Published online May 28, 2014. doi: 10.4329/wjr.v6.i5.169
Revised: March 6, 2014
Accepted: March 13, 2014
Published online: May 28, 2014
Processing time: 145 Days and 11.8 Hours
A fistula is an abnormal vascular connection leading to diversion of blood from a high resistance arterial circuit to low resistance venous circuit. Coronary artery fistulas are abnormal communications of the coronary artery with a chamber of the heart, or with any segment of systemic or pulmonary circulation, bypassing the myocardial capillaries. Other unusual fistulas include connection between aorta and the right atrium/superior vena cava, aorta and the inferior vena cava or between a coronary artery bypass graft and a cardiac vein. Abnormal connections also include origin of the coronary artery from the pulmonary artery. In this article, we review the imaging, particularly computed tomography and magnetic resonance imaging of unusual fistulas and connections involving the cardiovascular system, particularly the coronary arteries and the aorta.
Core tip: Computed tomography and magnetic resonance imaging are very useful imaging modalities in determining the type and demonstrating the anatomy of these fistulas, which is essential for surgical/interventional planning. Careful analysis in multiple planes and three-dimensional reconstruction is required for comprehensive evaluation of fistulas. Treatment depends on the symptoms and the anatomy.
- Citation: Ghandour A, Rajiah P. Unusual fistulas and connections in the cardiovascular system: A pictorial review. World J Radiol 2014; 6(5): 169-176
- URL: https://www.wjgnet.com/1949-8470/full/v6/i5/169.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i5.169
An arteriovenous (AV) fistula is an abnormal communication between an artery and vein, which results in the shunting of blood from the high-resistance arterial circuit to the low-resistance venous circuits. AV fistulas involving the coronary arteries and the aorta are rare. AV fistulas can be either congenital or acquired secondary to surgeries or interventions. In this article, we review the imaging, particularly computed tomography (CT) and magnetic resonance imaging (MRI) of unusual fistulas and connections involving the cardiovascular system, particularly the coronary arteries and the aorta.
AV fistula leads to decreased peripheral resistance and increased venous resistance, venous pressure and volume. This results in higher heart rate, stroke volume, cardiac output and cardiac work in order to compensate for the lack of perfusion. If left untreated, this may be followed by myocardial hypertrophy, dilation, and then hyper-dynamic cardiac failure. Also, the decreased arterial perfusion distal to fistula may raise the renal venous pressure and decrease the arterial perfusion pressure resulting in activation of the renin-angiotensin system, increasing aldosterone and causing plasma expansion. This creates an abnormal low-resistance circuit that steals from the high-resistance normal capillary bed circuit which can cause tissue ischemia.
Imaging plays an important role in the diagnosis and management of these fistulas. Coronary angiography is the traditional technique used in the evaluation of coronary fistulas, although identification of the exact site of drainage is often difficult, since it is usually into a low pressure chambers, with significant dilution of radiographic contrast[1]. Coronary angiography is an invasive procedure and does not provide three-dimensional (3D) information, particularly the exact anatomy and relationship to adjacent structures, which is vital in surgical planning. Echocardiography has a limited role in the evaluation of coronary fistulas, especially in demonstration of the anatomy and drainage. Transesophageal echocardiography has higher accuracy than transthoracic echocardiography. Color-flow Doppler demonstrates flow abnormalities within the fistula.
Cross sectional modalities such as CT and MRI are the most useful imaging modalities in diagnosis and accurate characterization of cardiovascular fistulas. Both these modalities have high spatial and temporal resolutions, multi-planar imaging/reconstruction capabilities and wide field of view. CT is more widely available in many centers and can be performed rapidly, often without the need for any sedation/anesthesia even in children. CT angiography has high spatial and temporal resolution and is performed with intravenous contrast and with ECG gating to avoid motion artifacts. Radiation dose can be minimized by using several techniques such as prospective ECG triggering, retrospective ECG gating with tube current modulation, automatic tube current modulation, low kilovoltage, and iterative reconstruction algorithms. Multi-planar reformats and 3D reconstruction techniques such as volume rendering and surface shading enable exquisite demonstration of the anatomy of the fistulas, which is essential for surgical/interventional planning. CT scan has been shown to have high sensitivity of up to 87% compared to coronary angiography in the detection of coronary artery fistulas, with a lower sensitivity of 58% for fistulas draining in cardiac chambers[2]. Disadvantages of CT include the use of ionizing radiation and use of potentially nephrotoxic contrast media.
MRI does not involve the use of ionizing radiation or potentially nephrotoxic contrast medium. Anatomic information of the vasculature is obtained with MR angiography, which typically requires the use of Gadolinium-based contrast agent but equivalent information can be obtained without the use of contrast medium through navigator gated 3D whole-heart steady state free precession (SSFP) sequences. Anatomical information can be obtained using black blood (double inversion recovery) or bright blood (cine SSFP) sequences. Flow can be quantified using phase contrast velocity encoded sequences, through the pulmonary artery and aorta, thus giving an estimate of the Qp:Qs ratio (pulmonary to systemic flow). Presence of myocardial ischemia can be identified using stress perfusion imaging and infarcts can be identified using delayed enhancement MRI sequences[3].
Coronary artery fistula is an abnormal communication between the coronary artery with a chamber of the heart (coronary cameral) or with any segment of systemic or pulmonary circulation (coronary AV fistula) close to the heart, bypassing the myocardial capillaries. Coronary artery fistulas are typically congenital but may also be acquired following trauma or invasive cardiac procedures (pacemaker, endomyocardial biopsy, coronary angiography, septal myomectomy). Congenital coronary artery fistulas are very rare, accounting for 0.2%-0.4% of congenital cardiac abnormalities[4]. It has been reported in 0.05%-0.25% of patients undergoing coronary angiography, with an estimated prevalence of 0.002% in the general population[5]. Pathologically, it is characterized by dilation of involved vessel, thinned fistulous wall, thrombosis, atherosclerotic changes, myocardial hypertrophy and fibrosis[6].
As discussed above, the communication of the coronary artery could be with the lumen of a cardiac chamber (right ventricle -41%, right atrium -26%, left atrium -5%, left ventricle -3%), coronary sinus (7%), superior vena cava (SVC) (1%), pulmonary artery (17%) or the pulmonary vein. 90% of venous drainage is seen into the systemic venous side[7]. The right coronary artery is the most common artery involved in fistulous formation (50% of cases) and is often symptomatic. Left coronary artery accounts for 42% of fistulas and is often asymptomatic. Both right coronary artery (RCA) and left coronary artery (LCA) are involved in 5% of cases[5]. Majority of fistulas are single, but multiple and complex fistulas have been reported. Cardiovascular anomalies are associated in 5%-30% of cases[6].
Left-to-right shunt is seen in 90% of cases[7]; however, the shunt ratio is not large, as a result of which most patients are asymptomatic, especially in the adult population. Pediatric patients are usually symptomatic[7]. Clinical presentations include-continuous heart murmur, dyspnea, orthopnea, right ventricular dysfunction/failure, fatigue, chest pain, endocarditis, stroke, arrhythmias, myocardial ischemia/infarction due to coronary steal, pericardial effusion or sudden death. Larger shunts are complicated by cardiac failure, pulmonary hypertension, thrombosis, rupture, or aneurysm.
Coronary artery fistulas are conservatively treated with imaging follow up in asymptomatic patients. Spontaneous thrombosis has been reported in few patients (1%-2%)[5]. Medical treatment includes anti platelet therapy and antibiotic prophylaxis for infective endocarditis. Some centers perform closure even in asymptomatic patients to avoid long term morbidity and mortality[2]. Definitive repair can be performed using surgical ligation or percutaneous techniques such as transcatheter embolization of the fistulous connection using detachable platinum coils or polytetrafluoroehylene-covered coronary artery stent grafts, both of which have been shown to have similar outcomes[8]. Surgery is performed in larger fistulas, larger flow, multiple fistulas, complex fistulas, aneurysm, or large vascular branches. Embolization is performed in single drainage, proximal fistulous vessel, termination away from coronary arteries, absence of associated disorders requiring surgery, and older age. Imaging is used for follow up after surgery/intervention to ensure there is complete closure of fistula and no recanalization[5].
This is the most common type of coronary artery fistula, resulting in communication between a coronary artery and a cardiac chamber. This is mostly congenital and believed to be persistent embryonic intratrabecular spaces and sinusoids[8]. As discussed above, the most frequent chamber involved in this fistula is the right ventricle, followed by right atrium, then left atrium and finally the left ventricle.
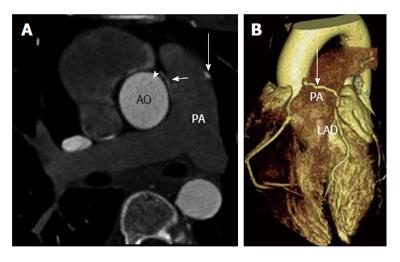
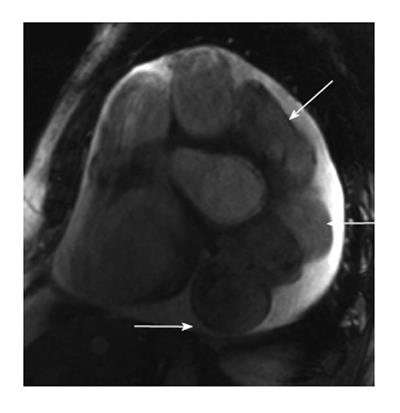
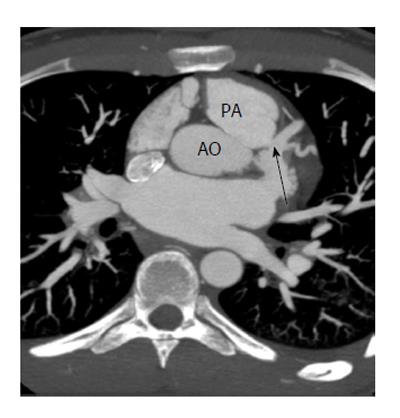
A fistulous communication between the coronary artery and the pulmonary artery is the second most common type of coronary artery fistula, accounting for 17% of these cases. Some studies have shown this to be as high as 30% of all fistulas[9]. The RCA is more often involved than the LCA as a site of origin. This fistula is often asymptomatic and is only incidentally discovered in imaging. CT and MRI show the coronary artery or its branches draining into the pulmonary artery (Figure 1). On CT, demonstration of this fistula requires good contrast opacification of the coronary arteries and non-contrast opacification of the pulmonary artery. Evaluation might be challenging due to low pressure circulation of these fistula causing low blood flow and small caliber of fistula[10,11]. Asymptomatic fistulas are managed conservatively by follow up whereas symptomatic fistulas require surgery or transcatheter closure.
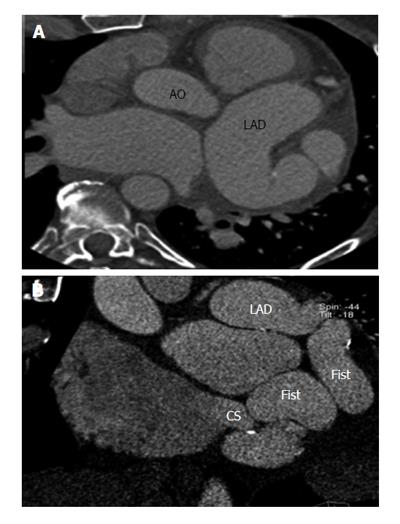
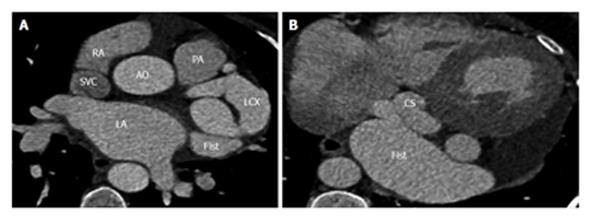
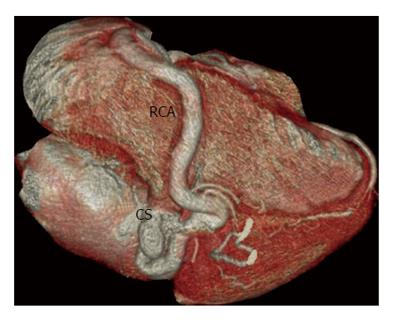
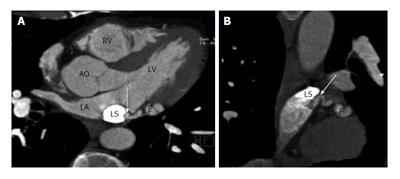
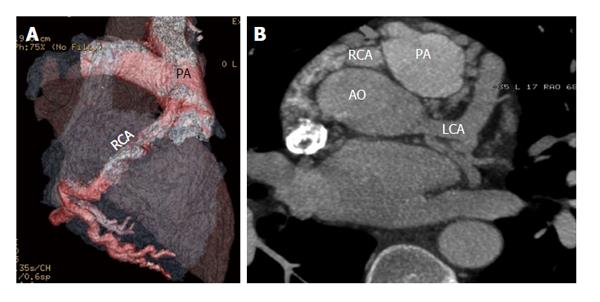
Fistulous communication between the coronary artery and the coronary sinus is the third commonest type of coronary artery fistula, accounting for 7% of cases (Figures 2, 3 and 4). On CT and MRI, the coronary artery or arteries are dilated and tortuous and a communication with the coronary sinus which is dilated can be demonstrated (Figure 5). When there is a severely dilated coronary sinus, coronary artery fistula should be in the differential diagnosis. Other causes of coronary sinus dilation include tricuspid regurgitation, tricuspid stenosis, pulmonary hypertension and right heart dysfunction. Coronary sinus is also dilated in the presence of a persistent left superior vena cava (LSVC) draining into the coronary sinus, interrupted inferior vena cava (IVC) with hemiazygos connection to LSVC or hepatic veins to coronary sinus (CS) connection, all of which are not associated with a left-to-right shunt or unroofed coronary sinus (partial or complete) or total anomalous pulmonary venous connection, both of which are associated with left-to-right shunt[12]. In symptomatic patients, fistulas are repaired by surgery or percutaneous technique, since spontaneous closure is rare[5].
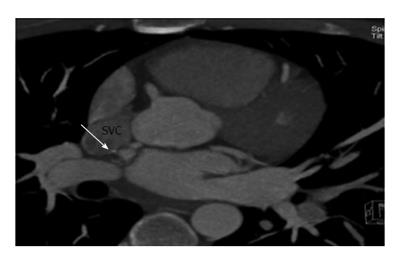
Fistulous communication between coronary artery and the SVC is rare, accounting for 1% of these fistulas[13]. Majority of these fistulas drain into a normal right sided SVC (Figure 6), typically originating from the right coronary artery and less commonly the left circumflex artery. Occasionally, these fistulas drain into a persistent left SVC (Figure 7). Majority of these fistulas originate from the left circumflex artery[14]. CT and MRI are ideal imaging modalities in the demonstration of these rare fistulas.
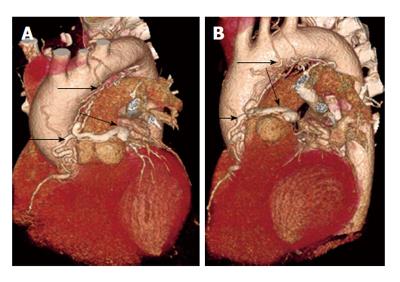
Occasionally coronary artery fistulas can be complex, with multiple sites of origin and drainage, which may be challenging to diagnose. Multiple fistulas can be seen in 11%-16% of cases[9]. One such case is shown in Figure 8, which is an arterial- arterial fistula originating from the proximal right coronary artery and 1st diagonal branch, which terminates in the pulmonary artery. CT and MRI with multi-planar and 3D reconstructions are vital in precise characterization of the fistula anatomy, which is essential for surgical planning.
Anomalous origin of left coronary artery from pulmonary artery (ALCAPA) is a rare (0.25%-0.5%) congenital cardiac anomaly[15], with the most common form being Bland White Garland syndrome (LCA from pulmonary artery) (Figure 9). There are two types of ALCAPA, the infant and adult types. Physiologically, ALCAPA is not a major problem during fetal and early neonatal life since during this period; the pulmonary artery pressure is similar to systemic arterial pressure, resulting in antegrade coronary flow. However, after birth, the pulmonary arterial pressure decreases resulting in retrograde flow from the LCA to the pulmonary artery. Presentation and hemodynamics depends on the artery involved, myocardial distribution, pulmonary resistance, and number and size of collaterals. In the more common infant type, there is no development of collaterals between the RCA and the LCA, as a result of which these vessels are of normal caliber. Retrograde flow from the LCA to the low pressure pulmonary artery is maintained, producing a left-to-right shunt. The myocardial hypoperfusion results in myocardial infarction and congestive cardiac failure, leading to death in 90% within one year. Adult type is less common and is characterized by extensive collateral vessel formation between RCA and the LCA, resulting in dilation and tortuosity of these vessels. The anomalous vessel acts as a vein diverting flow from the normal coronary artery to the pulmonary artery, the so called “steal” phenomenon. This often results in chronic myocardial ischemia, LV dysfunction, mitral regurgitation and arrhythmias, which may cause sudden death, seen in 80%-90% of these cases[16].
CT and MRI demonstrate the origin of the left coronary artery from the main pulmonary artery, usually from its left inferolateral aspect just beyond the valvular level. In addition, reverse flow from the left coronary artery to the pulmonary artery can be demonstrated using phase contrast velocity encoded MRI image or with SSFP sequence. In neonates, the coronary arteries are of normal size, but in adults the coronary arteries are dilated and tortuous due to shunting of blood from RCA to LCA and into pulmonary artery. Prominent intercoronary collateral vessels may also be seen. Myocardial damage can be evaluated using delayed enhancement sequences, which will estimate the extent of viable myocardium, especially in adult patients. Presence of scarring is also a substrate for arrhythmias. Other MRI findings include LV hypertrophy/dilation, wall motion abnormalities and mitral regurgitation. Dilated bronchial arteries may also be seen. Ideal treatment is reestablishment of a two coronary artery system, either using coronary button transfer (preferred in infants), Takeuchi procedure, or placement of coronary artery bypass graft (CABG) after ligation of LCA origin (preferred in adults). Single-coronary system repair such as ligation of LCA is not preferred due to higher complications. Percutaneous closure and cardiac transplantation are other options[13].
In ARCAPA, there is anomalous origin of the right coronary artery from the pulmonary artery (Figure 10). This has an incidence of 0.002% in general population compared to 0.008% for ALCAPA[17]. Occasionally, a left anterior descending or left circumflex coronary arterial branch may originate from the pulmonary artery. Very rarely, both coronary arteries may originate from the pulmonary artery.
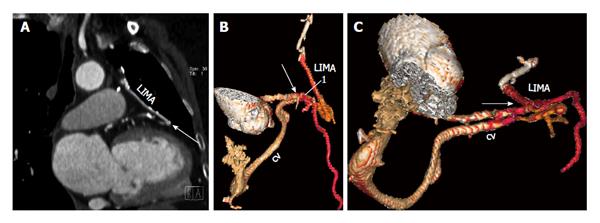
A fistulous communication between a CABG and cardiac vein is extremely rare, with less than 25 cases reported in the literature[18]. It happens more frequently with aortocoronary venous bypass grafts than with arterial grafts. The fistulous communication can occur either in the immediate post-operative period or may manifest after several months or even years[18,19]. This may be asymptomatic or present with heart failure, angina, or continuous precordial murmur. CT or MRI demonstrates the communication between a coronary artery bypass graft and the cardiac vein (Figure 11). Careful analysis is required since the communication may be subtle and may be confused for a normal anastomosis with a coronary artery. Treatment for small shunts is medical management, whereas large shunts which are usually symptomatic require a repeat CABG or embolization of the fistula.
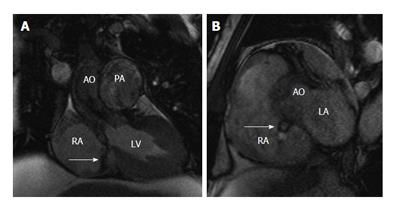
A fistulous communication between the aorta and the right atrium is rare. This often results from an intimal tear near the aortic root, especially in patients with prior cardiac surgery (1/3 rd of all cases). Dense pericardial adhesions resulting from the previous surgery contain the free rupture and contribute to the formation of the aorto-right atrial fistula. Also, this may be associated with right coronary artery disruption. Rarely, it occurs in patients with infective endocarditis (native or prosthetic valve), causing rapid bacterial invasion with tissue destruction. High intra-aortic pressure promotes progression of the tear towards the outer side of aortic wall draining into the right atrium. This type of fistula may be seen in any of the sinuses, but less common in the non-coronary sinus[20]. It has been reported after transcatheter closure of septal defect[20] and aortic dissection repair[21]. Sixty percent of patients develop heart failure and 40% eventually die. On CT, a defect can be demonstrated between the aorta and the right atrium. MRI is the ideal modality for demonstration of this fistula, with a dark jet of flow demonstrated on cine SSFP or GRE images (Figure 12). The fistula can be closed either by surgery or by transcatheter technique.
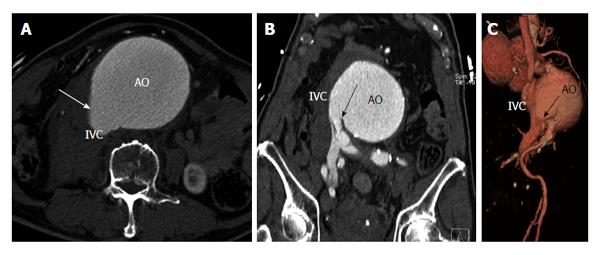
A fistula between the aorta and the IVC is very rare. It can be due to trauma, post-surgery (laminectomy), ruptured abdominal aortic aneurysm, infection, connective tissue disorders (Marfans, Ehler Danlos), or neoplasms. 80% of these fistulas are secondary to ruptured abdominal aortic aneurysm. This fistula is seen in 3%-6% of ruptured abdominal aortic aneurysms[22] and may or may not be associated with retroperitoneal hemorrhage. Clinical features include tachycardia, congestive cardiac failure, leg swelling, abdominal thrill, machinery type bruit, renal failure, or peripheral ischemia. CT and MRI demonstrate early contrast opacification of a dilated IVC, at the same time and with similar attenuation to that of abdominal aorta. Loss of normal anatomic plane between the aorta and IVC is also shown (Figure 13). Occasionally, a direct communication can be demonstrated between the aorta and IVC[23]. The most common site is the distal posterolateral aorta and adjacent IVC. Other sites are the aorta and iliac veins and aorta and renal vein. Identification of the exact site of the fistulous communication is essential for pre-surgical planning and to avoid massive blood loss. Surgery is performed immediately, with closure of fistula done from the aneurismal sac or ligation of infrarenal IVC/iliac veins. Complications include dislodgement of atheromatous debri or embolization across the fistula leading to pulmonary embolism[24]. Endovascular treatment of these fistulas has also been reported[25].
In this review, we have discussed several unusual types of cardiovascular fistulas and abnormal connections involving the coronary arteries and the aorta. CT and MRI are very useful imaging modalities in determining the type and demonstrating the anatomy of these fistulas, which is essential for surgical/interventional planning. Careful analysis in multiple planes and 3D reconstruction is required for comprehensive evaluation of fistulas. Treatment depends on the symptoms and the anatomy.
P- Reviewers: Hsu JL, Júnior EA, Nagamachi S S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Schmitt R, Froehner S, Brunn J, Wagner M, Brunner H, Cherevatyy O, Gietzen F, Christopoulos G, Kerber S, Fellner F. Congenital anomalies of the coronary arteries: imaging with contrast-enhanced, multidetector computed tomography. Eur Radiol. 2005;15:1110-1121. [PubMed] |
| 2. | Kacmaz F, Isiksalan Ozbulbul N, Alyan O, Maden O, Demir AD, Atak R, Senen K, Erbay AR, Balbay Y, Olcer T. Imaging of coronary artery fistulas by multidetector computed tomography: is multidetector computed tomography sensitive? Clin Cardiol. 2008;31:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Parga JR, Ikari NM, Bustamante LN, Rochitte CE, de Avila LF, Oliveira SA. Case report: MRI evaluation of congenital coronary artery fistulae. Br J Radiol. 2004;77:508-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Chen CC, Hwang B, Hsiung MC, Chiang BN, Meng LC, Wang DJ, Wang SP. Recognition of coronary arterial fistula by Doppler 2-dimensional echocardiography. Am J Cardiol. 1984;53:392-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Zenooz NA, Habibi R, Mammen L, Finn JP, Gilkeson RC. Coronary artery fistulas: CT findings. Radiographics. 2009;29:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Said SA, Lam J, van der Werf T. Solitary coronary artery fistulas: a congenital anomaly in children and adults. A contemporary review. Congenit Heart Dis. 2006;1:63-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Wilcox WD, Neal MJ, Alpert BS, Taylor AB, Dooley KJ. Localized occurrence of congenital coronary artery fistula in the southeast United States. Am J Cardiol. 1986;57:361-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Gowda RM, Vasavada BC, Khan IA. Coronary artery fistulas: clinical and therapeutic considerations. Int J Cardiol. 2006;107:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 262] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Dodge-Khatami A, Mavroudis C, Backer CL. Congenital Heart Surgery Nomenclature and Database Project: anomalies of the coronary arteries. Ann Thorac Surg. 2000;69:S270-S297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Tomasian A, Lell M, Currier J, Rahman J, Krishnam MS. Coronary artery to pulmonary artery fistulae with multiple aneurysms: radiological features on dual-source 64-slice CT angiography. Br J Radiol. 2008;81:e218-e220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Zeina AR, Blinder J, Rosenschein U, Barmeir E. Coronary-pulmonary artery fistula diagnosed by multidetector computed tomography. Postgrad Med J. 2006;82:e15. [PubMed] |
| 12. | Shah SS, Teague SD, Lu JC, Dorfman AL, Kazerooni EA, Agarwal PP. Imaging of the coronary sinus: normal anatomy and congenital abnormalities. Radiographics. 2012;32:991-1008. [PubMed] |
| 13. | Galbraith AJ, Werner D, Cutforth RH. Fistula between left coronary artery and superior vena cava. Br Heart J. 1981;46:99-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Yanagihara K, Ueno Y, Kobayashi T, Isobe J, Watanabe S, Itoh M. Coronary artery fistula into a persistent left superior vena cava: report of a case. Surg Today. 1997;27:966-968. [PubMed] |
| 15. | Pfannschmidt J, Ruskowski H, de Vivie ER. [Bland-White-Garland syndrome. Clinical aspects, diagnosis, therapy]. Klin Padiatr. 1992;204:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Peña E, Nguyen ET, Merchant N, Dennie G. ALCAPA syndrome: not just a pediatric disease. Radiographics. 2009;29:553-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Gupta R, Marwah A, Shrivastva S. Anomalous origin of right coronary artery from pulmonary artery. Ann Pediatr Cardiol. 2012;5:95-96. [PubMed] |
| 18. | Farand P, Brochu MC, Belzile F, Benko A, Dalery K. Fistula between a coronary artery bypass graft pseudoaneurysm and the coronary sinus. Can J Cardiol. 2008;24:920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Khunnawat C, Mukerji S, Abela GS, Thakur RK. Unusual complications of coronary artery bypass graft surgery. Am J Cardiol. 2006;98:1665-1666. [PubMed] |
| 20. | Chandra S, Vijay S, Kaur D, Dwivedi S. Congenital aorta right atrial fistula: successful transcatheter closure with the Amplatzer occluder. Pediatr Cardiol. 2011;32:1057-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Hsu RB, Chien CY, Wang SS, Chu SH. Aorto-right artrial fistula: a rare complication of aortic dissection. Tex Heart Inst J. 2000;27:64-66. [PubMed] |
| 22. | Schmidt R, Bruns C, Walter M, Erasmi H. Aorto-caval fistula--an uncommon complication of infrarenal aortic aneurysms. Thorac Cardiovasc Surg. 1994;42:208-211. [PubMed] |
| 23. | Cinara IS, Davidovic LB, Kostic DM, Cvetkovic SD, Jakovljevic NS, Koncar IB. Aorto-caval fistulas: a review of eighteen years experience. Acta Chir Belg. 2005;105:616-620. [PubMed] |
| 24. | Ravi R, Peter SB, Swamination TS, Chandrasekar V. Spontaneous Aortocaval Fistula due to Aneurysm Rupture- A Case Report. Ind J Radiol Imaging. 2006;16:453-456. |
| 25. | Vetrhus M, McWilliams R, Tan CK, Brennan J, Gilling-Smith G, Harris PL. Endovascular repair of abdominal aortic aneurysms with aortocaval fistula. Eur J Vasc Endovasc Surg. 2005;30:640-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |