follow up it has progressed into a frank penetrating ulcer (B: arrow).
PAU most commonly occurs in the descending thoracic aorta (90%) and is associated with type B IMH in the majority of cases. It is seen as a focal contrast-filled outpouching into the aortic wall on MDCT (Figure 10)[37,38]. Other MDCT features include overhanging edges with a focal bulge of the external aortic contour. It can be differentiated from a benign atherosclerotic ulcer as the latter would not have contrast extending into the aortic media and would not be associated with an IMH[39]. Given the relationship with IMH the use of unenhanced images can improve visualisation of crescentic high attenuation haemorrhage in the aortic wall along with displacement of intimal calcifications[1,22].
Figure 10 A 62-year-old with acute chest pain.
A computed tomography aortogram (A-coronal) shows a penetrating atherosclerotic ulcer at the proximal descending aorta with contrast seen within the aortic media (black arrows) and non-opacifying hyperdensity throughout the rest of the descending thoracic aortic wall compatible with intramural haematoma (white arrow). The axial image (B) shows the contrast extending within the aortic wall and splitting the same. The sagittal oblique reconstruction (C) gives a better demonstration of the penetrating atherosclerotic ulcer at the distal aortic arch (black arrow) with intramural hematoma and progressing into an aortic dissection (white arrow).
The natural history and management of patients with PAU remains controversial. PAU tends to have a worse prognosis than dissection with increased incidence of rupture even if the ulcer is limited to the descending thoracic or abdominal aorta[25]. Most patients are symptomatic although in approximately a quarter of cases PAU may present incidentally[1,40]. The condition typically occurs in elderly patients with multiple co-morbidities and risk factors for atherosclerosis[41]. These factors, especially underlying coronary and peripheral arterial atherosclerosis, may preclude open surgical repair in this group of patients[42]. Open surgical repair, typically with a synthetic graft is performed in patients with ascending aortic PAU (these are rare) and in patients with haemodynamic instability and high risk of rupture, e.g., rapid enlargement of the lesion[1,18]. Endovascular graft placement in patients with symptomatic PAU is an alternative with potentially lower morbidity and mortality[43-45]. Sometimes, conservative management with antihypertensive therapy may be adequate or may be the best option in PAU especially in asymptomatic patients with involvement of the descending aorta and in those who are poor candidates for any form of invasive treatment. The medically treated patients need at least an annual follow-up to assess for disease progression, which has a strong likelihood in initially symptomatic patients[19,44,46,47].
UNSTABLE THORACIC AORTIC ANEURYSM
Thoracic aortic aneurysms are relatively uncommon and defined as the permanent dilatation of the aorta to more than 150% of its usual diameter or greater than 5 cm. True aneurysms tend to be fusiform and involve all three layers of the aortic wall[48]. False or pseudoaneurysms are typically saccular and often limited by the adventitia alone (Figure 11). The most common cause for both types is atherosclerosis (approximately 70%), although false saccular aneurysms often arise following surgery, trauma or due to an infective/inflammatory aetiology[1,19,49].
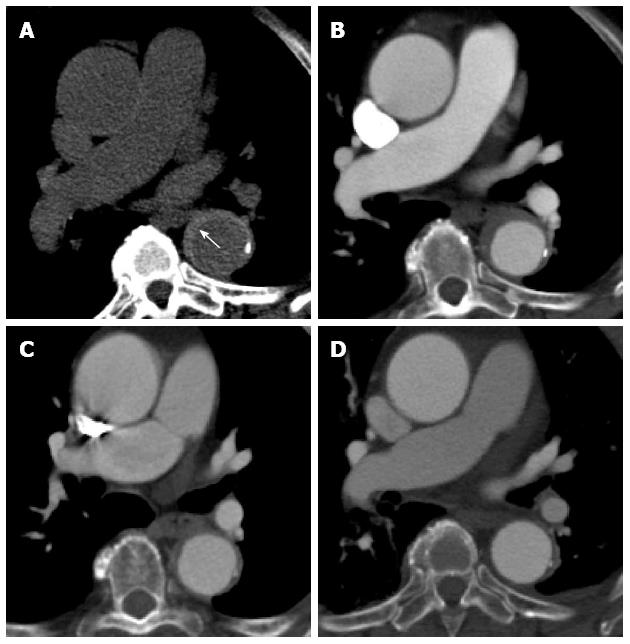
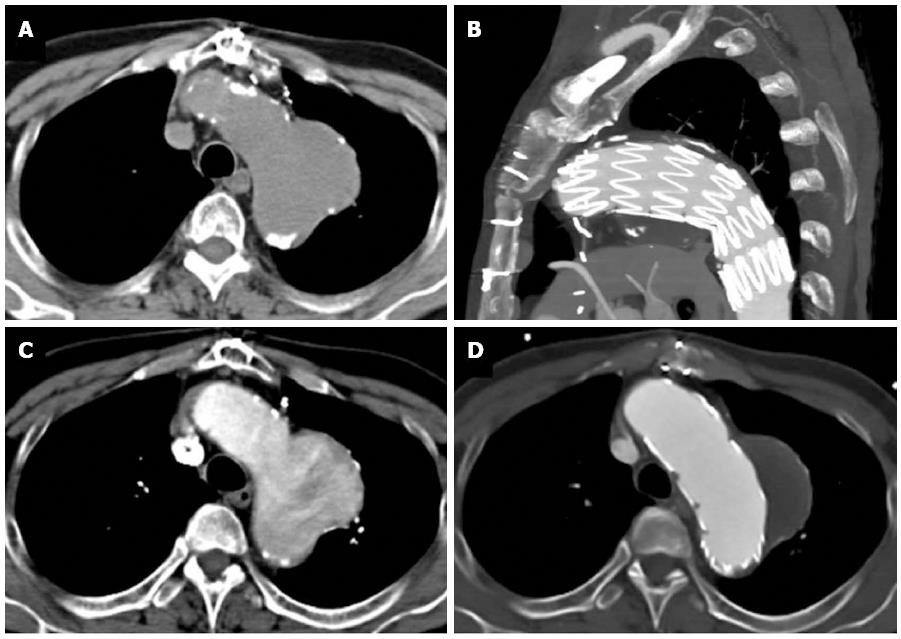
Figure 11 A 68-year-old with chest pain and long standing hypertension.
A saccular aneurysm is seen arising from the lateral aortic arch just distal to the origin of the left subclavian artery on this computed tomography aortogram. Atherosclerotic calcifications are seen at the periphery of the aneurysm (A) and there is heterogeneous contrast opacification secondary to turbulent flow on the arterial phase (B). No evidence of rupture is apparent. The aneurysm was excluded using an aortic stent graft as seen in the sagittal plane (C). The excluded sac has completely thrombosed with no endoleak as seen on an axial image (D).
Thoracic aortic aneurysms are often clinically asymptomatic but they are considered unstable when showing rapid increase in size or evidence of contained or impending rupture on MDCT. Unstable thoracic aortic aneurysms may be considered an AAS, especially when symptomatic as they are clinically indistinguishable from dissection, IMH or PAU[4]. The risk of rupture is related to the diameter of the aneurysm sac, with a significantly higher risk for those in the ascending aorta measuring greater than 6 cm, and 7.2 cm in the descending aorta[50]. On serial CT scans an annual increase in diameter of the thoracic aortic aneurysm of at least 1 cm is also considered a sign of increased likelihood of rupture[19,51]. Other morphological MDCT signs of impending rupture should also be taken into account for therapeutic decision making. These include associated IMH with a high attenuating crescent in the aortic wall, discontinuity of circumferential intimal atherosclerotic calcifications within a fusiform aneurysm, a “draped” aortic appearance conforming to the anterolateral contour of an adjacent vertebral body, eccentric or nipple like contour to the aorta, and poor visualisation of the posterior aortic wall[1,19,52,53]. Frank rupture of a thoracic aortic aneurysm on MDCT is suggested by peri aneurysmal fat stranding, mediastinal haematoma, haemothorax most commonly on the left, hemopericardium or pericardial effusion and evidence of haemodynamic compromise, e.g., collapsed IVC[1,19].
Therapy should be considered for all symptomatic aneurysms regardless of size due to the high risk of rupture. Otherwise surgical or endovascular therapy should be considered for ascending thoracic aortic aneurysms measuring greater than 5.5 cm, and those in the descending thoracic aorta measuring greater than 6 cm. Similar to dissection open surgical or hybrid surgical and endovascular techniques are the mainstay of ascending thoracic aortic aneurysm therapy[13]. Smaller aneurysms, typically less than 5.5 cm in diameter can be followed up with serial CT or MRI on an annual basis. However those with underlying genetic connective tissue predispositions to aneurysm formation such as Marfan’s syndrome should be considered for therapy at smaller diameters. Overall decision on when to intervene should be tailored to the individual clinical scenario and involve an experienced multidisciplinary team[1,19,54,55].
OTHER IMAGING MODALITIES AND TECHNIQUES
This article has focused on the use of MDCT in the diagnosis and management of AASs while other imaging modalities including conventional angiography, transoesophageal or transthoracic echocardiography and MRI have a complimentary/alternative role in dealing with this clinical problem. MDCT also has some disadvantages including radiation exposure and potential risk of contrast induced nephropathy, which can be overcome by using alternative methods. Conventional angiography is historically the gold standard for evaluation of AASs. However it is invasive, unable to assess for IMH, and can lead to false negative exclusion of dissection. Transthoracic echocardiography can be used in the initial evaluation of suspected AASs in the emergency department. The technique can sometimes visualise the proximal extent of an aortic dissection but can provide vital secondary evidence of aortic root/valve dysfunction and pericardial effusions or tamponade. Invasive transoesophageal echocardiography can then be considered for further detailed evaluation, and is especially valuable in unstable patients as it can be performed at the bedside. In experienced hands the technique has high sensitivity and specificity for ascending aortic dissection. It has the ability to detect the entry and re-entry site of dissection and can evaluate for the direction of flow within the false lumen. Additional evaluation of the aortic valvular and left ventricular function is useful to exclude proximal extension of the dissection into the aortic root and left coronary artery respectively. Transoesophageal echocardiography is of limited use at the proximal aortic arch since artefacts from air in the adjacent right main bronchus interferes with echocardiographic imaging. Other limitations of the technique include inability to visualise surrounding structures in the mediastinum, and inadequate assessment of anatomical detail required for planning endovascular or hybrid therapy[1-3].
MRI is a useful modality in the assessment of AASs but has limited scope in the emergency setting due to the longer acquisition times and potentially limited availability[1,2]. In stable patients it has a role in confirming IMH, when CT is indeterminate. It can provide high resolution multiphasic images of the aorta without the use of ionising radiation[56]. Real time examination of the aortic root and valve function can also be performed with MRI. This modality is likely to play an increasing role in the follow-up of patients with AASs allowing for a comprehensive assessment. MRI is also the modality of choice for patients with contraindications to CT and iodinated contrast[57].
CONCLUSION
MDCT is the modality of choice for the evaluation of suspected AAS in the emergency setting. High sensitivity, rapid acquisitions and easy access to the technique are its major advantages over transoesophageal echocardiography and MRI. There is a wide base of expertise available in interpreting MDCT with limited interpersonal variability in the inference. Most radiologists and vascular surgeons are comfortable and confident of using this modality in diagnosing and treating AAS. Detailed assessment of the morphology of the aorta using MDCT allows for the classification of the interlinked AASs and helps in determining the treatment. It permits assessment of life-threatening complications associated with acute aortic conditions. It is also useful in identifying some of the other conditions such as acute pulmonary embolism and pneumothorax that can mimic AAS.
P- Reviewers: Kato M, Kirali K Paraskevas KI S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN