Revised: October 31, 2013
Accepted: November 15, 2013
Published online: January 28, 2014
Processing time: 229 Days and 16.2 Hours
Hepatocellular carcinoma (HCC) is the sixth most common neoplasm and the third cause of cancer death worldwide. Contrast enhanced ultrasound (CEUS) has been applied for more than ten years and plays increasingly important roles in the management of HCC. On the basis of the Guideline and Good Clinical Practice Recommendations for CEUS in the liver-update 2012 and related literature about the management of HCC, we summarize the main roles and applications of CEUS in the management of HCC, including HCC surveillance, diagnosis, CEUS-guided treatment, treatment response evaluation and follow-up. The diagnostic algorithm for HCC is also suggested. Meanwhile, the comparisons between CEUS and contrast enhanced computed tomography/magnetic resonance imaging (CECT/CEMRI) in these areas are made. Although CEUS is subject to the same limitation as ordinary US and is inferior to CECT/CEMRI in some aspects, CEUS has proved to be of great value in the management of HCC with inherent advantages, such as sufficient high safety profile making it suitable for patients with renal failure or allergic to iodine, absence of radiation, easy reproducibility and high temporal resolution. The tremendous application of CEUS to the diagnosis and treatment of HCC provides more opportunities for patients with HCC diagnosed at different stages.
Core tip: Whether contrast enhanced ultrasound (CEUS) is comparable to contrast enhanced computed tomography/magnetic resonance imaging (CECT/CEMRI) in the management of hepatocellular carcinoma (HCC) is a controversial topic recently. Regarding to this issue, we list almost all the updated applications of CEUS in this paper and discuss the main role of CEUS in the management of HCC by comparison with CECT/CEMRI.
- Citation: Zheng SG, Xu HX, Liu LN. Management of hepatocellular carcinoma: The role of contrast-enhanced ultrasound. World J Radiol 2014; 6(1): 7-14
- URL: https://www.wjgnet.com/1949-8470/full/v6/i1/7.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i1.7
Hepatocellular carcinoma (HCC) is the sixth most common neoplasm and the third cause of cancer death worldwide[1,2]. In most cases, HCC develops within an established background of chronic liver disease (70%-90% of all patients) and most of the patients have a background of liver cirrhosis[3]. The development of HCC is thought to occur through a multistep process in about 90% of cases in the following sequence: large regenerative nodule (RN), low- or high-grade dysplastic nodule (DN), DN with a focus of HCC, well differentiated HCC, and moderately to poorly differentiated HCC[4]. On a histopathologic basis, portal tracts, including the portal vein and normal hepatic artery, are decreased with increasing grade of malignancy and are almost absent in HCC. On the other hand, abnormal arteries due to tumour angiogenesis progressively increase in the course of hepatocarcinogenesis. This progressive neo-angiogenesis provides the clue for clinical diagnosis of HCC using imaging techniques.
With the progress in HCC research and the application of new techniques, the management of HCC is updated frequently and patients with HCC at various stages have more optional therapeutic strategies. The patients can benefit from more effective treatments that will dramatically improve their survival. Among these new techniques, contrast enhanced ultrasound (CEUS) plays increasingly important roles in the management of HCC[2,5-8]. Since the advent of the second generation microbubble contrast agents (such as SonoVue, Definity and Luminity) and nonlinear harmonic contrast imaging technique, CEUS has been applied to HCC management for more than ten years, including HCC surveillance, diagnosis, CEUS-guided treatment, treatment response evaluation and follow-up[9]. A series of CEUS application modes, such as 2D or 3D transabdominal CEUS and intraoperative CEUS (IO-CEUS), are developed and applied clinically for management of HCC[8]. Beside the above-mentioned vascular change, HCC tends to lack Kupffer cells (reticuloendothelial cells), particularly in case of progressive dedifferentiation from well to moderately and poorly differentiated HCC. This has become of particular importance with the introduction of Sonazoid, a new contrast agent which can be engulfed by Kupffer cells. This contrast agent has a postvascular phase, which begins 10 min after contrast administration and lasts for 60 min. HCC always shows an enhancement defect in the postvascular phase due to the lack of Kupffer cells whereas benign lesions always show sustained enhancement. The advent of Sonazoid brings CEUS into the era of cell functional imaging or molecular US imaging[7,10-12].
Here, on the basis of the Guideline and Good Clinical Practice Recommendations for CEUS in the liver -update 2012[7] and other literature about the management of HCC, we summarize the main roles and applications of CEUS in the management of HCC.
In cirrhotic livers, the risk of HCC increases with the increase in nodule size. Nodules < 1 cm are rarely malignant. Attention should be paid to nodules > 1 cm. The rate of HCC is 66% in nodules 1-2 cm in diameter, about 80% in nodules 2-3 cm and 92%-95% in nodules > 3 cm. The most challenging situation for imaging techniques is the diagnosis of nodules 1-3 cm in diameter[7]. It has been proven that surveillance for HCC can decrease the disease-related mortality. For screening HCC in high-risk patients with viral-related cirrhosis or chronic alcoholic liver disease, US follow-up (at 3-6 mo intervals) is recommended, according to the AASLD guidelines[2,6], due to its simple, non-invasive, cost-effective and real-time features, although the tumor detectability by US is not high enough[8,13]. CEUS has not yet been recommended as the sole imaging tool for screening HCC, which is largely ascribed to that examining the entire liver during the arterial phase to find hyperenhancing nodules is difficult or impossible by CEUS because of the short duration of the arterial phase. Washout in the portal or late phase is helpful for detection, whereas it is only observable in about 50% of cases. Sonazoid, unlike SonoVue, is different from pure blood-pool UCA, and its postvascular phase lasts up to 60 min, which may improve the detection rate of HCC by CEUS. CEUS with Sonazoid detects liver malignancies as defects in the postvascular phase with a sensitivity of 95%, specificity of 93%, positive predictive value (PPV) of 99%, and negative predictive value (NPV) of 97%[4,7,14-16].
HCC has generally been regarded as a hypervascular tumor. Conventional color Doppler US and power Doppler US have limited ability to depict intralesional vascularity, because both of them are insensitive to slow-flowing, deeply located blood vessels and are usually associated with many artifacts. Thus, their diagnostic capability for HCC is limited. On CEUS, HCC in cirrhotic livers typically exhibits arterial hyper-enhancement in comparison with the surrounding liver tissue, which is encountered in 93.5%-97% of cases[17-20]. Hyper-enhancement in the arterial phase is usually homogeneous and intense in HCC, but may be inhomogeneous in larger nodules (> 5 cm), because of the regions of necrosis. A thin, perilesional, rim-like hyperenhancement is seen in about 5%-34.6% of HCC cases, which may represent the tumor capsule or blood vessels around the lesion[17-20]. In most of the cases, HCC always shows earlier enhancement than the surrounding liver tissue. The detection rates of hyper-enhancement in lesions ≤ 1.0 cm, 1.1-2.0 cm, and 2.1-3.0 cm are 67%, 83%-88%, and 92%-100%, respectively[19-23]. Apparently, CEUS has a relatively low ability to determine the characteristics of smaller lesions.
Washout in the late phase is observed overall in about half of the cases of HCC but more rarely in small nodules (20%-30% in those 1-2 cm)[7]. This is more frequent in patients in Eastern countries, because washout is found in 80.4% of HCC cases in the portal phase and in 95.3% of HCC cases in the late phase[17,18]. However, in those 1-2 cm, only 53.5% exhibited washout in the portal phase and 69%-90.7% in the late phase[19,24]. Washout is observed more frequently and quickly in HCC with poorer grades of differentiation than in well-differentiated HCC, which tend to be iso-enhanced in the late phase[25-27]. In comparison with other liver malignancies such as intrahepatic cholangiocarcinoma (ICC) and metastatic liver cancer, washout in the late phase is usually less marked in HCC[7,28-32]. In addition, washout tends to start later in HCC, usually not before 60 s after UCA injection, and in about 25% of cases, appearing only after 180 s; therefore, it is important to observe nodules in cirrhosis until very late (> 4 min) to increase the sensitivity for the diagnosis of HCC[7].
The tumor thrombus in the portal or hepatic vein is an important sign of HCC progression and determines HCC stage and therapeutic strategy. Thus, it is crucial to differentiate the malignant thrombus from benign thrombosis. CEUS is also valuable in evaluating the presence and extension of the portal or hepatic vein thrombosis caused by tumor invasion. Hyper-enhancement of the thrombus in the arterial phase indicates malignant thrombosis whereas non-enhancement indicates benign thrombosis. CEUS seems to be superior to CT in detection (100% vs 68%) and characterization (98% vs 68%) of the portal thrombosis complicating HCC[33]. The tumor source of the malignant portal vein thrombus may be invisible on US, especially in the case of diffuse HCC in which portal vein thrombus may be the only visible clue. Moving the transducer from the thrombus to the adjacent liver tissue is recommended to find if there is any washout region and the washout regions should undergo reinjection to observe the arterial hyper-enhancement[7].
In cirrhotic livers, arterial hyperenhancement with subsequent washout facilitates the diagnosis of HCC when other lesions such as hemangioma, ICC, abscess, and hypervascular liver metastasis are excluded. On the other hand, arterial hyperenhancement without subsequent washout is also highly suspicious for HCC, mainly well-differentiated HCC, but is not conclusive[7,34-36]. An inconclusive CEUS pattern should prompt other contrast imaging (CECT or CEMRI), and if these are still inconclusive, biopsy is recommended. In general, the sensitivity, specificity, and PPV of CEUS in diagnosing HCC are 88.8%, 89.2% and 91.3%, respectively[18]. The diagnostic ability is highly associated with the nodular size; the sensitivities in nodules 1.0-2.0, 2.1-3.0 and 3.1-5.0 cm are 69%-80%, 97% and 100%, respectively, and the accuracies are 82%-87%, 97% and 100%, respectively[17,19,24].
The 2005 American Association for the Study of Liver Diseases (AASLD) guidelines has accepted CEUS as a reference imaging for the diagnosis of HCC just like contrast-enhanced CT or MRI[6]. CEUS is still a part of the Japanese guideline on HCC and the Asian Pacific Association for the Study of the Liver consensus recommendations on HCC but has been removed from the latest American guidelines[6]. This is partly justified by the fact that no UCA is licensed for the liver in the United States and additionally because of perceived possibility of false-positive HCC diagnosis in patients with ICC when CEUS is used alone. The role of CEUS in differential diagnosis between HCC and ICC is still controversial. ICC always enhances later and more slightly and washes out more quickly than HCC on CEUS. In fact, in experienced hands, CEUS has the same accuracy as contrast-enhanced CT in diagnosing ICC and the likelihood of misdiagnosis is minimal[29-32].
The other major concern in cirrhotic livers is to make a distinction between HCC and other nodules such as large RN, low-grade and high-grade DN. Pathologically, large RN and low-grade DN generally show arterial and capillary supply similar to that detected in the adjacent cirrhotic nodules, whereas high-grade DN and HCC may show abnormally increased arterial supply. About 33.3%-60% of high grade DN cases show arterial hyper-enhancement whereas 40%-66.7% show hypo-enhancement[21,24]. Washout is seldom found in the late phase for high grade DN, in contrast to typical HCC. Occasionally, cancerous foci of very well differentiated HCC is encountered within DN, which is called nodule-in-nodule lesion or DN with a focus of HCC. Differentiation between HCC and these nodules is always a major concern in cirrhotic livers, as the appearance in BUS may be similar but their prognosis is substantially different from each other. CEUS facilitates the detection of HCC portion in DN, because HCC portion generally shows arterial hyper-enhancement.
HCC in cirrhotic livers usually does not harbor reticuloendothelial (Kupffer) cells, different from normal and cirrhotic liver parenchyma and from most solid benign liver lesions. The absence of Kupffer cells causes a defect in Sonazoid uptake in the postvascular phase. The diagnostic capability of CEUS with Sonazoid in the postvascular phase is similar to that of MRI with superparamagnetic iron oxide (SPIO) and has been endorsed in the Japanese guidelines for the management of HCC[10-12,37,38].
Furthermore, CEUS has an inherent advantage over CECT or CEMRI: its real-time imaging can not only dynamically display the vascular perfusion of HCC, but also carry on quantitative analysis for FLL on the basis of time intense curve, which indeed helps radiologists to read CEUS images accurately and to avoid misdiagnose due to some subjective biases[2,5,7,8,19].
Little information is available for the role of CEUS in the diagnosis of HCC in non-cirrhotic livers. On CEUS, the enhancement pattern has no difference in comparison with that in cirrhotic livers. However, the lesions that should be differentiated may be different, especially for those appearing arterial hyper-enhancement and subsequent sustained enhancement. Under such circumstance, the lesions need to be differentiated from those including focal nodular hyperplasia, liver adenoma, and some small hemangioma. For the lesions that show arterial hyper-enhancement and subsequent washout, attention also should be paid to exclude liver metastasis and ICC, because the latter two lesions are often encountered in non-cirrhotic livers[29-32,39,40].
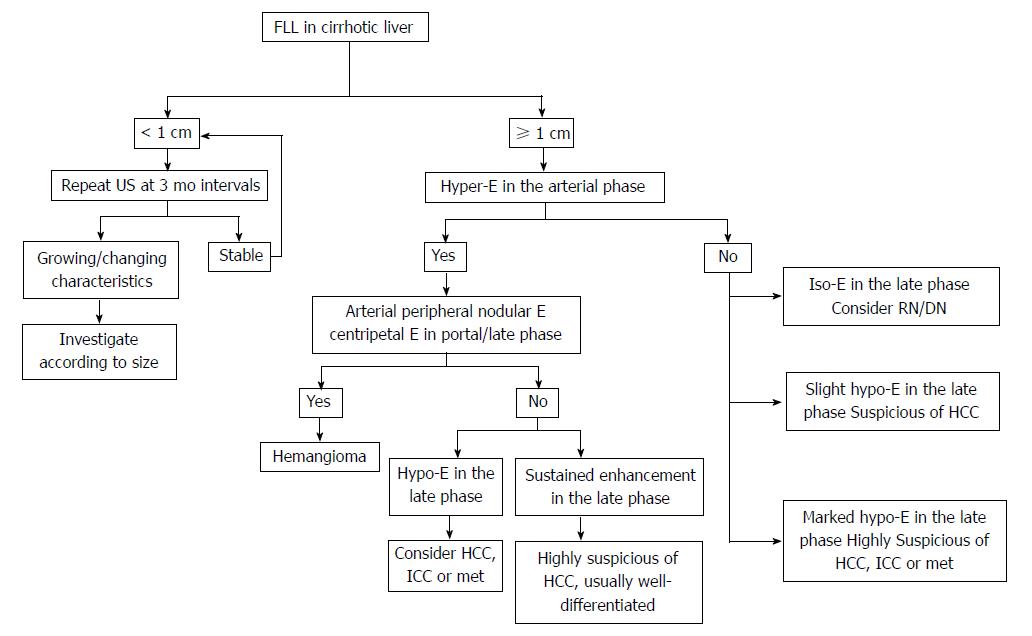
The diagnosis of HCC should be based not only on the imaging findings but also on clinical background, such as liver cirrhosis or hepatitis. Recent studies show that alpha-fetoprotein determination lacks adequate sensitivity and specificity for effective surveillance and diagnosis[41,42]. Thus, diagnosis of HCC should be based on imaging techniques and/or biopsy. The application of dynamic imaging criteria should be applied only to patients with cirrhosis of any etiology and to patients with chronic hepatitis B who may not have fully developed cirrhosis or have regressed cirrhosis[6]. In view of the updated AASLD guideline and the 2012 liver CEUS guideline, the diagnosis algorithm for HCC is suggested as following (Figure 1).
Clinically, percutaneous biopsy and therapy are commonly operated under the guidance of US or CT, whereas a part of HCC in patients with cirrhotic livers or repeated treatment history usually cannot be figured out on unenhanced US[7,13,43]. Although CT-guided percutaneous treatment is a well-established technique and a useful method for HCC lesions undetected by US, its inconvenience and radiation exposure have to be taken into account[13].
In order to get the definite pathologic diagnosis before therapy, US-guided percutaneous biopsy has already been regarded as the preferred choice for the cases that cannot show typical manifestations on imaging. CEUS prior to biopsy procedures can increase the diagnostic yield by 10% and decrease the false negative rate, especially in large tumors with areas of necrosis. CEUS can localize the site for biopsy more accurately by demonstrating regions of the vascularized viable tumor, which should be targeted, and regions of necrosis, which should be avoided[7,44].
Besides surgical resection and liver transplantation, local percutaneous therapy, such as ethanol ablation (EA), radiofrequency ablation (RFA), microwave ablation (MWA) and cryotherapy, is always the primary choice in the management of HCC as a minimally invasive method, especially for small, recurrent, and residual HCC lesions after local ablation or transcatheter arterial chemoembolization (TACE)[5]. Survival after ablation in Child-Pugh A patients is 50%-70% at 5 years, paralleling the outcome after surgical resection[2,45,46]. As a new guidance tool, CEUS represents a significant improvement in all steps of HCC percutaneous therapy. Prior to the percutaneous therapy, CEUS can be used to assess the HCC lesion size, number, margins and its relationship with the surrounding structures, which is helpful to devise the best therapeutic strategy, to reduce the risk of complications, and to compare the patterns before and after treatment[7]. CEUS can not only accurately tell the location of HCC but also guide real-time puncture during the arterial phase, or its sufficient long portal-venous and late phases. Moreover, UCA can be administrated repeatedly to guide percutaneous therapy at multiple sites or for multi-lesions[7,8,47].
With respect to the evaluation of HCC treatment response, currently, the use of contrast enhanced imaging to detect the viable tumor or recurrent HCC is widely accepted[8,47-49]. Previously, CECT or CEMRI has been regarded as the reference standard for treatment response evaluation after local therapies. Recently, several studies have proven that CEUS has the same ability to evaluate treatment response as CECT or CEMRI, thus CEUS can be performed as an alternative method to spare CECT or CEMRI for this purpose[8,50-53]. CEUS, similar to CECT or CEMRI, also follows the guideline of the modified Response Evaluation Criteria in Solid Tumor (mRECIST)[54]. In this guideline, viable HCC is defined as uptake of contrast agent in the arterial phase of CEUS; while complete response is defined as disappearance of any intratumoral arterial enhancement in HCC lesions[54]. In past decades, the assessment of HCC treatment response is mainly on the basis of change in tumor size, but it disregards the extent of necrosis and seems unsuitable for TACE and some targeted therapies, such as Sorafenib or monoclonal antibodies, in which anti-tumor effect mainly results from destroying the tumor blood vessel or suppressing angiogenesis. The change of microvascularization is also confirmed as the criterion for treatment response evaluation in mRECIST. Thus, on CEUS, the disappearance of intratumor arterial enhancement indicates internal necrosis after the treatment of HCC[54,55].
It should be noted that the time point of treatment response evaluation by CEUS is not limited except for percutaneous therapy. Because on CEUS the reactive hyperemia generated in the procedure of percutaneous therapy, such as RFA, EA and MWA, will cover the ablated HCC lesions in several days, the residual viable tumor may be missed. Within 2 wk after percutaneous therapy, the reactive hyperemia around the ablated lesion is apt to be misdiagnosed as false positive residual HCC. Given the above-mentioned phenomena, some experts have recommended that CEUS should be performed in a month after percutaneous therapy. However, there is still controversy over the role of CEUS vs CECT in the treatment response evaluation of HCC after ablation, which is probably influenced by the individual opinion and familiarity with the techniques[16]. Frieser et al[56] has concluded that CEUS is equal to CECT in evaluating treatment response; Gallotti et al[57] found that CEUS was excellent in evaluating treatment response after RFA, whereas it was inadequate for evaluating treatment response after EA.
After the treatment of HCC, contrast enhanced imaging in a month may fail to detect the tiny viable tumor tissue, especially when neoangiogenesis is not obvious. Therefore, the follow-up should aim to detect the progression of incomplete necrotic HCC and find the intrahepatic recurrence as early as possible, so that the patients can benefit timely from additional and effective therapy that will improve their survival, save the costs and reduce the side effects[15,58,59].
According to the previous studies, compared with CECT after HCC ablation, the sensitivity, specificity, PPV, NPV and overall accuracy of follow-up CEUS in detecting local tumor progression were 67.5%, 97.4%, 81.8%, 94.4% and 92.3%, respectively, and for detection of new intrahepatic recurrence were 77.7%, 92.0%, 92.4%, 76.7% and 84.0%, respectively[15,16]. Thus, CEUS is not comparable to CECT, because, similar to surveillance, it is difficult to provide an overview of the liver to detect all possible HCC progressions and intrahepatic recurrences on CEUS even if reinjection of UCA is carried out[5,7,15,16]. But when follow-up CECT or CEMRI is contraindicated or not inclusive, CEUS as a substitute or complementary method can be applied to assess the HCC progression and intrahepatic recurrence[7,15].
Thus, for the treatment response assessment of HCC, CEUS can be equal to CECT/CEMRI. And as a complement to CECT/CEMRI, CEUS may be used in follow-up protocols.
IO-CEUS is commonly used for intraoperatively detecting and locating HCC before resection and has attracted more attention of surgeons. Generally, CECT or CEMRI are considered as standard and basic imaging tools for preoperative staging and detection of metastasis or primary liver tumors. However, intraoperative ultrasound (IOUS) is increasingly used by most centers as several studies showed that up to 40% of malignant lesions are missed by the preoperative cross-sectional imaging[60,61]. Even though the cross-sectional imaging techniques have continuously improved, IOUS was still able to detect additional liver lesions in 10% of patients[60]. The use and value of IO-CEUS during surgery was reported firstly by Leen et al[62] in 2006, and IO-CEUS also showed even significantly better sensitivity in detecting liver metastasis compared to CECT, CEMRI and IOUS with an alteration of surgical management in almost 30% of cases[60,62]. Recently, Loss et al[60] reported that more than 50% additional liver lesions were found on IO-CEUS compared to preoperative imaging and IOUS, and that with IO-CEUS equipped with a high frequency linear transducer, some liver lesions even smaller than 10 mm in diameter can be detected and characterized[60]. The improved sensitivity of IO-CEUS was also confirmed by other studies[63-65]. Though most of the above-mentioned studies were not focused on HCC, IO-CEUS remains to be of potential value. It is now recognized that the more aggressive the surgical approach adopted, the higher the impact of IO-CEUS becomes[7,62].
In conclusion, although CEUS is subject to the same limitation as baseline US and is inferior to CECT/CEMRI in some aspects, CEUS is proved to be of great value in management of HCC with inherent advantages, such as sufficient high safety profile suitable for patients with renal failure or allergic to iodine, absence of radiation, easy repeatability and high temporal resolution. The tremendous application of CEUS to the diagnosis and treatment of HCC provides more opportunities for patients with HCC at different stages.
P- Reviewer: Yoshida S S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11837] [Article Influence: 845.5] [Reference Citation Analysis (4)] |
| 2. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3597] [Article Influence: 276.7] [Reference Citation Analysis (4)] |
| 3. | Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 4. | International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 589] [Article Influence: 36.8] [Reference Citation Analysis (2)] |
| 5. | Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D’Onofrio M. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 499] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 6. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 7. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 8. | Xu HX. Contrast-enhanced ultrasound: The evolving applications. World J Radiol. 2009;1:15-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 9. | Bauer A, Hauff P, Lazenby J, von Behren P, Zomack M, Reinhardt M, Schlief R. Wideband harmonic imaging: a novel contrast ultrasound imaging technique. Eur Radiol. 1999;9 Suppl 3:S364-S367. [PubMed] |
| 10. | Hatanaka K, Kudo M, Minami Y, Ueda T, Tatsumi C, Kitai S, Takahashi S, Inoue T, Hagiwara S, Chung H. Differential diagnosis of hepatic tumors: value of contrast-enhanced harmonic sonography using the newly developed contrast agent, Sonazoid. Intervirology. 2008;51 Suppl 1:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Hatanaka K, Kudo M, Minami Y, Maekawa K. Sonazoid-enhanced ultrasonography for diagnosis of hepatic malignancies: comparison with contrast-enhanced CT. Oncology. 2008;75 Suppl 1:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Inoue T, Kudo M, Hatanaka K, Takahashi S, Kitai S, Ueda T, Ishikawa E, Hagiwara S, Minami Y, Chung H. Imaging of hepatocellular carcinoma: qualitative and quantitative analysis of postvascular phase contrast-enhanced ultrasonography with sonazoid. Comparison with superparamagnetic iron oxide magnetic resonance images. Oncology. 2008;75 Suppl 1:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Maruyama H, Yoshikawa M, Yokosuka O. Current role of ultrasound for the management of hepatocellular carcinoma. World J Gastroenterol. 2008;14:1710-1719. [PubMed] |
| 14. | Maruyama H, Takahashi M, Ishibashi H, Yoshikawa M, Yokosuka O. Contrast-enhanced ultrasound for characterisation of hepatic lesions appearing non-hypervascular on CT in chronic liver diseases. Br J Radiol. 2012;85:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Zheng SG, Xu HX, Lu MD, Xie XY, Xu ZF, Liu GJ, Liu LN. Role of contrast-enhanced ultrasound in follow-up assessment after ablation for hepatocellular carcinoma. World J Gastroenterol. 2013;19:855-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Liu LN, Xu HX, Zhang YF, Xu JM. Hepatocellular carcinoma after ablation: the imaging follow-up scheme. World J Gastroenterol. 2013;19:797-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of small focal liver lesions using real-time contrast-enhanced sonography: diagnostic performance analysis in 200 patients. J Ultrasound Med. 2006;25:349-361. [PubMed] |
| 18. | Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J Clin Ultrasound. 2006;34:261-272. [PubMed] |
| 19. | Xu HX, Xie XY, Lu MD, Liu GJ, Xu ZF, Zheng YL, Liang JY, Chen LD. Contrast-enhanced sonography in the diagnosis of small hepatocellular carcinoma & lt; or =2 cm. J Clin Ultrasound. 2008;36:257-266. [PubMed] |
| 20. | Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420-430. [PubMed] |
| 21. | Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, Venturi AM, Piscaglia F. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27-34. [PubMed] |
| 22. | Gaiani S, Celli N, Piscaglia F, Cecilioni L, Losinno F, Giangregorio F, Mancini M, Pini P, Fornari F, Bolondi L. Usefulness of contrast-enhanced perfusional sonography in the assessment of hepatocellular carcinoma hypervascular at spiral computed tomography. J Hepatol. 2004;41:421-426. [PubMed] |
| 23. | Chen MH, Dai Y, Yan K, Fan ZH, Yin SS, Yang W, Wu W, Wang YB, Li JY. The role of contrast-enhanced ultrasound on the diagnosis of small hepatocellular carcinoma (& lt; /=3cm) in patients with cirrhosis. Hepatol Res. 2006;35:281-288. [PubMed] |
| 24. | Xu HX, Lu MD, Liu LN, Zhang YF, Guo LH, Xu JM, Liu C. Discrimination between neoplastic and non-neoplastic lesions in cirrhotic liver using contrast-enhanced ultrasound. Br J Radiol. 2012;85:1376-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Fan ZH, Chen MH, Dai Y, Wang YB, Yan K, Wu W, Yang W, Yin SS. Evaluation of primary malignancies of the liver using contrast-enhanced sonography: correlation with pathology. AJR Am J Roentgenol. 2006;186:1512-1519. [PubMed] |
| 26. | Iavarone M, Sangiovanni A, Forzenigo LV, Massironi S, Fraquelli M, Aghemo A, Ronchi G, Biondetti P, Roncalli M, Colombo M. Diagnosis of hepatocellular carcinoma in cirrhosis by dynamic contrast imaging: the importance of tumor cell differentiation. Hepatology. 2010;52:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Xu H, Lu M. The current status of contrast-enhanced ultrasound in China. J Med Ultrason. 2010;37:97-106. |
| 28. | Barreiros AP, Piscaglia F, Dietrich CF. Contrast enhanced ultrasound for the diagnosis of hepatocellular carcinoma (HCC): comments on AASLD guidelines. J Hepatol. 2012;57:930-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Chen LD, Xu HX, Xie XY, Xie XH, Xu ZF, Liu GJ, Wang Z, Lin MX, Lu MD. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol. 2010;20:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Chen LD, Xu HX, Xie XY, Lu MD, Xu ZF, Liu GJ, Liang JY, Lin MX. Enhancement patterns of intrahepatic cholangiocarcinoma: comparison between contrast-enhanced ultrasound and contrast-enhanced CT. Br J Radiol. 2008;81:881-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Xu HX, Chen LD, Liu LN, Zhang YF, Guo LH, Liu C. Contrast-enhanced ultrasound of intrahepatic cholangiocarcinoma: correlation with pathological examination. Br J Radiol. 2012;85:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Xu HX, Lu MD, Liu GJ, Xie XY, Xu ZF, Zheng YL, Liang JY. Imaging of peripheral cholangiocarcinoma with low-mechanical index contrast-enhanced sonography and SonoVue: initial experience. J Ultrasound Med. 2006;25:23-33. [PubMed] |
| 33. | Rossi S, Ghittoni G, Ravetta V, Torello Viera F, Rosa L, Serassi M, Scabini M, Vercelli A, Tinelli C, Dal Bello B. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma. Eur Radiol. 2008;18:1749-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97-104. [PubMed] |
| 35. | Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898-906. [PubMed] |
| 36. | Leoni S, Piscaglia F, Golfieri R, Camaggi V, Vidili G, Pini P, Bolondi L. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am J Gastroenterol. 2010;105:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Kudo M, Hatanaka K, Kumada T, Toyoda H, Tada T. Double-contrast ultrasound: a novel surveillance tool for hepatocellular carcinoma. Am J Gastroenterol. 2011;106:368-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Moriyasu F, Itoh K. Efficacy of perflubutane microbubble-enhanced ultrasound in the characterization and detection of focal liver lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol. 2009;193:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Zheng SG, Xu HX, Liu LN, Wang Y, Zhang YF, Guo LH, Liu C, Xu JM, Sun LP, Wu J. Parametric imaging with contrast-enhanced ultrasound: Usefulness for characterization of dynamic effects of microvascularization for hepatocellular carcinoma and focal nodular hyperplasia. Clin Hemorheol Microcirc. 2013;55:375-389. [PubMed] |
| 40. | Lin MX, Xu HX, Lu MD, Xie XY, Chen LD, Xu ZF, Liu GJ, Xie XH, Liang JY, Wang Z. Diagnostic performance of contrast-enhanced ultrasound for complex cystic focal liver lesions: blinded reader study. Eur Radiol. 2009;19:358-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 41. | Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 573] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 42. | Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, Morgan TR, Kim HY, Lee WM, Bonkovsky HL. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 451] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 43. | Miyamoto N, Hiramatsu K, Tsuchiya K, Sato Y. Contrast-enhanced sonography-guided radiofrequency ablation for the local recurrence of previously treated hepatocellular carcinoma undetected by B-mode sonography. J Clin Ultrasound. 2010;38:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Wu W, Chen MH, Yin SS, Yan K, Fan ZH, Yang W, Dai Y, Huo L, Li JY. The role of contrast-enhanced sonography of focal liver lesions before percutaneous biopsy. AJR Am J Roentgenol. 2006;187:752-761. [PubMed] |
| 45. | Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 46. | Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 47. | Xu HX. Era of diagnostic and interventional ultrasound. World J Radiol. 2011;3:141-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Xu HX, Lu MD, Xie XH, Xie XY, Kuang M, Xu ZF, Liu GJ, Wang Z, Chen LD, Lin MX. Treatment response evaluation with three-dimensional contrast-enhanced ultrasound for liver cancer after local therapies. Eur J Radiol. 2010;76:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Xu HX, Lu MD, Xie XH, Xie XY, Xu ZF, Chen LD, Liu GJ, Liang JY, Lin MX, Wang Z. Three-dimensional contrast-enhanced ultrasound of the liver: experience of 92 cases. Ultrasonics. 2009;49:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Salvaggio G, Campisi A, Lo Greco V, Cannella I, Meloni MF, Caruso G. Evaluation of posttreatment response of hepatocellular carcinoma: comparison of ultrasonography with second-generation ultrasound contrast agent and multidetector CT. Abdom Imaging. 2010;35:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Lu MD, Yu XL, Li AH, Jiang TA, Chen MH, Zhao BZ, Zhou XD, Wang JR. Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring percutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol. 2007;33:1736-1749. [PubMed] |
| 52. | Andreana L, Kudo M, Hatanaka K, Chung H, Minami Y, Maekawa K, Ruggiero G. Contrast-enhanced ultrasound techniques for guiding and assessing response to locoregional treatments for hepatocellular carcinoma. Oncology. 2010;78 Suppl 1:68-77. [PubMed] |
| 53. | Inoue T, Kudo M, Hatanaka K, Arizumi T, Takita M, Kitai S, Yada N, Hagiwara S, Minami Y, Sakurai T. Usefulness of contrast-enhanced ultrasonography to evaluate the post-treatment responses of radiofrequency ablation for hepatocellular carcinoma: comparison with dynamic CT. Oncology. 2013;84 Suppl 1:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3303] [Article Influence: 220.2] [Reference Citation Analysis (36)] |
| 55. | Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, Reig M, Bianchi L, Llovet JM, Bruix J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable. Cancer. 2009;115:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 339] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 56. | Frieser M, Kiesel J, Lindner A, Bernatik T, Haensler JM, Janka R, Hahn EG, Strobel D. Efficacy of contrast-enhanced US versus CT or MRI for the therapeutic control of percutaneous radiofrequency ablation in the case of hepatic malignancies. Ultraschall Med. 2011;32:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Gallotti A, D’Onofrio M, Ruzzenente A, Martone E, De Robertis R, Guglielmi A, Pozzi Mucelli R. Contrast-enhanced ultrasonography (CEUS) immediately after percutaneous ablation of hepatocellular carcinoma. Radiol Med. 2009;114:1094-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Liu LN, Xu HX, Lu MD, Xie XY. Percutaneous ultrasound-guided thermal ablation for liver tumor with artificial pleural effusion or ascites. Chin J Cancer. 2010;29:830-835. [PubMed] |
| 59. | Frampas E, Lassau N, Zappa M, Vullierme MP, Koscielny S, Vilgrain V. Advanced Hepatocellular Carcinoma: early evaluation of response to targeted therapy and prognostic value of Perfusion CT and Dynamic Contrast Enhanced-Ultrasound. Preliminary results. Eur J Radiol. 2013;82:e205-e211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Loss M, Schneider J, Uller W, Wiggermann P, Scherer MN, Jung W, Schlitt HJ, Stroszczynski C, Jung EM. Intraoperative high resolution linear contrast enhanced ultrasound (IOUS) for detection of microvascularization of malignant liver lesions before surgery or radiofrequeny ablation. Clin Hemorheol Microcirc. 2012;50:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Sietses C, Meijerink MR, Meijer S, van den Tol MP. The impact of intraoperative ultrasonography on the surgical treatment of patients with colorectal liver metastases. Surg Endosc. 2010;24:1917-1922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Leen E, Ceccotti P, Moug SJ, Glen P, MacQuarrie J, Angerson WJ, Albrecht T, Hohmann J, Oldenburg A, Ritz JP. Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: an essential investigation before resection. Ann Surg. 2006;243:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 63. | Fioole B, de Haas RJ, Wicherts DA, Elias SG, Scheffers JM, van Hillegersberg R, van Leeuwen MS, Borel Rinkes IH. Additional value of contrast enhanced intraoperative ultrasound for colorectal liver metastases. Eur J Radiol. 2008;67:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Jung EM, Ross CJ, Rennert J, Scherer MN, Farkas S, von Breitenbuch P, Schnitzbauer AA, Piso P, Lamby P, Menzel C. Characterization of microvascularization of liver tumor lesions with high resolution linear ultrasound and contrast enhanced ultrasound (CEUS) during surgery: First results. Clin Hemorheol Microcirc. 2010;46:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Larsen LP, Rosenkilde M, Christensen H, Bang N, Bolvig L, Christiansen T, Laurberg S. The value of contrast enhanced ultrasonography in detection of liver metastases from colorectal cancer: a prospective double-blinded study. Eur J Radiol. 2007;62:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |