Published online Jul 28, 2013. doi: 10.4329/wjr.v5.i7.259
Revised: May 23, 2013
Accepted: July 4, 2013
Published online: July 28, 2013
Processing time: 120 Days and 14.1 Hours
AIM: To evaluate the value of administration of hyoscine-N-butyl-bromide (HBB) for image quality magnetic resonance imaging (MRI) of the prostate.
METHODS: Seventy patients were retrospectively included in the study. Thirty-five patients were examined with administration of 40 milligrams of HBB (Buscopan®; Boehringer, Ingelheim, Germany); 35 patients were examined without HBB. A multiparametric MRI protocol was performed on a 3.0 Tesla scanner without using an endorectal coil. The following criteria were evaluated independently by two experienced radiologists on a five-point Likert scale: anatomical details (delineation between peripheral and transitional zone of the prostate, visualisation of the capsule, depiction of the neurovascular bundles); visualisation of lymph nodes; motion related artefacts; and overall image quality.
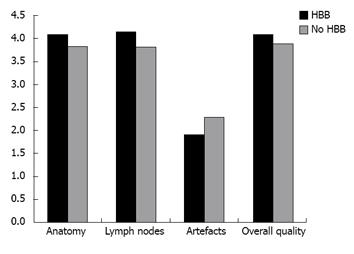
RESULTS: Comparison of anatomical details between the two cohorts showed no statistically significant difference (3.9 ± 0.7 vs 4.0 ± 0.9, P = 0.54, and 3.8 ± 0.7 vs 4.2 ± 0.6, P = 0.07) for both readers. There was no significant advantage regarding depiction of local and iliac lymph nodes (3.9 ± 0.6 vs 4.2 ± 0.6, P = 0.07, and 3.8 ± 0.9 vs 4.1 ± 0.8, P = 0.19). Motion artefacts were rated as “none” to “few” in both groups and showed no statistical difference (2.3 ± 1.0 vs 1.9 ± 0.9, P = 0.19, and 2.3 ± 1.1 vs 1.9 ± 0.7, P = 0.22). Overall image quality was rated “good” in average for both cohorts without significant difference (4.0 ± 0.6 vs 4.0 ± 0.9, P = 0.78, and 3.8 ± 0.8 vs 4.2 ± 0.6, P = 0.09).
CONCLUSION: The results demonstrated no significant effect of HBB administration on image quality. The study suggests that use of HBB is not mandatory for MRI of the prostate at 3.0 Tesla.
Core tip: The study demonstrated no significant effect of hyoscine-N-butyl-bromide (HBB) (butylscopolamine) administration on image quality of prostate magnetic resonance imaging (MRI) at 3.0 Tesla without using an endorectal coil. The results suggest that the use of HBB is not generally mandatory for MRI of the prostate.
- Citation: Roethke MC, Kuru TH, Radbruch A, Hadaschik B, Schlemmer HP. Prostate magnetic resonance imaging at 3 Tesla: Is administration of hyoscine-N-butyl-bromide mandatory? World J Radiol 2013; 5(7): 259-263
- URL: https://www.wjgnet.com/1949-8470/full/v5/i7/259.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i7.259
Magnetic resonance imaging (MRI) is an emerging modality for detection and staging of prostate cancer. To date, multiparametric imaging protocols for MRI of the prostate apply morphological high-spatial resolution T2-weighted sequences complemented by functional imaging techniques: diffusion weighted imaging, dynamic contrast-enhanced imaging, and MR-spectroscopy. A mandatory next step for further acceptance of this technique is to simplify and to standardize the MR-protocols for prostate MRI.
A majority of MR-studies of the prostate are performed with glucagon or hyoscine-N-butyl-bromide (HBB, butylscopolamine) because administration of an anti-peristaltic drug is recommended for many oncologic MR-examinations of the pelvis[1,2]. The rationale behind it consists of motion reduction of prostate surrounding structures (bladder, rectum, and small bowels) that may cause motion related artefacts which potentially degrade signal-to-noise ratio (SNR) and image quality. However, a recent study by Wagner et al[3] found no benefit for HBB in prostate MRI at 1.5 Tesla. The authors contended that the prostate is located in the lower pelvis, between pelvic floor muscles, bladder and rectum, distant to small bowel structures, and thus it is not affected by peristaltic artefacts. Consequently, they suggested waiving of spasmolytic drug administration. At present, MRI of the prostate at 3.0 Tesla is becoming the state-of-the art examination, because the increased field strength at 3.0 Tesla potentially improves image quality by increased SNR[4,5]. Nevertheless, non-significant artefacts due to peristaltic bowel motion on 1.5 Tesla MR imaging may become more exaggerated on 3.0 Tesla MR imaging and result in reduced image quality.
Hence, purpose of the study was to evaluate the value of administration of HBB for image quality in prostate MRI at 3.0 Tesla.
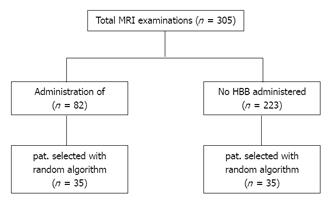
This retrospective, single institutional study was approved by local ethics committee. Patients were included into the study from October 2010 to June 2011. All patients were referred for prostate MRI from the university hospital by the department of urology with clinical suspicion of prostate cancer. Patients with prior radiation therapy or adjuvant hormone ablative therapy were not included into the study. The standard MR-protocol provided administration of HBB. HBB was not administered in the presence of contraindications such as glaucoma, cardiac arrhythmia and/or ischemic heart disease, myasthenia gravis, and apparent benign prostatic hyperplasia with potential urinary retention[6]. Patients who used their car after the examination did not receive HBB for safety reasons. The data of the included patients were transferred into pseudonymous data and allocated to a database into a HBB-group and into a non-HBB-group. Then, the patients in both groups were sorted according to age in an ascending sequence and into 5-year intervals. To avoid a selection bias, a random number generator software function was used (Excel; Microsoft, Redmond, WA, United States): first, a patient was selected out of the HBB-group. Then, a patient from the non-HBB-group was randomly drawn out of the corresponding age-interval. In total, two datasets with 35 patients from each group were generated (Figure 1). Finally, the resulting groups were tested for statistically significant age difference.
Patients in the HBB group were administered 40 mg of drug approved HBB (Buscopan®; Boehringer, Ingelheim, Germany) by a venous access directly before the examination. All examinations were performed on a 3.0 Tesla scanner (Siemens Magnetom Trio; Siemens Healthcare, Erlangen, Germany) using the manufacturer standard multi-channel body coil and integrated spine phased-array coil. The MR-protocol started with T2-weighted half-fourier acquisition turbo spin echo localizer sequences. An transverse T1-weighted 3-dimensional gradient echo sequence (FLASH-3D) was obtained for lymph node staging and detection of haemorrhage with the following imaging parameters: repetition time (TR): 6.66 ms, echo time (TE): 2.55 ms, echo train length: 1, averages: 1, section thickness: 3 mm, no intersection gap, matrix: 317 × 512, field of view: 24 cm × 35 cm, acquisition time: 1:40 min. Sequences included high-spatial resolution T2-weighted turbo spin echo MR imaging in the transverse and coronal plane with the following imaging parameters: TR: 5120 ms, TE: 143 ms, echo train length: 13, averages: 4, section thickness: 3 mm, no intersection gap, matrix: 254 × 448, field of view: 21.2 cm × 30.0 cm, acquisition time: 4:14 min. Axial diffusion-weighted images were acquired using a single-shot echo-planar imaging pulse sequence: TR 12500 ms, TE 65 ms; averages, 4; matrix size, 176 × 176; FOV 450 × 306 mm2; slice thickness, 5 mm; parallel imaging GRAPPA factor 2; b-values: 0, 50, 100, 150, 200, 250, and 800 s/mm2). Three orthogonal diffusion directions were acquired. ADC maps were implicitly calculated on the scanner with the standard software provided by the manufacturer using all measured b-values.
DCE was performed with a high-spatial resolution T1-weighted 3-dimensional gradient echo sequence with a temporal resolution of 9.9 s: TR 4.42 ms, TE 2.2 ms; flip angle 15°; matrix size 176 × 265, FOV 400 × 275 mm2; slice thickness 1.5 mm. As contrast agent, weight-adjusted (0.1 mmol/kg) gadobutrol (Gadovist®; Bayer Healthcare, Leverkusen, Germany) was administered.
The acquired datasets were analysed by two board-certified radiologists with 5 and 12 years of experience in reading prostate MRI studies. Both radiologists were blinded to administration of HBB. The readers scored the cases independently and in random order on Likert five-point scales. First, anatomical details (delineation between peripheral and transitional zone of the prostate, visualisation of the capsule, depiction of neurovascular bundles) and visualisation of local and iliac lymph nodes were assigned on a five-point scale referring to imaging criteria described by Wagner et al[3]: (1) non-diagnostic: structures cannot be evaluated; (2) poor visualization: heavily blurred appearance of structures; (3) moderate visualization: moderate blurring; (4) good delineation: slight blurring; and (5) excellent visualization: sharp delineation. Motion related artefacts in the prostatic area were scored on the following five-point scale: (1) no artefacts; (2) few artefacts; (3) moderate artefacts; (4) considerable artefacts; and (5) severely affected. Finally, the readers documented their perception of overall image quality: (1) non-diagnostic; (2) poor; (3) satisfactory; (4) good; and (5) excellent.
A two-tailed Wilcoxon rank-sum test was used to determine significant difference between the two cohorts. Additionally, Bonferroni correction was applied. Weighted kappa (κ) statistics were used for evaluation of interobserver agreement. The following intervals were defined for interpretation of the kappa values: 0.0-0.2 = poor, 0.21-0.4 = fair, 0.41-0.6 = moderate, 0.61-0.8 = substantial, 0.81-1.00 high to almost perfect agreement. A P-value of 0.05 or less was considered as statistically significant. All analyses were performed with SAS/STAT software (SAS Institute, Cary, NC, United States).
305 patients matched the predefined criteria. Out of this cohort 82 patients were eligible for administration of HBB and 223 patients did not receive HBB. The two resulting groups of 35 patients each showed no statistical difference regarding age distribution (P = 0.26) with an average age in the HBB-group of 64.9 [95%CI: 62.4- 67.4] and 67.0 years [95%CI: 64.7-68.6] in the non-HBB group, respectively.
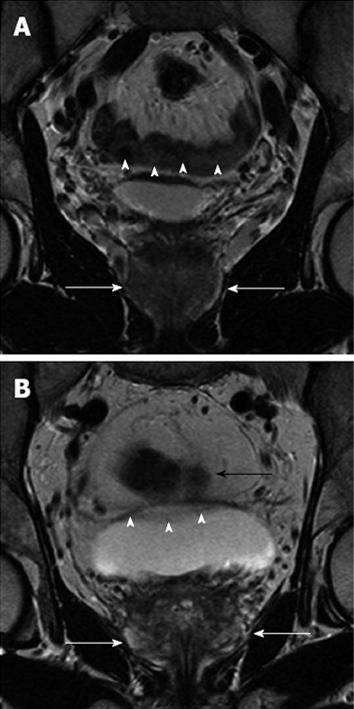
Qualitative analysis demonstrated good results regarding the scored criteria for both groups (Figure 2, Table 1). Comparison of anatomical details between the two cohorts showed no statistical significant difference (P = 0.54 and P = 0.07) for both readers. There was no statistically significant advantage regarding depiction of local and iliac lymph nodes (P = 0.07 and P = 0.19). Motion artefacts were rated as “no” to “few” in both groups. Analysis showed no statistical difference (P = 0.19 and P = 0.22) between the two groups. Two examples of the evaluated patient sets are demonstrated in Figure 3A and B. Scoring of overall image quality showed no significant difference (P = 0.78 and P = 0.09).
| Non-HBB group | HBB group | |||
| A | B | A | B | |
| Prostate anatomy | 3.9 ± 0.7 | 3.8 ± 0.7 | 4.0 ± 0.9 | 4.2 ± 0.6 |
| Iliac lymph nodes | 3.9 ± 0.6 | 3.8 ± 0.9 | 4.2 ± 0.6 | 4.1 ± 0.8 |
| Related artefacts | 2.3 ± 1.0 | 2.3 ± 1.1 | 1.9 ± 0.9 | 1.9 ± 0.7 |
| Overall quality | 4.0 ± 0.6 | 3.8 ± 0.8 | 4.0 ± 0.9 | 4.2 ± 0.6 |
Evaluation of inter-observer agreement was moderate for all criteria (range: κ = 0.44-0.53) except for a fair result with κ = 0.37 for assessment of overall image quality. However, a difference of more than one scale interval between the two readers occurred in only 3 of 280 scores (1.07%).
The results demonstrated no significant effect of administration of HBB on visualisation of the prostate, iliac lymph nodes, periprostatic artefacts, and overall image quality. The findings correspond to the results of a study by Wagner et al[3] at 1.5 Tesla with ERC that found no significant effect of intravenous or intramuscular administration of HBB for visualisation of the prostate, neurovascular bundles, pelvic lymph nodes, and overall image quality. Studies promoting the use of HBB for pelvic MRI emphasize the advantage of suppressing bowel peristalsis to reduce motion artefacts[4,7,8], which is particularly the case in MR-sequences with long acquisition times such as T2w TSE sequences[9]. Nevertheless, motion artefacts were comparably low in both of our assessed groups, which could be explained by the anatomical distance between the prostate and small bowel. Thus, the prostate is not directly affected by small bowel motion artefacts (Figure 3A and B). Choosing a posteroanterior phase-encoding direction, which sufficiently suppresses peristaltic artefacts caused by small and larger intestine, is an explanation for visualisation of iliac lymph nodes being marginally affected by administration of HBB[10]. In contrast to this study, an initial study by Johnson et al[11] found improved visualisation of the prostate in about 40% of the patients after HBB administration. However, the number of patients was considerably small with 23 men and there was no dedicated MR-protocol for prostate imaging at a 1.0 Tesla system.
In opposite to this study, Wagner et al[3] used an ERC for prostate imaging at 1.5 Tesla. To date, after introduction of 3.0 Tesla scanners, the increased SNR is frequently used to exclude the ERC from prostate examinations, mainly in order to prevent discomfort from patients and to avoid the additional costs for the ERC[12-17]. The use of an ERC provides a wide-ranging immobilization of the prostate in the lower pelvis, which contributes to reduction of motion related artefacts in that specific area[3]. On the other hand, the inflated ERC provides a mechanical stimulus for rectal motion artefacts that can hardly be suppressed by HBB. That is further the case for air that processes through the rectum during the examination. Nevertheless, studies at 1.5 Tesla found that image quality of prostate MRI without ERC to be equal to those with ERC without increased motion artefacts[5,12,13]. A few studies assessed the additional value of the use of an ERC for prostate MRI at 3.0 Tesla. These studies demonstrated an incremental benefit for image quality using an ERC[4,7,8]. However, it remains unclear if further improved SNR at 3.0 Tesla by an ERC improves diagnostic performance[9].
Although there was no significant effect of HBB administration on image in our study, the authors believe that there are indications for administration of an anti-peristaltic drug in MR of the prostate, e.g., for patients with hyper-motile intestine or flatulence. In these cases, an anti-peristaltic drug can be administered subsequently after detection on initial T1- or T2-weighted sequences. Furthermore, it would be interesting to investigate the effect of an anti-peristaltic agent on spectral noise in MR-spectroscopy.
The study has some limitations. First, the study was performed in a retrospective design. A larger prospective study would substantiate the findings of this study. Secondly, there was no intra-individual comparison between HBB and no HBB administration. This methodical drawback was compensated by a sufficient number of patients and adjustment of the age characteristics in both patient groups. At present, there is no evidence for a difference between the HBB and the non-HBB group caused by a methodical bias.
In conclusion, the results of the study demonstrated no significant effect of HBB administration on image quality of prostate MRI at 3.0 Tesla without ERC. The results of the study suggest that the use of HBB is not mandatory for MRI of the prostate.
A mandatory next step for further acceptance for prostate magnetic resonance imaging (MRI) is to simplify and to standardize MR-protocols. Like many other oncologic MR-examinations of the pelvis, a majority of MR-studies of the prostate are performed using an anti-peristaltic drug. However, recent studies indicate that the additional value of bowel motion suppressing agents is limited. Thus, purpose of this study was to evaluate the value of administration of an anti-peristaltic agent for prostate MRI at 3.0 Tesla.
A current topic of research consists of standardizing reading, reporting, and conduction of multiparametric MRI of the prostate. At present, for prostate MRI, there are different recommendations regarding the use of an anti-peristaltic agent for bowel motion suppression.
A recent study by Wagner et al. found no benefit for administration of an anti-peristaltic agent for prostate MRI at 1.5 Tesla. However, a contrary study by Johnson et al. found improved visualisation of the prostate at 1.0 Tesla in about 40% of the patients after administration of an anti-peristaltic agent. Furthermore, the use of an endorectal coil may have an influence on prostate MRI. Currently, a major number of examinations at state-of-the-art 3.0 Tesla scanners are carried out without using an endorectal coil.
The study demonstrated no significant effect of hyoscine-N-butyl-bromide (HBB) (butylscopolamine) administration on image quality of prostate MRI at 3.0 Tesla. The results suggest that the use of HBB is not generally mandatory for MRI of the prostate. This may help to further facilitate and to simplify prostate MRI.
HBB, an anti-peristaltic agent commonly used for suppression of bowel motion, e.g., for pelvic imaging. Endorectal coils are used to gain increased signal from the prostate. However, modern pelvic phased-array surface coils deliver excellent signal, especially at higher field strengths. Therefore, many sites perform prostate MRI without using an endorectal coil.
The authors evaluated the role of administration of HBB in improving the image quality of MRI of the prostate at 3.0 Tesla. The results demonstrated no significant effect of HBB administration on image quality, and suggest that the use of HBB is not mandatory for MRI of the prostate at 3.0 Tesla. This is a direct and carefully-designed study with relatively large number of patients. The conclusion drew from the study is clear and convincing. The study was carried out thoroughly and the paper is written nicely.
P- Reviewers Chen F, El-Ghar MA, Maintz D S- Editor Gou SX L- Editor A E- Editor Lu YJ
| 1. | Dosdá R, Martí-Bonmatí L, Ronchera-Oms CL, Mollá E, Arana E. Effect of subcutaneous butylscopolamine administration in the reduction of peristaltic artifacts in 1.5-T MR fast abdominal examinations. Eur Radiol. 2003;13:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Fütterer JJ. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1898] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 3. | Wagner M, Rief M, Busch J, Scheurig C, Taupitz M, Hamm B, Franiel T. Effect of butylscopolamine on image quality in MRI of the prostate. Clin Radiol. 2010;65:460-464. [PubMed] |
| 4. | Bloch BN, Rofsky NM, Baroni RH, Marquis RP, Pedrosa I, Lenkinski RE. 3 Tesla magnetic resonance imaging of the prostate with combined pelvic phased-array and endorectal coils; Initial experience(1). Acad Radiol. 2004;11:863-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Sosna J, Pedrosa I, Dewolf WC, Mahallati H, Lenkinski RE, Rofsky NM. MR imaging of the prostate at 3 Tesla: comparison of an external phased-array coil to imaging with an endorectal coil at 1.5 Tesla. Acad Radiol. 2004;11:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Dyde R, Chapman AH, Gale R, Mackintosh A, Tolan DJ. Precautions to be taken by radiologists and radiographers when prescribing hyoscine-N-butylbromide. Clin Radiol. 2008;63:739-743. [PubMed] |
| 7. | Heijmink SW, Fütterer JJ, Hambrock T, Takahashi S, Scheenen TW, Huisman HJ, Hulsbergen-Van de Kaa CA, Knipscheer BC, Kiemeney LA, Witjes JA. Prostate cancer: body-array versus endorectal coil MR imaging at 3 T--comparison of image quality, localization, and staging performance. Radiology. 2007;244:184-195. [PubMed] |
| 8. | Hambrock T, Somford DM, Hoeks C, Bouwense SA, Huisman H, Yakar D, van Oort IM, Witjes JA, Fütterer JJ, Barentsz JO. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183:520-527. [PubMed] |
| 9. | Seitz M, Shukla-Dave A, Bjartell A, Touijer K, Sciarra A, Bastian PJ, Stief C, Hricak H, Graser A. Functional magnetic resonance imaging in prostate cancer. Eur Urol. 2009;55:801-814. [PubMed] |
| 10. | Wood ML, Runge VM, Henkelman RM. Overcoming motion in abdominal MR imaging. AJR Am J Roentgenol. 1988;150:513-522. [PubMed] |
| 11. | Johnson W, Taylor MB, Carrington BM, Bonington SC, Swindell R. The value of hyoscine butylbromide in pelvic MRI. Clin Radiol. 2007;62:1087-1093. [PubMed] |
| 12. | Park BK, Kim B, Kim CK, Lee HM, Kwon GY. Comparison of phased-array 3.0-T and endorectal 1.5-T magnetic resonance imaging in the evaluation of local staging accuracy for prostate cancer. J Comput Assist Tomogr. 2007;31:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Torricelli P, Cinquantini F, Ligabue G, Bianchi G, Sighinolfi P, Romagnoli R. Comparative evaluation between external phased array coil at 3 T and endorectal coil at 1.5 T: preliminary results. J Comput Assist Tomogr. 2006;30:355-361. [PubMed] |
| 14. | Beyersdorff D, Winkel A, Hamm B, Lenk S, Loening SA, Taupitz M. MR imaging-guided prostate biopsy with a closed MR unit at 1.5 T: initial results. Radiology. 2005;234:576-581. [PubMed] |
| 15. | Liu X, Zhou L, Peng W, Qian M. Effect of intravenous gadolinium-DTPA on diffusion-weighted imaging for prostate lesions and normal tissue at 3.0-Tesla magnetic resonance imaging. Acta Radiol. 2011;52:575-580. [PubMed] |
| 16. | Kim CK, Park BK. Update of prostate magnetic resonance imaging at 3 T. J Comput Assist Tomogr. 2008;32:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Augustin H, Fritz GA, Ehammer T, Auprich M, Pummer K. Accuracy of 3-Tesla magnetic resonance imaging for the staging of prostate cancer in comparison to the Partin tables. Acta Radiol. 2009;50:562-569. [PubMed] |