Published online Jun 28, 2014. doi: 10.4329/wjr.v6.i6.230
Revised: April 22, 2014
Accepted: May 15, 2014
Published online: June 28, 2014
Processing time: 182 Days and 8.6 Hours
Hydrostatic pulmonary edema is as an abnormal increase in extravascular water secondary to elevated pressure in the pulmonary circulation, due to congestive heart failure or intravascular volume overload. Diagnosis of hydrostatic pulmonary edema is usually based on clinical signs associated to conventional radiography findings. Interpretation of radiologic signs of cardiogenic pulmonary edema are often questionable and subject. For a bedside prompt evaluation, lung ultrasound (LUS) may assess pulmonary congestion through the evaluation of vertical reverberation artifacts, known as B-lines. These artifacts are related to multiple minimal acoustic interfaces between small water-rich structures and alveolar air, as it happens in case of thickened interlobular septa due to increase of extravascular lung water. The number, diffusion and intensity of B lines correlates with both the radiologic and invasive estimate of extravascular lung water. The integration of conventional chest radiograph with LUS can be very helpful to obtain the correct diagnosis. Computed tomography (CT) is of limited use in the work up of cardiogenic pulmonary edema, due to its high cost, little use in the emergencies and radiation exposure. However, a deep knowledge of CT signs of pulmonary edema is crucial when other similar pulmonary conditions may occasionally be in the differential diagnosis.
Core tip: Acute decompensated heart failure (ADHF) is a frequent emergency condition that represents a diagnostic challenge for the emergency physicians. Imaging has a fundamental role in the diagnosis of heart failure, but the efficacy of the diagnostic process is highly dependent from the ability to integrate information drawn from lung ultrasound (LUS), chest radiography and computed tomography (CT). Chest radiography and LUS are the most used diagnostic tools: the first one combining relative low cost with the panoramic view that allows exclusion of many pulmonary conditions that comes into the differential diagnosis; otherwise the second one has higher sensitivity in the diagnosis of the early signs of pulmonary congestion and permit to perform the examination at bedside during the first clinical approach. CT scan is the best method to have a panoramic thoracic view and CT scan is a powerful method but it has many limitations due to costs, availability in emergency situations and relatively high radiation exposure. The modern clinician and radiologist should be aware of the potential and limitations of these diagnostic tools and be prepared to integrate information derived from a correct use of ultrasound, conventional radiology and CT.
- Citation: Cardinale L, Priola AM, Moretti F, Volpicelli G. Effectiveness of chest radiography, lung ultrasound and thoracic computed tomography in the diagnosis of congestive heart failure. World J Radiol 2014; 6(6): 230-237
- URL: https://www.wjgnet.com/1949-8470/full/v6/i6/230.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i6.230
Acute decompensated heart failure (ADHF) is a frequent emergency condition that, often represents a diagnostic challenge for the emergency physicians. Accurate assessment of effectiveness of medical treatment on reducing pulmonary congestion, which is the consequence of elevated cardiac filling pressure, is a basic step for a correct management of patients with ADHF. Most patients hospitalized for ADHF are not submitted to invasive hemodynamic measurements, and clinical improvement relies on change in physical findings, radiologic imaging, and hormone levels. Physical findings of elevated filling pressure are often inadequate and rarely decisive to assess real clinical improvement when considered alone[1-3].
Chest X-ray (CXR) is the traditional first line procedure to assess pulmonary congestion, but interpretation of radiologic signs, such as vascular opacity redistribution and interstitial edema, are often questionable and subjective, while different levels of expertize of the readers may cause high inter-observer variability.
In doubtful cases, lung ultrasound (LUS) has been shown to be of value in assessing pulmonary congestion by the evaluation of vertical comet tail artifacts, named B-lines. These artifacts represent easy-to-acquire and highly reproducible bedside signs of diffuse interstitial syndrome, but their limitation is the low specificity[4,5].
B-lines are caused by a change of the normal balance between the air and fluid pulmonary content, when air is lost and fluid are increased. The multiple and small air-fluid interfaces due to small water-rich structures surrounded by air presenting in the lung periphery, create a reverberation phenomenon represented on the screen by multiple B-lines[6]. However, this phenomenon is irrespective of the cardiogenic or pulmonary origin of the condition.
Concerning thoracic computed tomography (CT) scan, it is rarely used for diagnosing pulmonary congestion, unless highly selected cases where other pulmonary interstitial conditions come into the differential. Signs of hydrostatic pulmonary edema on high-resolution CT should always be recognized, even if edema may sometimes be misdiagnosed and the differential diagnosis not always is easily read by the radiologist. Indeed, sometimes signs of pulmonary congestion on CT imaging represent an unexpected condition on patients investigated for other diseases[7].
This review describes the specific signs of cardiogenic pulmonary edema of these three main imaging techniques and discuss their role in the diagnostic process.
In the acute phase of decompensated heart failure, the early pulmonary alteration is congestion of the vascular bed due to progressive increase of capillary hydrostatic pressure. When pressure increases further and the lymphatic vessels become congested, fluids begin to accumulate in the interstitium around arteries, veins, and airways, and particularly in the interlobular septa. In the early phase, this mechanism protects the lung against the final stage of congestion, that is leakage of fluids into the alveolar spaces, the alveolar edema. The radiologic findings at chest radiography reflect the anatomo-pathologic alterations.
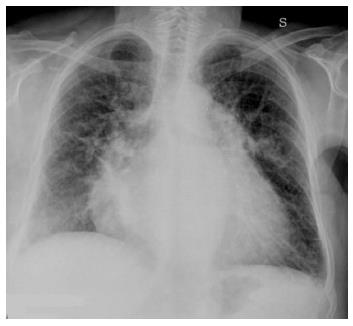
As severity of congestion increases, the sequence of signs visible on chest radiographs are: (1) vascular opacity “redistribution” towards the upper lobes and distention of the upper pulmonary veins; (2) enlargement and loss of definition of hilar structures; (3) septal lines in the lower lung, indicated as Kerley A and B lines; (4) peribronchial and perivascular cuffing with widening and blurring of the margins; and (5) thickening of interlobar fissures with subpleural fluid accumulation (Figure 1)[8,9].
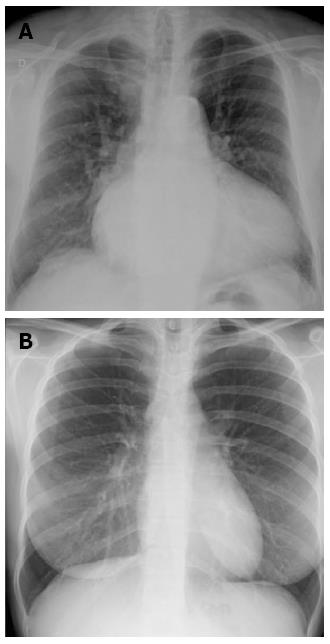
Redistribution, known also as cephalization, occurs only in the setting of chronic pulmonary venous hypertension, very often encountered in mitral stenosis (Figure 2). Cardiomegaly and pleural effusions are adjunctive radiologic findings quite frequently detected in cardiogenic pulmonary congestion. When congestion increases and becomes alveolar edema, chest radiography shows bilateral and usually symmetric parenchymal opacities, with a central or basilar distribution, without air bronchogram[10] (Figure 3).
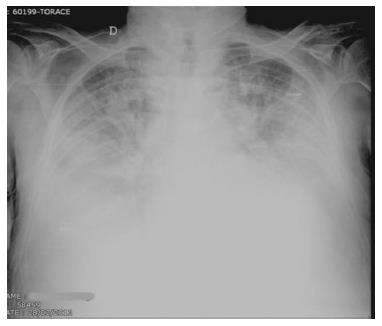
The distribution of alveolar edema may be influenced by gravity. In this case performing the examination in supine or orthostatic position and right or left decubitus, may consistently change the radiologic pattern. Moreover, a coexistent condition of chronic obstructive lung disease may influence irregular distribution of edema as fluids tend to leak in the area of the lung where the structure of the organ is less subverted.
In the emphysematous lung, edema of the alveolar spaces will not be imaged because of alveolar destruction in over-inflated areas, while accentuation of interstitial signs of congestion may still being detected at CXR.
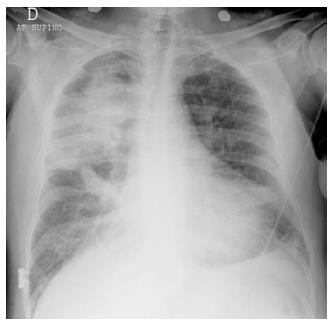
In case of large, acute myocardial infarction (MI) that involves the function of the mitral valve, a regional asymmetric distribution of pulmonary edema may produce atypical radiologic patterns that mimic non-cardiogenic edema or, in some cases, even pneumonia (Figure 4).
This pattern is caused by the flow vector due to mitral regurgitation, which may be massively directed toward the right superior pulmonary vein[11]. However, opacities due to alveolar edema may rapidly change their dimension and even dissolve on the effect of treatment. Thus, radiologic follow-up may sometimes contribute to resolve the diagnostic dilemma.
Signs of pulmonary congestion at chest radiography may even precede clinical symptoms. Conversely, pulmonary edema may be still visible radiographically for hours or even days after hemodynamic recovery[12].
To date, CXR represents the first line imaging exam in patients presenting to the emergency department (ED) complaining of acute dyspnea. The possibility of correct diagnosis at CXR is greater the more severe and prolonged will be pulmonary congestion, because the radiologic signs are more accurate and clearly visible. Relating the diagnosis of cardiogenic pulmonary congestion, CXR is moderately specific (specificity 76%, 83%), but not very sensitive (50%-68%)[13].
Therefore, CXR does not have a direct role in the pathway for the diagnosis of heart failure, where the standard of care is cardiac and LUS. The main reason of this limitation is that CXR is not sensitive enough, because heart failure cannot be ruled out with certainty in the presence of a normal radiologic pattern. However, our opinion is that CXR is highly useful to diagnose alternative diagnoses when they are, together with decompensated heart failure in the differential.
Quite recently, LUS opened new perspectives in the bedside evaluation of pulmonary congestion. Many authors produced a growing number of papers showing the power of LUS in diagnosing pulmonary diseases[14-20].
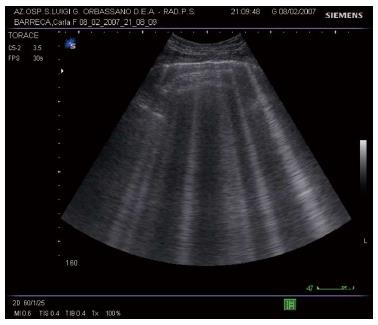
Rather than from technologic progress, development of modern LUS is mainly based on discovering the significance of sonographic artifacts[21]. Particularly, some vertical echogenic linear artifacts, known as B-lines, are simple, noninvasive signs of pulmonary interstitial fluid that can be easily evaluated at bedside. B-lines originate from multiple small subpleural air/fluid acoustic interfaces, due to the fact that air and water are two elements with opposite values of acoustic impedance[22]. This phenomenon is related to the contrast between air-filled and water-rich structures, which generate multiple reverberation of the ultrasound beam that is visualized on the screen as linear vertical artifacts, the B-lines (Figure 5).
In the normally aerated lung, only a very few B-lines can be detected by sonography[23].
When the water content increases and air decreases due to disease, the thickened interlobular septa and fluid into the alveolar spaces cause the appearance of multiple and diffuse B-lines (Figure 6)[4,5].
Any condition of the lung where alveolar air is partially lost and interstitial fluids or cellularity are diffusely increased, causes the appearance of B-lines at LUS. B-lines underlines the so called interstitial syndrome.
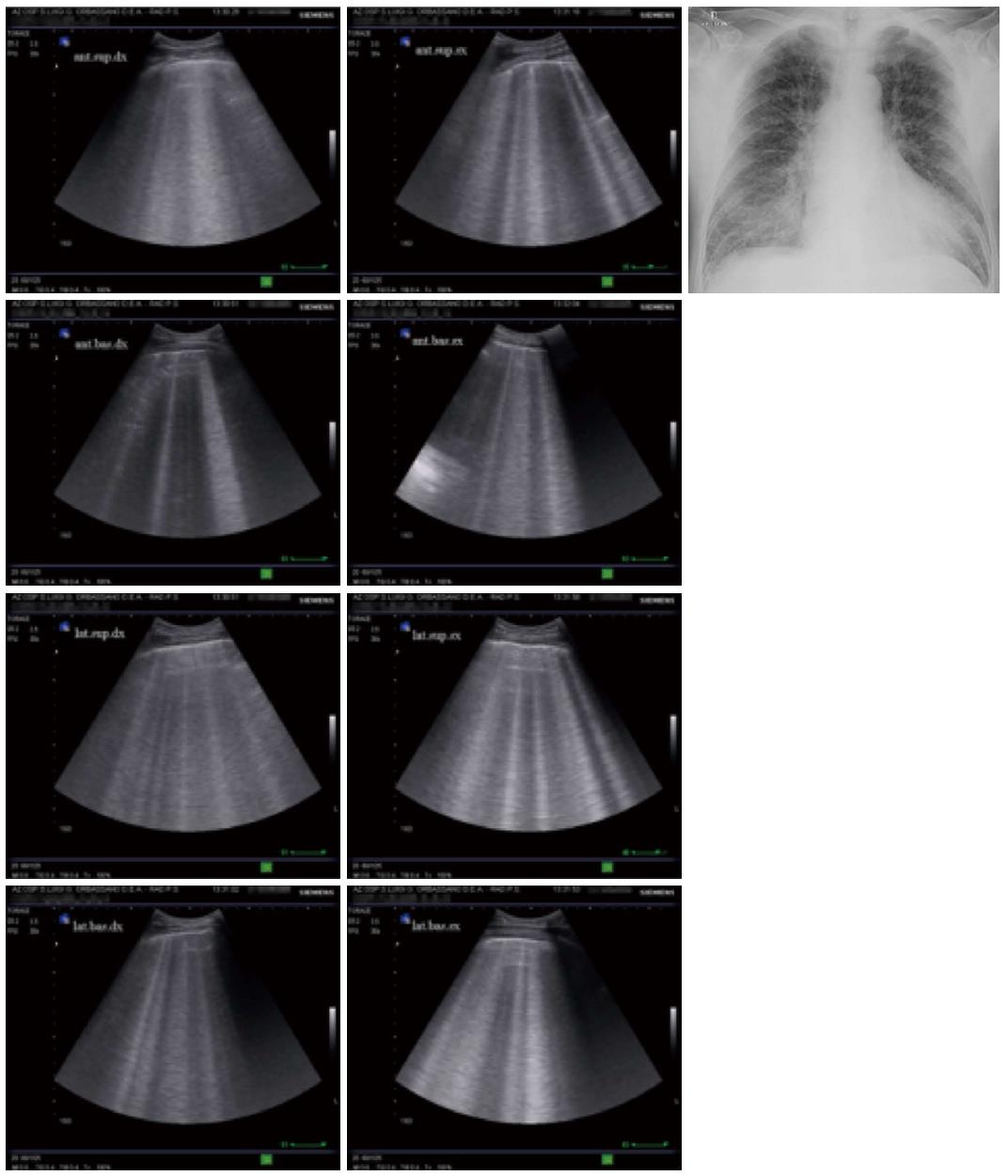
The fundamental technique for diagnosing interstitial syndrome consists of examining the anterior and lateral chest using four intercostal scans per side, corresponding to the upper and inferior areas anteriorly and the upper and basal areas laterally. A positive scan is characterized by a minimum of three B-lines, whereas a positive examination is defined by at least two positive areas per side[5,17] (Figure 6).
Simple detection of B-lines does not allow differentiation of the disease involving the lung interstitium, but other organ ultrasound signs can be used to confirm the diagnosis of pulmonary congestion in decompensated heart failure. For convenience a focused cardiac sonography can be performed using the same probe used for lung examination, looking for global left ventricle function impairment, which will be detected in about 50% of cases with acute decompensated heart failure[24].
Regarding LUS, other signs than B-lines may be evaluated for differentiating similar patterns of interstitial syndrome from cardiogenic and non-cardiogenic causes. These includes evaluation of pleural sliding and irregularities, distribution of B-lines and sub-pleural consolidations. Some studies showed the reliability of these signs in differentiating signs of cardiogenic pulmonary edema from ARDS and pulmonary fibrosis[25].
The primary diagnosis of pulmonary interstitial fluid in the emergency setting is crucial for the differential diagnosis between a cardiogenic and non-cardiogenic respiratory failure. Some studies showed the usefulness of B-lines as a primary diagnostic test in acute respiratory failure patients[20,26]. Lung ultrasound appears to be particularly useful in differentiating between exacerbation of chronic obstructive pulmonary disease (COPD), a condition that does not show B-lines, and decompensated heart failure. In a study performed in dyspneic patients in the emergency department, diffuse B-lines were detected in 100% of patients with cardiogenic pulmonary oedema but was absent in 92% of cases with exacerbation of COPD and 98.75% of those with normal lungs[26]. Conclusion of the study was that sonographic detection of B-lines might help distinguish pulmonary edema from exacerbation of COPD.
Other studies showed the correlation between B-lines and natriuretic peptides in the primary evaluation of acute decompensated heart failure in the Emergency department[27]. Pulmonary interstitial fluid, sonographically demonstrated by B-lines, was strictly correlated with natriuretic hormones level. Conclusions of these studies was that LUS can be used alone or can provide additional predictive power to natriuretic peptides in the immediate evaluation of dyspneic patients to diagnose the cardiac origin of the symptom.
Another great potential of LUS is that B-lines are highly sensitive to the resolution of lung congestion in patients admitted to the hospital for acute decompensated heart failure. Clearance of B-lines represents a direct sign of effective treatment, but also may be useful to specify the diagnosis in cases where the origin of B-lines cannot be differentiated at a first examination[28].
Finally, LUS may be also useful to diagnose unsuspected conditions when it is performed in combination with other tools, showing similar performances as compared to other more panoramic chest diagnostic imaging tools[29-32].
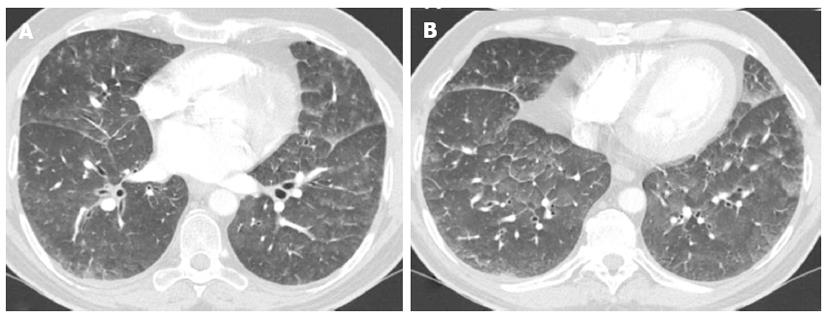
On high resolution computed tomography (HRCT), signs of hydrostatic edema generally results in a combination of septal thickening and ground-glass opacities. Incidence and predominance of these signs is individually variable[33-39] (Figure 7).
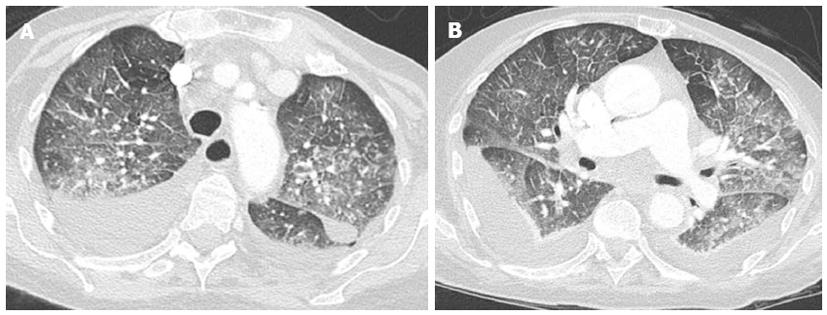
Crazy paving and consolidation are also frequently imaged. In some patients, ill-defined perivascular and centrilobular opacities may also be detected, or ground-glass opacity may appear lobular and patchy with a tendency to have a parahilar and gravitational distribution (Figure 8)[40].
There is some evidence that a parahilar or bat wing distribution of edema is typically found in patients who have a rapid accumulation of fluid[40]. Occasionally edema may have unilateral distribution, as may happen in patients with a prolonged lateral decubitus, or asymmetric and even with bizarre distribution in patients with regional emphysema[29]. In studies on hydrostatic edema in dog lungs, high resolution CT patterns showed predominantly central, peribronchovascular, and posterior distribution of edema, associated with an apparent increased thickness of bronchial walls[30,31].
Imaging has a fundamental role in the diagnosis of heart failure, but the efficacy of the diagnostic process is highly dependent from the ability to integrate information drawn from LUS, chest radiography and CT (Table 1).
| Lung ultrasound | Chest X-ray | Chest CT |
| First line in emergency and critically ill monitoring and to assess pulmonary congestion in typical clinical presentation | Second line to confirm doubtful cases in emergency or critically ill after haemodynamic recovery | Third step differential diagnosis of Pulmonary Embolism |
Chest radiography has the great advantage of combining relative low cost with the panoramic view that allows exclusion of many pulmonary conditions that comes into the differential diagnosis. CT scan is the best method to have a panoramic thoracic view, and much more sensitive than chest radiography for the first diagnosis of many conditions, like pulmonary embolism and early phase of cardiogenic pulmonary edema. However, it has many limitations due to costs, availability in emergency situations and relatively high radiation exposure. However, in recent years technological advances have made it possible to improve the modulation of dose exposure to follow the principles of radiological protection. Besides radiation exposure, low availability and feasibility are other fundamental limitations. CT scan cannot be performed as routine technique in heart failure because of the high prevalence of this disease and high costs of use.
However, while LUS and chest radiography are the first choice imaging technique in most cases, in selected cases where multiple conditions are in the differential, CT scan may become the method of reference. This is the case in acutely dyspneic patients when the differential diagnosis with pulmonary embolism is a challenge. In other cases, when the differential diagnosis includes diffuse parenchymal lung diseases, the high-resolution CT of the chest may be useful to rule-out or confirm pulmonary congestion.
Lung ultrasound has the limitation of being a surface imaging technique far less panoramic than chest radiography and CT scan. However, the great advantages of LUS are a higher sensitivity than chest radiography in the diagnosis of the early signs of interstitial thickening due to pulmonary congestion, and the possibility to perform the examination at bedside during the first clinical approach (Table 2).
| Sensitivity | |
| X-ray | 56% |
| US | 100% |
In the diagnostic imaging of pulmonary congestion due to decompensated heart failure, LUS and CXR are the most used diagnostic tools. Lung ultrasound does not fully replace CXR but may be of great help in some specific situations, like in the emergency setting when a prompt diagnostic evaluation of dyspneic patients at bedside is needed and also for monitoring clinical evolution. Moreover, LUS outperforms conventional radiology for the diagnosis of early signs of pulmonary congestion and should always be considered when radiologic signs are not detected on CXR but heart failure is still considered a possibility. However, LUS standing alone has a limited specificity for cardiogenic pulmonary congestion. Indeed, the main ultrasound signs of the interstitial syndrome, the B lines, are also detected in other pulmonary conditions, even chronic, characterized by loss of aeration and increase in fluids. Moreover, CXR is superior to LUS as a panoramic imaging modality that allows an immediate and comprehensive evaluation of the thoracic structures. CT scan is a powerful method for the evaluation of the thorax and even more panoramic, but is of limited use in the first diagnosis of decompensated heart failure in comparison to CXR and LUS. However, in selected cases it may be of help in the differential diagnosis of interstitial lung diseases or other causes of respiratory failure. Very often, in cases when a CT study is performed to investigate other conditions, the diagnosis of pulmonary congestion is incidental.
Integration of information obtained by the correct use of these three thoracic imaging, may improve the accuracy of the diagnostic process for cardiogenic pulmonary edema. The modern clinician and radiologist should be aware of the potential and limitations of these diagnostic tools and be prepared to integrate information derived from a correct use of ultrasound, conventional radiology and CT.
We thanks for his support Professor Andrea Veltri, Chief of Radiology Unit, AOUS Luigi Gonzaga, Orbassano (TO)/IT.
P- Reviewers: Tripoliti EE, Yew DT S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
| 1. | Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 101] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Chakko S, Woska D, Martinez H, de Marchena E, Futterman L, Kessler KM, Myerberg RJ. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. Am J Med. 1991;90:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 181] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Badgett RG, Lucey CR, Mulrow CD. Can the clinical examination diagnose left-sided heart failure in adults? JAMA. 1997;277:1712-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 702] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 5. | Volpicelli G, Mussa A, Garofalo G, Cardinale L, Casoli G, Perotto F, Fava C, Frascisco M. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 392] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 6. | Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 270] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Storto ML, Kee ST, Golden JA, Webb WR. Hydrostatic pulmonary edema: high-resolution CT findings. AJR Am J Roentgenol. 1995;165:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Heitzman ER, Ziter FM. Acute interstitial pulmonary edema. Am J Roentgenol Radium Ther Nucl Med. 1966;98:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Milne EN, Pistolesi M, Miniati M, Giuntini C. The radiologic distinction of cardiogenic and noncardiogenic edema. AJR Am J Roentgenol. 1985;144:879-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 155] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Maffessanti M, Lucangelo U, Pellegrin A. Radiologia toracica in terapia intensiva. Radiol Med. 2010;115:S34-S44. |
| 11. | Cardinale L, Volpicelli G, Lamorte A, Martino J. Revisiting signs, strengths and weaknesses of Standard Chest Radiography in patients of Acute Dyspnea in the Emergency Department. J Thorac Dis. 2012;4:398-407. [PubMed] |
| 12. | Pistolesi M, Miniati M, Milne EN, Giuntini C. The chest roentgenogram in pulmonary edema. Clin Chest Med. 1985;6:315-344. [PubMed] |
| 13. | Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, Mant D, McManus RJ, Holder R, Deeks J. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess. 2009;13:1-207, iii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 251] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | Lichtenstein DA. Ultrasound in the management of thoracic disease. Crit Care Med. 2007;35:S250-S261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Volpicelli G, Silva F, Radeos M. Real-time lung ultrasound for the diagnosis of alveolar consolidation and interstitial syndrome in the emergency department. Eur J Emerg Med. 2010;17:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Volpicelli G. Lung sonography. J Ultrasound Med. 2013;32:165-171. [PubMed] |
| 17. | Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1882] [Article Influence: 144.8] [Reference Citation Analysis (0)] |
| 18. | Bouhemad B, Zhang M, Lu Q, Rouby JJ. Clinical review: Bedside lung ultrasound in critical care practice. Crit Care. 2007;11:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 19. | Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 20. | Volpicelli G, Cardinale L, Garofalo G, Veltri A. Usefulness of lung ultrasound in the bedside distinction between pulmonary edema and exacerbation of COPD. Emerg Radiol. 2008;15:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Lichtenstein DA, Mezière G, Lascols N, Biderman P, Courret JP, Gepner A, Goldstein I, Tenoudji-Cohen M. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 379] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 22. | Soldati G, Copetti R, Sher S. Sonographic interstitial syndrome: the sound of lung water. J Ultrasound Med. 2009;28:163-174. [PubMed] |
| 23. | Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F, Frascisco MF. Detection of sonographic B-lines in patients with normal lung or radiographic alveolar consolidation. Med Sci Monit. 2008;14:CR122-CR128. [PubMed] |
| 24. | Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 964] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 25. | Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 369] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 26. | Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 246] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Gargani L, Frassi F, Soldati G, Tesorio P, Gheorghiade M, Picano E. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: a comparison with natriuretic peptides. Eur J Heart Fail. 2008;10:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 28. | Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F, Frascisco MF. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Maffessanti M, Dalpiaz G. Pulmonary Edema in Diffuse Lung Disease: Clinical Features, Pathology, HRCT. Milan: Springer 2004; 186-189. |
| 30. | Forster BB, Müller NL, Mayo JR, Okazawa M, Wiggs BJ, Paré PD. High-resolution computed tomography of experimental hydrostatic pulmonary edema. Chest. 1992;101:1434-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Scillia P, Delcroix M, Lejeune P, Mélot C, Struyven J, Naeije R, Gevenois PA. Hydrostatic pulmonary edema: evaluation with thin-section CT in dogs. Radiology. 1999;211:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Volpicelli G, Melniker LA, Cardinale L, Lamorte A, Frascisco MF. Lung ultrasound in diagnosing and monitoring pulmonary interstitial fluid. Radiol Med. 2013;118:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Ketai LH, Godwin JD. A new view of pulmonary edema and acute respiratory distress syndrome. J Thorac Imaging. 1998;13:147-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Gluecker T, Capasso P, Schnyder P, Gudinchet F, Schaller MD, Revelly JP, Chiolero R, Vock P, Wicky S. Clinical and radiologic features of pulmonary edema. Radiographics. 1999;19:1507-1531; discussion 1507-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 169] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Todo G, Herman PG. High-resolution computed tomography of the pig lung. Invest Radiol. 1986;21:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Webb WR, Stein MG, Finkbeiner WE, Im JG, Lynch D, Gamsu G. Normal and diseased isolated lungs: high-resolution CT. Radiology. 1988;166:81-87. [PubMed] |
| 37. | Bessis L, Callard P, Gotheil C, Biaggi A, Grenier P. High-resolution CT of parenchymal lung disease: precise correlation with histologic findings. Radiographics. 1992;12:45-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Malagari K, Nikita A, Alexopoulou E, Brountzos E, Papathanasiou M, Mitromaras J, Zakynthinos E, Papiris S, Kelekis DA. Cirrhosis-related intrathoracic disease. Imaging features in 1038 patients. Hepatogastroenterology. 2005;52:558-562. [PubMed] |
| 39. | Tanaka N, Matsumoto T, Miura G, Emoto T, Matsunaga N. HRCT findings of chest complications in patients with leukemia. Eur Radiol. 2002;12:1512-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Mukhopadhyay P, Rezzoug F, Webb CL, Pisano MM, Greene RM. Suppression of chondrogenesis by Id helix-loop-helix proteins in murine embryonic orofacial tissue. Differentiation. 2009;77:462-472. [PubMed] |