Published online Apr 28, 2013. doi: 10.4329/wjr.v5.i4.156
Revised: February 8, 2013
Accepted: March 6, 2013
Published online: April 28, 2013
Processing time: 139 Days and 12.4 Hours
AIM: To review the literature on the assessment of venous vessels to estimate the penumbra on T2*w imaging and susceptibility-weighted imaging (SWI).
METHODS: Literature that reported on the assessment of penumbra by T2*w imaging or SWI and used a validation method was included. PubMed and relevant stroke and magnetic resonance imaging (MRI) related conference abstracts were searched. Abstracts that had overlapping content with full text articles were excluded. The retrieved literature was scanned for further relevant references. Only clinical literature published in English was considered, patients with Moya-Moya syndrome were disregarded. Data is given as cumulative absolute and relative values, ranges are given where appropriate.
RESULTS: Forty-three publications including 1145 patients could be identified. T2*w imaging was used in 16 publications (627 patients), SWI in 26 publications (453 patients). Only one publication used both (65 patients). The cumulative presence of hypointense vessel sign was 54% (range 32%-100%) for T2* (668 patients) and 81% (range 34%-100%) for SWI (334 patients). There was rare mentioning of interrater agreement (6 publications, 210 patients) and reliability (1 publication, 20 patients) but the numbers reported ranged from good to excellent. In most publications (n = 22) perfusion MRI was used as a validation method (617 patients). More patients were scanned in the subacute than in the acute phase (596 patients vs 320 patients). Clinical outcome was reported in 13 publications (521 patients) but was not consistent.
CONCLUSION: The low presence of vessels signs on T2*w imaging makes SWI much more promising. More research is needed to obtain formal validation and quantification.
Core tip: Thrombolytic therapy with intravenous tissue plasminogen activator is the only approved therapy for acute ischemic stroke. The detection of hypointense venous vessels with blood oxygenation level dependent (BOLD) imaging to assess the amount of penumbral tissue in acute ischemic stroke has emerged as a little noticed alternative imaging technique. In the present state the combined use of perfusion and BOLD imaging would provide further complementary information to help visualize and understand the role of the ischemic penumbra.
- Citation: Jensen-Kondering U, Böhm R. Asymmetrically hypointense veins on T2*w imaging and susceptibility-weighted imaging in ischemic stroke. World J Radiol 2013; 5(4): 156-165
- URL: https://www.wjgnet.com/1949-8470/full/v5/i4/156.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i4.156
Assessment of penumbral tissue is a key concept to identify patients who may benefit from acute stroke therapy. One has to keep in mind that the penumbra cannot be regarded as a homogenous single entity but rather a gradient from ischemic core to normal tissue[1]. Therefore, whatever imaging method is used for penumbra detection, one or more arbitrary cut-off values need to be chosen to segment the brain image into normal tissue and ischemic core, with one or more penumbras in between. Despite oxygen-15 positron emission tomography (15O-PET) being the gold standard to define the penumbra[2], its clinical use is very limited due to logistical and practical reasons. As a substitute, magnetic resonance imaging (MRI) with diffusion-weighted image (DWI)-perfusion-weighted image (PWI) mismatch is commonly used in the clinical work-up of stroke patients[3]. Although widely utilized, it has some methodological limitations. The DWI lesion does not seem to represent irreversibly damaged tissue[4], nor can PWI differentiate between penumbra and benign oligemia[5]. Recently, the use of blood oxygenation level dependent (BOLD) imaging as an alternative to DWI-PWI mismatch has stirred a lot of interest[6]. It is sensitive to an increased concentration of deoxyhemoglobin (DHb) and may therefore be an indirect marker of oxygen metabolism. There are two approaches to detect an increased oxygen extraction fraction (OEF) using BOLD: assessment of tissue and assessment of draining veins containing an increased fraction of DHb.
As the latter has been described by several authors, we will focus this review on the assessment of venous vessels to estimate the amount of penumbral tissue. We provide an overview on published data, take a glimpse at experimental studies and critically appraise the method’s clinical value and suggest future research.
Publications that reported on the assessment of the penumbra in ischemic stroke by hypointense vessels on T2*w imaging and/or SWI and performing at least one validation method were included. Search terms in PubMed were “T2*”, “GRE”, “SWI”, “susceptibility weighted imaging”, “leptomeningeal vessels/veins”, “hypointense”, “stroke”. Full text articles and published abstracts from major stroke and MRI related conferences (International Stroke Conference, European Stroke Conference, World Stroke Congress, World Congress of Neurology, ISMRM, ASNR, etc.) were included in this review. Only literature published in English and dealing with human subjects was included, patients with Moya-Moya syndrome were disregarded. The reference section of retrieved publications was manually searched for further relevant literature. Abstracts with obviously overlapping content with full text articles that were probably first presented as an abstract and subsequently published as full text articles were excluded.
Data is presented as cumulative absolute and relative percentage values. Ranges are given where appropriate.
Forty-three papers, conference abstracts and reports of cases (partially from review articles) with a total of 1145 patients were identified[7-49]. For details of the reviewed studies with relevant information, see Tables 1 and 2. Sixteen publications (627 patients) were identified using T2*w imaging (Table 1)[7-22]. SWI was used in 26 publications (453 patients, Table 2)[23-48]. In one publication both T2* and SWI was used (65 patients, Table 2)[49].
| Ref. | n | Field strength | Occluded vessel | Time from onset | Validation method | Results |
| Hermier et al[7] | 49 | 1.5 T | Anterior circulation | 6 h | DWI; DSC-MRI | AVV obvious in 8/49, moderate in 15/49 patients, inter- and intra-observer reliability r > 0.9; Correlated with higher baseline NIHSS, larger DWI and PWI lesions, worse outcome, intracranial haemorrhage and more severe hemodynamic impairment |
| Liebeskind et al[8] | 91 | NS | MCA | NS | MRA; DWI | Hypointense vessels in or adjacent to the infarct in 40/91 patients |
| Liebeskind et al[9] | 83 | NS | MCA | Median 2 d | MRA | Unilateral hypointensity of the basal vein of Rosenthal was noted in 27/83 patients on the side of the occlusion |
| Hermier et al[10] | 48 | 1.5 T | NS | 6 h | DWI; DSC-MRI | AVLV present in 17/48 patients, within TTP lesion, not concordant with DWI lesion; No impact on clinical status and final stroke volume |
| Seo et al[11] | 20 | 3 T | ICA, MCA | 6 h | DSC-MRI; DWI | HVS present in 13/20 patients. Patients with asymmetrical HVS had better NIHSS improvement (8.1 ± 5.7 vs 2 ± 4.2) |
| Sohn et al[12] | 86 | NS | ICA, MCA | 12 h | DSC-MRI; DWI | Present in 59/86 patients; HypoTCV associated with large perfusion defect, but low cholesterol and haemoglobin level may obscure its visibility |
| Ha et al[13] | 22 | 3 T | MCA | 6 h | DSA | Present in 7/22 patients. Patients with HLV showed larger baseline NIHSS (16.9 ± 3.4 vs 11.7 ± 5.3) and major improvement (≥ 8 points) was observed more often. It corresponded with delayed venous wash-out on DSA |
| Morita et al[14] | 24 | 3 T | ICA, MCA | 12 h | DWI; FAIR | CVS present in all patients, BS present in 23/24 patients, good interobserver agreement (κ = 0.7). Area defined by CVS/BS similar to hypoperfused area |
| Harada et al[15] | 24 | 3 T | NS | 12 h | FAIR; DWI | κ for cortical and deep vessel signs 0.84 and 0.72, respectively |
| Ha et al[16] | 35 | NS | MCA | 6 h | DSC-MRI; DWI; DSA | HLV present in 12/35 patients. Patients with HLV had larger NIHSS improvement at 7 d (6.5 ± 4.6 d vs 0.5 ± 6.7 d) and bigger TTP-DWI mismatch. HLV corresponded with delayed venous wash-out |
| Kaya et al[17] | 20 | 3 T | Large arteries | 3 h | DWI; DSC-MRI | Present in all patients. Very good correlation of RMHV with final infarct (r = 0.91) and MTT/rCBV lesion (r = 0.96); very high interobserver correlation (ICC = 0.99) |
| Kinoshita et al[18] | NS (case series) | 1.5 T | NS | NS | 15O-PET | Enhanced venous contrast (hypointensity and enlargement of veins), ipsilateral corresponding increased OEF |
| Harada et al[19] | 33 | 3 T | ICA, MCA | 3 h | FAIR; DWI | IschV present in 79% (κ = 0.83); Not correlated with worse outcome |
| Tada et al[20] | 2 | 3 T | MCA (stenosis) | NS | DWI; DSC-MRI; 123I-IMP SPECT | Area defined by ischemic signs was similar to area of hypoperfusion on MRI and SPECT |
| Rosso et al[21] | 60 | 3 T | Anterior circulation | 6 h | DWI; MRI | VTV present in 58.3% (κ = 0.895), correlated with larger infarcts and haemorrhage but not with baseline or follow-up NIHSS |
| Ryoo et al[22] | 30 | NS | ICA, MCA | 6 h | Clinical | GRE vein present in 15/30 patients. Early neurological improvement (ΔNIHSS ≥ 8 or NIHSS ≤ 2 at 24 h) more frequently observed with GRE vein (8 patients vs 1 patients, P = 0.014) |
| Ref. | n | Field strength | Occluded vessel | Time from onset | Validation method | Results |
| Ida[23] | 62 | 1.5 T | NS | 24 h | DSC-MRI; DWI | IVC were noted in 77.4%, agreement with perfusion defect in all patients |
| Tong et al[24] | 1 | NS | MCA | 3 d | ADC | Prominent asymmetric medullary veins exceeding the area of the DWI lesion |
| Mittal et al[25] | 1 | 1.5 T | MCA | 2 h | DWI; DSC-MRI | Prominently hypointense cortical veins exceeding the area of the DWI lesion, similar to PWI lesion |
| Santhosh et al[26] | 1 | 1.5 T | MCA | NS | DWI | Prominent veins exceeding the area of the DWI lesion |
| Tsui et al[27] | 1 | NS | MCA | NS | DWI | Prominent hypointense veins exceeding the area of the DWI lesion |
| Christoforidis et al[28] | 6 | 3 T | MCA | NS | DSC-MRI; DWI | MHV were noted within the MTT lesion while they absent within the DWI lesion |
| Hingwala et al[29] | 1 | NS | MCA | NS | DWI | Prominent veins exceeding the area of the DWI lesion |
| Huisman et al[30] | 1 | NS | MCA | NS | ADC/DWI | Prominent intramedullary veins exceeding the area of the DWI lesion |
| Mittal et al[31] | 2 | 1.5 T | MCA | NS | ADC/DWI; DSC-MRI | Prominent hypointense veins exceeding the area of the DWI lesion, similar to PWI lesion |
| Yen et al[32] | 1 | NS | MCA | 4 d | DWI; DSC-MRI | Prominent venous hypointensities exceeding the area of the DWI lesion, similar to PWI lesion |
| Kesavadas et al[33] | 2 | NS | MCA | NS | DSC-MRI; ADC/DWI | Prominent veins exceeding the area of the DWI lesion, similar to PWI lesion |
| Park et al[34] | 82 | NS | ICA, MCA | 3 d | DSC-MRI; DWI | MHV visible in 73/82 patients, excellent agreement with TTP maps |
| Lin et al[35] | 53 | NS | ICA, MCA | 12 h | Traditional MRI | Hypointense transmedullary veins predisposed to worse outcome (OR = 2.2) |
| Meoded et al[36] | 2 | NS | MCA, ACA | NS | Conventional MRI; DWI | Prominent intramedullary veins were noted within the DWI lesion. In one case prominent sulcal veins matched the area of infarct growth. |
| Gasparotti et al[37] | 1 | 1.5 T | ICA | NS | DWI; DSC-MRI | SWI lesion exceeded DWI lesion and matched MTT lesion |
| Yamashita et al[38] | 30 | 3 T | MCA | 7 d | DWI; FAIR | Increased venous contrast in 22/30 patients, area similar to hypoperfused tissue |
| Huang et al[39] | 44 | 3 T | MCA | 2 d | DWI; MRA; CT | Prominent veins present in 15/44 patients; Not correlated with haemorrhage or outcome |
| Kao et al[40] | 15 | 1.5 T | MCA | 18 h | DWI; DSC-MRI | MTT-DWI and SWI-DWI mismatch similar to predict infarct growth |
| Tsai et al[41] | 49 | NS | NS | 3.5-8.5 h | MRI; Clinical | Presence of hypointense veins in all patients with worse outcome and haemorrhagic complications |
| Meoded et al[42] | 8 | NS | NS | 72 h | DWI | DWI > SWI mismatch found in 1/15 affected regions |
| Park et al[43] | 9 | NS | TIA | NS | DWI; DSC-MRI; MRA | 4/9 patients with DWI negative TIA showed asymmetric hypointense vessels, in accordance with perfusion deficit and stenosed/occluded vessel |
| Fujioka et al[44] | 1 | NS | ICA | 10 h | MRI | SWI lesion exceeding DWI lesion matured into infarction |
| Verschuuren et al[45] | 1 | NS | MCA, ACA | NS | ADC; CT | SWI lesion exceeding ADC lesion matured into infarction on CT |
| Meoded et al[46] | 1 | 1.5 T | MCA, PCA | NS | T2; ADC | SWI lesion matches ADC lesion; Mismatch also noted, indicating critical perfusion |
| Baik et al[47] | 19 | 1.5 Tand 3 T | ICA, MCA, BA | Median 2.5 h | DWI | Prominent veins present in affected territory which disappeared after recanalization (10/10 patients); After recanalization within DWI lesion: Equally prominent in 10/19 patients, small DWI lesions, good clinical outcome (7 d NIHSS median 3.5, 90 d mRS median 0) indicating normalisation; Less prominent in 5/19 patients, large DWI lesions, poor clinical outcome (7 d NIHSS median 13, 90 d mRS median 4) indicating futile reperfusion; Mixed in 4/19 patients, medium DWI lesions, relatively poor outcome (7d NIHSS median 13, 90 d mRS median 2) |
| Tsai[48] | 59 | NS | NS | NS | Imaging; Clinical | 34 patients improved or stable (clinical, imaging), 25 worse; SWI correlated with poor prognosis |
| Sohn et al1 [49] | 65 | NS | Anterior circulation | 12 h | Perfusion MRI | Asymmetrical HVS in 98% (SWI) and 74% (T2*w) |
The cumulative presence of hypointense vessels was 54% (range 32%-100%) of 668 patients for T2* and 81% (range 34%-100%) of 334 patients for SWI. However, more single patient case reports were identified for SWI.
Inter-rater agreement was reported in six publications (210 patients)[7,14,15,17,19,21] and ranged from good (κ = 0.7)[14] to excellent (intraclass correlation = 0.99)[17]. The agreement between penumbra on T2*w imaging and dynamic susceptibility contrast (DSC)-MRI was quantified in one publication (20 patients)[17] and was 0.92 [time to peak and relative cerebral blood flow (CBF)] to 0.96 (mean transit time and relative cerebral blood volume).
In most publications perfusion MRI either utilizing DSC-MRI (n = 17441 patients)[7,10-12,16,17,20,23,25,28,31-34,37,40,43] or flow sensitive alternating inversion recovery (n = 4111 patients)[14,15,19,38] combined with DWI/apparent diffusion coefficient (ADC) was used for validation (perfusion method not stated in one publication, 65 patients[49]). In three publications one additional method was used [digital subtraction angiography (DSA)[16], iodine-123 iodoamphetamine single-photon emission computed tomography[20] or magnetic resonance angiography (MRA)[43]]. Conventional MRI (DWI/ADC, MRA, structural imaging) was used in 14 publications (315 patients)[8,9,21,24,26,27,29,30,35,36,44,46,47], in three publications[39,41,45] computed tomography or clinical data were used as additional methods. Clinical information and unspecified imaging was used in one publication (59 patients)[48]. One publication used clinical data alone (30 patients)[48]. DSA alone was used in one publication (22 patients)[13]. In one case series gold standard 15O-PET was used for validation (number of patients not stated)[18].
Time of onset ranged from 3 h to 7 d with more publications reporting on the subacute (> 6 h, n = 15579 patients)[9,12,14,15,23,24,32,34,35,38,39,40,42,44,49] than the acute phase (≤ 6 h, n = 11337 patients)[7,10,11,13,16,17,19,21,22,25,47]. In one publication time from onset ranged from 3.5 to 8.5 h (49 patients)[41]. It was not provided in 16 publications (170 patients)[8,18,20,26-31,33,36,37,43,45,46,48].
Outcome was reported in 13 publications. In four publications (185 patients)[10,19,21,39] no correlation with clinical outcome was found. In four publications (107 patients)[11,13,16,22] a larger National Institute of Health Stroke Score improvement was observed, while in another four publications a worse outcome was found (210 patients)[7,35,41,48]. In one publication there was a better clinical outcome with normalized vessel appearance after successful recanalization (19 patients)[47].
The occluded vessel was located in the anterior circulation in 33 publications (864 patients)[7-9,11-14,16,19,21,22,24-40,44-47,49]. It was not stated in seven publications (250 patients)[10,15,18,23,41,42,48]. Other publications considered “large arteries” (20 patients)[17], the basilar artery (1 patient)[47], the posterior cerebral artery (1 patient)[46], TIA (9 patients)[43] and critical MCA stenosis (2 patients)[20].
Field strength was 1.5 T in 10 publications (180 patients)[7,10,18,23,25,26,31,37,40,46], 3 T in 11 publications (285 patients)[11,13-15,17,19-21,28,38,39] and not stated in 22 publications (662 patients)[8,9,12,16,22,24,27,29,30,32-36,41,42,43-45,48,49] while in one publication (19 patients)[47] both field strengths were used but not identified for individual patients.
For detailed differences between T2*w imaging and SWI see Table 3.
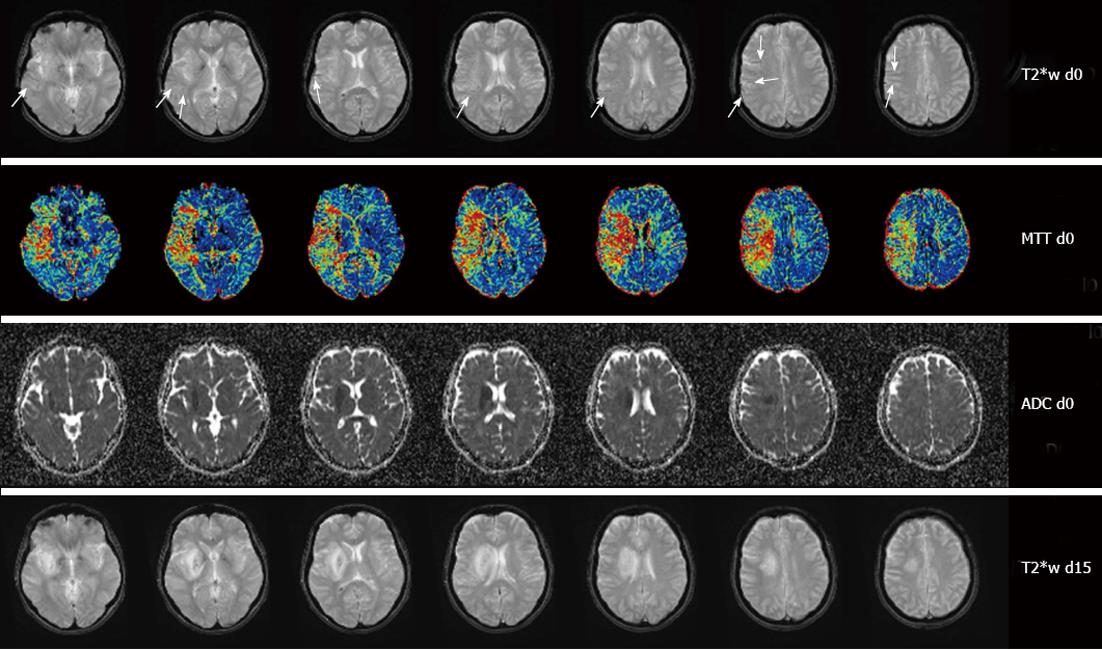
A number of terms have been used to describe the finding of hypointense venous vessels on T2*w or SWI images: Abnormal visualisation of leptomeningeal vessel, abnormal visualisation of transcerebral veins, cortical vessel sign, veins on gradient echo, hypointense leptomeningeal vessels, hypointense vessels, hypointense vessel sign, hypointense transcerebral or cortical veins, ischemic vessel signs, increased vessel contrast, multiple hypointense vessels and visibility of transcerebral veins have been used almost synonymously. A “region of multiple hypointense vessels” describes the area that is bordered and thus defined by hypointense vessels. The “brush sign” was described as a hypointense area along the course of subependymal and medullary veins in deep white matter.
| T2*w imagingpublications (patients) | SWIpublications (patients) | |
| Number of publications | 17 (692)1 | 27 (518)1 |
| Presence of vessel signs | 54% | 81% |
| 16 (668) | 21 (334) | |
| Interrater agreement | κ = 0.7 to > 0.9 and ICC = 0.99 | - |
| 6 (210) | ||
| Reliability assessment | r = 0.92-0.96 | - |
| 1 (20) | ||
| Occluded vessel | ||
| Anterior circulation | 12 (598)1 | 22 (331)1 |
| Large arteries | 1 (20) | - |
| Critical MCA stenosis | 1 (2) | - |
| TIA | - | 1 (9) |
| BA | - | 1 (1)2 |
| NS | 3 (72)3 | 4 (178) |
| Time from onset | ||
| ≤ 6 h | 9 (317) | 2 (18)4 |
| > 6 h | 5 (282) | 12 (364)1 |
| 3.5-8.5 h | - | 1 (49) |
| NS | 3 (93)3 | 13 (87) |
| Outcome | ||
| Worse outcome | 1 (49) | 3 (161) |
| Better NIHSS improvement | 4 (107) | - |
| Better outcome with normalisation | - | 1 (19) |
| No correlation | 3 (141) | 1 (44) |
| Validation method | ||
| DSC-MRI | 7 (260) | 10 (181) |
| FAIR | 3 (81) | 1 (30) |
| Conventional MRI | 3 (234) | 11 (242) |
| 15O-PET | 1 (NS) | - |
| DSA | 1 (22) | - |
| Field strength | ||
| 1.5 T | 3 (97) | 7 (83) |
| 3 T | 8 (205) | 3 (80) |
| NS | 6 (390)13 | 17 (355)15 |
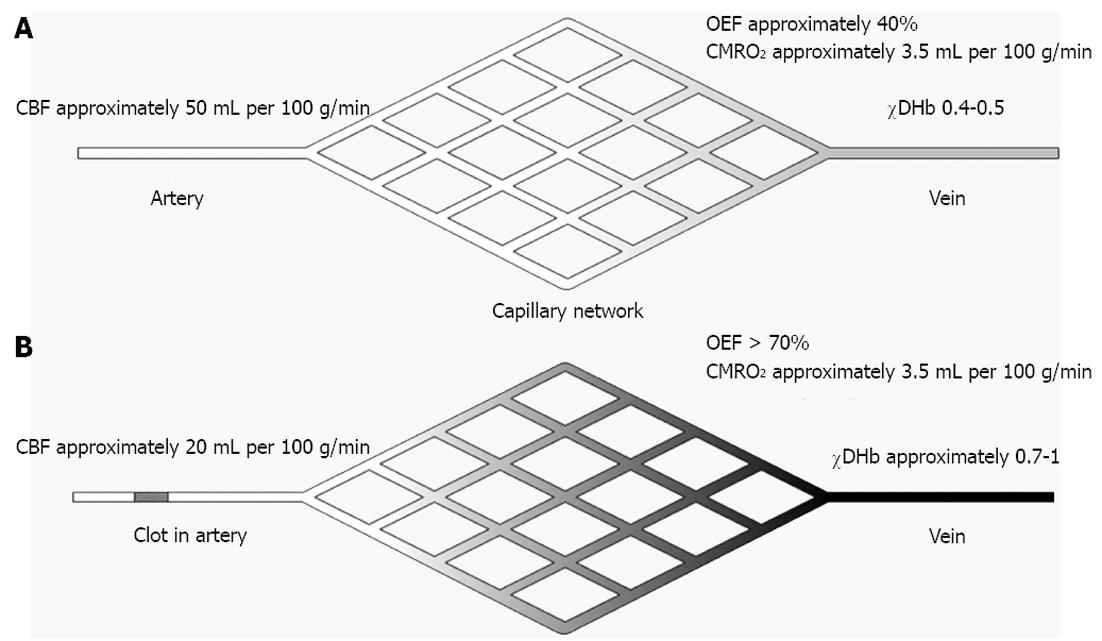
The occlusion of a brain-supplying artery causes a disruption in the supply of oxygen through decreased CBF. The threshold of 20 mL per 100 g/min is considered the threshold for penumbra but this value is likely to be time dependent[50]. The cerebral metabolism rate of oxygen (CMRO2) in viable, i.e., penumbral tissue is still at a near normal level (approximately 3.5 mL per 100 g/min) which causes the OEF to rise from its normal range to its theoretical maximum of 100%[51]. As can be seen in Equation 1 it is described as the ratio of CMRO2 and CBF multiplied with the oxygen content of blood (CaO2).
OEF = CMRO2/(CBF × CaO2).
While the increased OEF can keep CMRO2 stable over some time it causes the concentration of DHb to increase as the oxygen is transferred from oxyhemoglobin (OHb) to tissue and DHb is generated (Figures 1 and 2). However, as the BOLD signal is a composite of CBF, CMRO2 and cerebral blood volume (CBV) related signal changes their individual contributions can be difficult to establish. Dilatation of veins could thus in theory lead to an increased CBV (pooling of DHb) without concommitant change in overall flow or CMRO2 but may be detected by perfusion MRI. The phenomenon of darkening veins was already observed in early experimental stroke studies and was related to an increase in DHb[52].
Red blood cells carry haemoglobin which is present as OHb and DHb. Other cellular components of blood (leucocytes, platelets) do not contribute to oxygenation related signal changes. OHb has four outer electrons one of which is shared between the chelated hem iron and oxygen which makes OHb diamagnetic. In DHb, the four unpaired outer electrons are in a high-spin state which gives it paramagnetic properties[53].
This makes DHb an endogenous contrast agent which can be noninvasively imaged with appropriate MRI techniques. DHb induces local magnetic field inhomogeneity in vessels and surrounding tissue and causes faster transverse relaxation decay. T2*w images pick up that process which is translated into reduced T2* signal[54]. In addition to faster transverse relaxation decay, a phase dispersion is induced. This is exploited by SWI which combines both the magnitude and the phase information and thus further enhances differences in susceptibility than T2*w imaging alone. For an in-depth explanation of the SWI technique and further clinical examples please refer to the extensive reviews by Sehgal et al[55], Haacke et al[56] and Tong et al[24].
Quantitative susceptibility mapping (QSM) is a development of SWI which utilizes the phase data. It requires further postprocessing to obtain quantitative information on local susceptibility[57,58]. QSM may in the future be able to quantify oxygen saturation and thus provide fully quantitative and completely non-invasive information on oxygen metabolism.
The above stated pathophysiological considerations suggest that veins that appear hypointense and more pronounced in diameter may be used to assess oxygenation and in the face of acute ischemic stroke also may detect metabolically active tissue.
The depiction of hypointense veins can provide an approximation of the spatial extent of compromised oxygen metabolism. Although it has been qualitatively assessed, quantitative data is scarce. The published data however suggest that an estimation of the affected area is possible and reasonably reliable. The method does not deliver quantitative information and is only an indirect indicator of OEF. The main disadvantage is the fact that the exact relationship between discernible hypointensity within the vessels and increased OEF is unknown. Recently, SWI has been indirectly validated against arterial spin labelling[59] and has also been validated against 15O-PET in patients with chronic cerebrovascular disease[60]. However, formal validation is still missing and thus only subjective though reproducible[7,14,15,17,19,21] rater-dependent assessment is available.
There is only one publication directly comparing SWI and T2*w imaging in the same set of patients[49]. As expected, the sensitivity of T2*w imaging compared to SWI was significant (74% vs 98%). Accordingly, the pooled presence of vessel signs on T2* compared to SWI in the reviewed publications was considerably lower (54% vs 81%). The low presence of vessel signs in T2*w images is worrying and make SWI a much more suitable candidate sequence.
SWI has long been hampered by long acquisition times of about 10 min, making it unsuitable for acute stroke studies due to extreme susceptibility to movement artefacts. This is corroborated by the fact that only three publications using SWI in the acute clinical setting could be identified[25,41,47]. By the time of writing this article however, scanning time has been reduced to about 3 min for whole brain coverage, facilitating its usage in clinical protocols. More publications and patients were included using T2*w imaging. This is not completely surprising as T2*w imaging is already incorporated in MRI stroke protocols used in many institutions. However, their purpose is currently the detection of haemorrhage[61] and screening for blooming artefacts caused by thrombus in large vessel occlusion[62]. Attractively, both T2* and SWI combine information on haemorrhage that would preclude thrombolytic treatment, with information on penumbral tissue. Additionally, there is no need for the application of contrast agent in the light of potential albeit small risks of anaphylactic reactions and nephrogenic systemic fibrosis.
The findings regarding clinical outcome are heterogeneous. The reviewed publications either indicate larger improvement measured on the National Institute of Health Stroke Scale or worse outcomes. However, this finding is not necessarily a contradiction. Larger volumes of penumbral tissue may result in worse outcome if tissue at risk is not rescued by adequate therapy. The difference is thus likely an effect of successful therapy and not the volume of penumbral tissue itself.
As stated before, there is not one penumbra but a 4-dimensional gradient from necrosis to healthy tissue[1]. Depending on the occluded vessel, duration of ischemia, the tissue-specific vulnerability of certain areas of the brain, dynamics of reperfusion damage, etc., various necrotic and apoptotic pathways are activated. Recently, more modes of cell death have been identified[63]. Putative neuroprotective or neuroregenerative drugs will only work in a small window of this 4-dimensional space. Therefore, the need for MRI sequences that specifically visualize certain aspects of infarction and good segmentation algorithms are needed, so the effects of the drugs can be assessed correctly and not be masked by other processes that take place during infarction. Very little preclinical basic research[64] has been done so far on the imaging method presented in this review.
In conclusion, the detection of hypointense venous vessels with BOLD imaging to assess the amount of penumbral tissue in acute ischemic stroke has emerged as a little noticed alternative imaging technique. Although the data seems very encouraging and indirect validation has provided very convincing results, validation with gold standard PET or at least with complementary perfusion MRI in the acute phase is still missing. Further studies especially on SWI should be conducted since the data already available seem to merit further evaluation of this technique. A reliability assessment should be conducted and, in particular the possibilities of SWI in the acute clinical setting should be evaluated. It could prove useful in the non-acute setting or when no other imaging is available or when functional assessment of stenosis or occlusion is needed[65]. However, in the present state the combined use of perfusion and BOLD imaging would provide further complementary information to help visualize and understand the role of the ischemic penumbra.
We would like to thank Harry Ingleby, PhD for editing the manuscript.
Thrombolytic therapy with intravenous recombinant tissue plasminogen activator (rt-PA) is the only approved therapy for acute ischemic stroke. The target tissue for rt-PA therapy is the ischemic penumbra: electrically silent but viable and salvagable tissue. If no penumbra is present a given patient will only be subjected to the risks of rt-PA treatment. Thus, an accurate estimation of penumbral tissue to assess the risks and benefits of thrombolytic therapy is paramount.
In recent years magnetic resonance imaging (MRI) using the diffusion- and perfusion-weighted imaging mismatch concept was widely used to map the ischemic penumbra. It defines the penumbra as the difference between tissue that is terminally infarcted on diffusion weighted imaging and tissue that is undersupplied on perfusion MRI: the so-called mismatch. However, this concept proved to be an oversimplification. That is why an imaging protocol that provides insight into oxygen metabolism is needed.
Recently, blood oxygenation level dependent (BOLD) imaging has become a candidate sequence to map the ischemic penumbra. In short, it visualizes an increased deoxyhemoglobin (DHb) concentration. An increased DHb concentration is the signature of tissue that displays an elevated oxygen extraction fraction (OEF), a hallmark of the penumbra. Two approaches are generally used: Direct assessment of penumbral tissue, definition of penumbral tissue by draining veins. In this article the assessment of draining veins is reviewed.
Asymmetrically hypointense draining veins on T2*w and even more on SWI could serve as a surrogate marker for penumbral tissue. However, further validation and quantification is needed.
Penumbra: Tissue that has become undersupplied and electrically silent by an acute ischemic stroke. However, this tissue is still viable and can be salvaged by therapy; OEF: The percentage of oxygen extracted from the bloodstream by brain tissue. In normal tissue about 40%, elevated in penumbral tissue up to 100%; susceptibility weighted imaging: MRI sequence that combines the signal from T2*w imaging with phase information and thus enhances the contrast. BOLD imaging: Group of imaging methods that utilize the susceptibility difference between paramagnetic DHb and diamagnetic oxyhemoglobin. Any given change in oxygenation status will alter the signal.
This work represents a nice overview of what has been done in the field so far and points out that consistent reporting is necessary. However, it also points out that this is an important observation and should be more carefully studied as it could have a significant impact on the diagnosis and treatment of patients.
P- Reviewer Moser E S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:1011-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 304] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 2. | Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 342] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Chen F, Ni YC. Magnetic resonance diffusion-perfusion mismatch in acute ischemic stroke: An update. World J Radiol. 2012;4:63-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 4. | Guadagno JV, Warburton EA, Jones PS, Day DJ, Aigbirhio FI, Fryer TD, Harding S, Price CJ, Green HA, Barret O. How affected is oxygen metabolism in DWI lesions?: A combined acute stroke PET-MR study. Neurology. 2006;67:824-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Sobesky J, Zaro Weber O, Lehnhardt FG, Hesselmann V, Neveling M, Jacobs A, Heiss WD. Does the mismatch match the penumbra? Magnetic resonance imaging and positron emission tomography in early ischemic stroke. Stroke. 2005;36:980-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Jensen-Kondering U, Baron JC. Oxygen imaging by MRI: can blood oxygen level-dependent imaging depict the ischemic penumbra? Stroke. 2012;43:2264-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Hermier M, Nighoghossian N, Derex L, Adeleine P, Wiart M, Berthezène Y, Cotton F, Pialat JB, Dardel P, Honnorat J. Hypointense transcerebral veins at T2*-weighted MRI: a marker of hemorrhagic transformation risk in patients treated with intravenous tissue plasminogen activator. J Cereb Blood Flow Metab. 2003;23:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Liebeskind DS, Ances BM, Weigele JB, Hurst RW. Intravascular deoxygenation of leptomeningeal collaterals detected with gradient-echo MRI. Stroke. 2004;35:266. |
| 9. | Liebeskind DS, Ances BM, Weigele JB, Hurst R, Melhem ER. Gradient-echo T2*-weighted prominence of the basal vein of Rosenthal in MCA ischemia. ASNR 42th Annual Meeting; 2004 Jun 5-11; Seattle, WA. . |
| 10. | Hermier M, Nighoghossian N, Derex L, Wiart M, Nemoz C, Berthezène Y, Froment JC. Hypointense leptomeningeal vessels at T2*-weighted MRI in acute ischemic stroke. Neurology. 2005;65:652-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Seo SH, Oh HG, Park TW, Kim GM, Chung CS, Lee KH. Thrapeutic implications of hypointense vessel signs on gradient echo imaging in acute ischemic stroke. Joint World Congress on Stroke; 2006 Oct 26-29; Cape Town, South Africa). Int J Stroke. 2006;1:162-163. |
| 12. | Sohn SI, Park SW, Cho YW, Lee H, Lim JG, Yi SD, Sohn CH, Lee SH. Significances of hypointense transcerebral cortical veins on T2*-weighted gradient echo MR images in patients with acute cerebral artery occlusion. 15th European Stroke Conferenc; 2006 May 16-19; Brussels, Belgium. Cerebrovasc Dis. 2006;21 (Suppl 4). |
| 13. | Ha SY, Seo SH, Kim GM, Chung CS, Lee KH. Hypointense leptomeningeal vessels on T2*-weighted gradient echo imaging in acute ischemic stroke: A correlation with angiographic findings. 2008 International Stroke Conference; 2008 Feb 20-22; New Orleans, LA. Stroke. 2008;39:600. |
| 14. | Morita N, Harada M, Uno M, Matsubara S, Matsuda T, Nagahiro S, Nishitani H. Ischemic findings of T2*-weighted 3-tesla MRI in acute stroke patients. Cerebrovasc Dis. 2008;26:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Harada M, Kubo H, Nishitani H, Matsuda T. Evaluation of three ischemic signs and ischemic extent by T2*-weighted 3-Tesla MRI. ISMRM 17th Annual Meeting and Exhibition; 2009 Apr 18; Honolulu, HI. . |
| 16. | Ha SY, Seo SH, Bang OY, Kim GM, Chung CS, Lee KH. Hypointense leptomeningeal vessels on T2*-weighted gradient echo imaging in acute ischemic stroke is correlated with isolated focal swelling on CT. 2009 International Stroke Conference; 2009 Feb 18-20; San Diego, CA. Stroke. 2009;40:e163. |
| 17. | Kaya D, Dinçer A, Yildiz ME, Cizmeli MO, Erzen C. Acute ischemic infarction defined by a region of multiple hypointense vessels on gradient-echo T2* MR imaging at 3T. AJNR Am J Neuroradiol. 2009;30:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Kinoshita T, Toyoshima H, Ibaraki M, Nakamura K, Shinohara Y, Kinoshita F. Susceptibility-weighted MR imaging findings associated with misery perfusion on 15O Positron emission tomography in patients with chronic cerebrovascular disease. American Society of Neuroradiology 48th Annual Meeting; 2010 May 15-20; Boston, MA. . |
| 19. | Harada M, Kubo H, Morita N, Nishitani H, Matsuda T. Clinical significance of ischemic hypointense findings in vessels and tissue in gradient echo T2*-weighted images at 3 Tesla evaluated by simple visual estimation in stroke patients treated with intravenous rt-PA. Joint Annual Meeting ISMRM-ESMRMB; 2010 May 1-7; Seattle, WA. . |
| 20. | Tada Y, Uno M, Matsubara S, Suzue A, Shimada K, Morita N, Harada M, Nagahiro S. Reversibility of ischemic findings on 3-T T2*-weighted imaging after emergency superficial temporal artery-middle cerebral artery anastomosis in patients with progressive ischemic stroke. Neurol Med Chir (Tokyo). 2010;50:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Rosso C, Belleville M, Pires C, Dormont D, Crozier S, Chiras J, Samson Y, Bonneville F. Clinical usefulness of the visibility of the transcerebral veins at 3T on T2*-weighted sequence in acute stroke patients. Eur J Radiol. 2012;81:1282-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Ryoo S, Kim JH, Jeon P, Lee KH. The implication of FLAIR and GRE in hyperacute stroke. 8th World Stroke Congress; Oct 10-13; Brasilia, Brazil. . |
| 23. | Ida M. Susceptibility-weighted imaging: Clinical utility for hyperacute cerebral ischemia. ASNR 45th Annual Meeting; 2007 Jun 9-14; hicago, IL. . |
| 24. | Tong KA, Ashwal S, Obenaus A, Nickerson JP, Kido D, Haacke EM. Susceptibility-weighted MR imaging: a review of clinical applications in children. AJNR Am J Neuroradiol. 2008;29:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 25. | Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009;30:232-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 526] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 26. | Santhosh K, Kesavadas C, Thomas B, Gupta AK, Thamburaj K, Kapilamoorthy TR. Susceptibility weighted imaging: a new tool in magnetic resonance imaging of stroke. Clin Radiol. 2009;64:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Tsui YK, Tsai FY, Hasso AN, Greensite F, Nguyen BV. Susceptibility-weighted imaging for differential diagnosis of cerebral vascular pathology: a pictorial review. J Neurol Sci. 2009;287:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Christoforidis G, Slivka A, Mohammad Y, Yang M, Slone H. Susceptibility-weighted imaging in acute ischemic stroke. ASNR 48th Annual Meeting; 2010 May 15-20; Boston, MA. . |
| 29. | Hingwala D, Kesavadas C, Thomas B, Kapilamoorthy TR. Clinical utility of susceptibility-weighted imaging in vascular diseases of the brain. Neurol India. 2010;58:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Huisman TA, Singhi S, Pinto PS. Non-invasive imaging of intracranial pediatric vascular lesions. Childs Nerv Syst. 2010;26:1275-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Mittal P, Kalia V, Dua S. Pictorial essay: Susceptibility-weighted imaging in cerebral ischemia. Indian J Radiol Imaging. 2010;20:250-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Yen JC, Lai YJ, Chan L. Middle cerebral artery susceptibility sign and venous prominence in acute ischemic stroke. Neurol India. 2010;58:620-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Kesavadas C, Thomas B, Pendharakar H, Sylaja PN. Susceptibility weighted imaging: does it give information similar to perfusion weighted imaging in acute stroke? J Neurol. 2011;258:932-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Park KP, Lee W, Yang TI, Park MG, Oh SJ, Baik SK. Multiple hypointense vessels on susceptibility-weighted imaging represent diffusion-perfusion mismatch in acute ischemic stroke. 20th World Congress of Neurology; 2011 Nov 12-17; Marrakesh, Morocco. . |
| 35. | Lin C, Lai Y, Chen L, Guo W. Use of susceptibility-weighted imaging to predict the outcome of large vessel occlusion disease. ASNR 49th Annual Meeting; 2011 Jun 4-9; Seattle, WA. . |
| 36. | Lin C, Lai Y, Chen L, Guo W. Susceptibility-weighted imaging: A potential noninvasive imaging tool for characterizing ischemic brain injury? ASNR 49th Annual Meeting; 2011 Jun 4-9; Seattle, WA. . |
| 37. | Gasparotti R, Pinelli L, Liserre R. New MR sequences in daily practice: susceptibility weighted imaging. A pictorial essay. Insights Imaging. 2011;2:335-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Yamashita E, Kanasaki Y, Fujii S, Tanaka T, Hirata Y, Ogawa T. Comparison of increased venous contrast in ischemic stroke using phase-sensitive MR imaging with perfusion changes on flow-sensitive alternating inversion recovery at 3 Tesla. Acta Radiol. 2011;52:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Huang P, Chen CH, Lin WC, Lin RT, Khor GT, Liu CK. Clinical applications of susceptibility weighted imaging in patients with major stroke. J Neurol. 2012;259:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Kao HW, Tsai FY, Hasso AN. Predicting stroke evolution: comparison of susceptibility-weighted MR imaging with MR perfusion. Eur Radiol. 2012;22:1397-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Tsai FY, Kao HW, Chan W. Susceptibility-weighted (MR) imaging as prognostic indicator for patient selection with endovascular therapy of acute strok. ASNR 50th Annual Meeting; 2012 Apr 21-26; New York, NY. . |
| 42. | Meoded A, Poretti A, Tekes A, Huisman TAGM. Evaluation of the ischemic penumbra with susceptibility-weighted imaging in children affected with arterial ischemic stroke. ASNR 50th Annual Meeting; 2012 Apr 21-26; New York, NY. . |
| 43. | Park KP, Lee WH, Park MG, Yang TI, Oh SJ, Baik SK. Susceptibility-weighted imaging in hemispheric transient ischemic attack with negative diffusion-weighted imaging. 21st European Stroke Conference; 2012 May 22-25; Lisbon, Portugal. Cerebrovasc Dis. 2012;33 (Suppl 2). |
| 44. | Fujioka M, Takahashi M, Tada Y, Asai H, Iwamura A, Ito S, Watanabe T, Kawai Y, Seki T, Fukushima H. “DWI-SWI” mismatch may represent ischemic penumbra in acute stroke. 21st European Stroke Conference, 2012 May 22-25; Lisbon, Portugal. Cerebrovasc Dis. 2012;33 (Suppl 2). |
| 45. | Verschuuren S, Poretti A, Buerki S, Lequin MH, Huisman TA. Susceptibility-weighted imaging of the pediatric brain. AJR Am J Roentgenol. 2012;198:W440-W449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Meoded A, Poretti A, Northington FJ, Tekes A, Intrapiromkul J, Huisman TA. Susceptibility weighted imaging of the neonatal brain. Clin Radiol. 2012;67:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Baik SK, Choi W, Oh SJ, Park KP, Park MG, Yang TI, Jeong HW. Change in cortical vessel signs on susceptibility-weighted images after full recanalization in hyperacute ischemic stroke. Cerebrovasc Dis. 2012;34:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Tsai FY. Does mismatch of DWI and PWI mean penumbra with viable brain? 8th World Stroke Congress; 2012 Oct 10-13; Brasilia, Brazil. . |
| 49. | Sohn C, Kim J, Chang H, Sohn S, Chung E. Value of susceptibility-weighted imaging in acute ischemic stroke with perfusion defect. ASNR 47th Annual Meeting; 2009 May 16-21; Vancouver, BC. . |
| 50. | Baron JC. Mapping the ischaemic penumbra with PET: a new approach. Brain. 2001;124:2-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Baron JC. Mapping the ischaemic penumbra with PET: implications for acute stroke treatment. Cerebrovasc Dis. 1999;9:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 224] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 52. | Meyer JS. Circulatory changes following occlusion of the middle cerebral artery and their relation to function. J Neurosurg. 1958;15:653-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Pauling L, Coryell CD. The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin. Proc Natl Acad Sci USA. 1936;22:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29:1433-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 477] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 55. | Sehgal V, Delproposto Z, Haacke EM, Tong KA, Wycliffe N, Kido DK, Xu Y, Neelavalli J, Haddar D, Reichenbach JR. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005;22:439-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 309] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 56. | Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009;30:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 756] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 57. | Haacke EM, Tang J, Neelavalli J, Cheng YC. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. J Magn Reson Imaging. 2010;32:663-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 58. | Tang J, Liu S, Neelavalli J, Cheng YC, Buch S, Haacke EM. Improving susceptibility mapping using a threshold-based K-space/image domain iterative reconstruction approach. Magn Reson Med. 2013;69:1396-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 59. | Zaitsu Y, Kudo K, Terae S, Yazu R, Ishizaka K, Fujima N, Tha KK, Haacke EM, Sasaki M, Shirato H. Mapping of cerebral oxygen extraction fraction changes with susceptibility-weighted phase imaging. Radiology. 2011;261:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Kinoshita T, Kinoshita F, Shinohara Y. T2*-weighted gradient-echo type echo planar imaging in diagnosis of ischemic stroke. ASNR 48th Annual Meeting; 2010 May 15-20; Boston, MA. . |
| 61. | Schellinger PD, Jansen O, Fiebach JB, Hacke W, Sartor K. A standardized MRI stroke protocol: comparison with CT in hyperacute intracerebral hemorrhage. Stroke. 1999;30:765-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 158] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 62. | Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW. Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke. 2005;36:2379-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 63. | Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135:1161-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 413] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 64. | Christoforidis GA, Yang M, Mohammad YM, Abduljalil A, Heverhagen JT, Chakeres DW, Knopp MV. Imaging of Microvascularity During Acute Ischemic Stroke with and without Intravascular Contrast Agent on High-Resolution Ultra-High Field MRI in a Rodent Model with Histopathologic Correlation. ASNR 42nd Annual Meeting; 2004 Jun 5-11; Seattle, WA. . |
| 65. | Kesavadas C, Santhosh K, Thomas B. Susceptibility weighted imaging in cerebral hypoperfusion-can we predict increased oxygen extraction fraction? Neuroradiology. 2010;52:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |