Published online Apr 28, 2013. doi: 10.4329/wjr.v5.i4.143
Revised: January 30, 2013
Accepted: February 5, 2013
Published online: April 28, 2013
Processing time: 158 Days and 3 Hours
Carotid cavernous sinus fistulas are abnormal communications between the carotid system and the cavernous sinus. Several classification schemes have described carotid cavernous sinus fistulas according to etiology, hemodynamic features, or the angiographic arterial architecture. Increased pressure within the cavernous sinus appears to be the main factor in pathophysiology. The clinical features are related to size, exact location, and duration of the fistula, adequacy and route of venous drainage and the presence of arterial/venous collaterals. Noninvasive imaging (computed tomography, magnetic resonance, computed tomography angiography, magnetic resonance angiography, Doppler) is often used in the initial work-up of a possible carotid cavernous sinus fistulas. Cerebral angiography is the gold standard for the definitive diagnosis, classification, and planning of treatment for these lesions. The endovascular approach has evolved as the mainstay therapy for definitive treatment in situations including clinical emergencies. Conservative treatment, surgery and radiosurgery constitute other management options for these lesions.
Core tip: Carotid cavernous sinus fistulas (CCFs) are abnormal communications between the carotid arterial system and the cavernous sinus. The clinical presentation of CCFs, which is a direct consequence of elevation in intracavernous pressure and revised flow patterns, mostly comprises of ocular findings. Recent advances in endovascular techniques have resulted in several therapeutic modalities becoming available and the endovascular approach has evolved as the primary treatment option for the management of CCFs. This review provides detailed information about classification, etiology, pathophysiology, clinical presentation, diagnostic modalities, differential diagnosis, indications for emergency treatment, post-treatment follow-up and treatment modalities with emphasis on the endovascular approach in CCFs.
- Citation: Korkmazer B, Kocak B, Tureci E, Islak C, Kocer N, Kizilkilic O. Endovascular treatment of carotid cavernous sinus fistula: A systematic review. World J Radiol 2013; 5(4): 143-155
- URL: https://www.wjgnet.com/1949-8470/full/v5/i4/143.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i4.143
Carotid cavernous sinus fistulas (CCFs) are abnormal communications between the carotid arterial system and the cavernous sinus. Recent advances in endovascular techniques have resulted in several therapeutic modalities becoming available and the endovascular approach has evolved as the primary treatment option for the management of CCFs. This review provides an overview of various treatment modalities with emphasis on the endovascular approach. Furthermore, we will discuss the classification, etiology, and clinical presentation including pathophysiology and symptoms, diagnosis, indications for emergency treatment and post-treatment follow-up in CCFs.
Several classification schemes have categorized CCFs according to etiology (traumatic or spontaneous), hemodynamic features (high versus low flow), or the angiographic arterial architecture (direct or indirect). The angiographic classification defines the angioarchitecture of the lesion on which a therapeutic strategy can be planned. According to the angiographic findings, Barrow et al[1] provided a detailed anatomical classification which categorizes CCFs into four distinct types based on their arterial supply. Type A fistulas are direct communications between the internal carotid artery (ICA) and the cavernous sinus, usually associated with high flow rates. Indirect fistulas (types B, C and D) are dural arteriovenous fistulas fed by the meningeal arteries of the ICA, the external carotid artery (ECA), or both. Type B fistulas have dural ICA branches to the cavernous sinus, which are relatively uncommon. Type C fistulas are supplied solely by the dural branches of the ECA. The most prevalent form of indirect CCF is the type D fistula that has dural ICA and ECA branches to the cavernous sinus. Tomsick subclassified type D CCFs into type D1 or D2 depending on the presence of a unilateral or bilateral arterial supply[2].
Traumatic CCFs often demonstrate a single direct communication between the ICA and the cavernous sinus and are almost always found to be type A direct fistulas. However, spontaneous fistulas usually have multiple dural feeders and numerous microfistulas within the cavernous sinus wall[3]. Spontaneous CCFs may fall into any of the four angiographic categories defined by Barrow et al[1], because a type A shunt with high-flow characteristics can develop following spontaneous rupture of an intracavernous ICA aneurysm.
Most direct CCFs occur at the proximal horizontal intracavernous segment of the ICA in the vicinity of the inferolateral trunk. With decreasing frequency, CCFs are found to occur at the junction of the horizontal and intracavernous ascending segments, posterior ascending segment, junction of the anterior ascending and horizontal intracavernous segments, and the anterior ascending segment[4].
Traumatic disruption of the vessel wall is the most common etiological factor for direct CCFs. Blunt and penetrating head trauma as well as iatrogenic damage (trans-sphenoidal surgery, glycerol rhizotomy, Fogarty catheter manipulation for carotid angioplasty etc.) may lead to direct CCFs[5,6]. Approximately 20% of type A CCFs are not related to a history of trauma and regarded as spontaneous[7,8]. Spontaneous direct CCFs may stem from any condition that predisposes the ICA wall to weaken[4]. They are usually caused (formed) by the rupture of either a cavernous segment aneurysm or a weakened atherosclerotic artery[7,9]. Predisposing factors associated with the development of spontaneous type A CCFs are Ehlers-Danlos syndrome, fibromuscular dysplasia, and pseudoxanthoma elasticum[7,10,11]. Iatrogenic alterations in flow dynamics and vascular pressure are also suspected to contribute to spontaneous aneurysmal rupture (following prior contralateral ICA occlusion)[1].
Most direct type A CCFs are high-flow shunts. Flow rates in type A fistulas are variable and depend on the size of the ostium and venous drainage. Type A fistulas typically range from 1 to 5 mm in size (average = 3 mm), typically small enough to be treated with detachable balloons with a mean volume of 0.28 L, equivalent to an inflated balloon diameter of 7 to 9 mm[4,7,12,13].
Complete steal, which is defined as the complete absence of filling of the ICA above the fistula, occurs in 5% of patients at diagnosis[7]. The complete steal phenomenon deserves enormous interest because it confirms that the CCF is of high flow, that the rent is large, and that the patient has an excellent collateral flow through the circle of Willis if there are no contralateral deficits[11].
These lesions are usually unilateral although bilateral traumatic CCFs occur in approximately 1%-2% of patients with traumatic CCFs[4]. These unusual bilateral traumatic CCFs are generally associated with more severe head trauma, are more commonly fatal, and are therefore less frequent[6]. Additionally, unilateral fistulas may occur with bilateral or contralateral orbital symptoms, depending on the venous drainage route, via intercavernous communication[6,7,14]. Direct fistulae are less likely to resolve spontaneously and may require intervention if symptomatic.
Indirect CCFs (types B, C and D) are also called dural fistulas and typically have low flow rates. The major arterial supply to indirect fistulas arises from the internal maxillary, middle meningeal, accessory meningeal and ascending pharyngeal branches of the ECA, as well as cavernous segment branches of the ICA[15].
Indirect CCFs tend to occur more frequently in postmenopausal women. The cause of these lesions is still obscure, but infants presenting with dural fistulas in the literature furnish some evidence to congenital origin[4,7,16]. Factors that may predispose patients to the development of dural CCFs include hypertension, diabetes, pregnancy, trauma and straining, atherosclerotic disease, cavernous sinus thrombosis, sinusitis and collagen vascular disease. Trauma is less commonly associated with indirect CCFs[1,5,7].
Traumatic indirect CCFs differ from the spontaneous type because these lesions are usually single-hole fistulas in which the accessory meningeal artery and middle meningeal artery are found to be the most common feeders[17].
The cavernous sinus normally receives drainage from the superior and inferior ophthalmic veins as well as superiorly from the sphenoparietal sinus, sylvian veins, and cortical veins. The cavernous sinus drains posteriorly through the inferior petrosal sinus (IPS) and superior petrosal sinus to the jugular bulb, inferiorly through the pterygoid plexus via emissary veins, and contralaterally through the contralateral cavernous sinus[4,11].
A CCF allows highly pressurized arterial blood to be transmitted directly into the cavernous sinus and the draining veins, leading to venous hypertension. The clinical presentation of CCF is a direct consequence of elevation in intracavernous pressure and revised flow patterns. The revised venous drainage of the CCFs may head toward the ophthalmic venous system anteriorly; the superior petrosal sinus, the IPS, or the basilar plexus posteriorly; the sphenoparietal sinus laterally; the intercavernous sinus contralaterally; the pterygoid plexus via the vein of the foramen rotundum and the vein of the foramen ovale inferiorly. Most often, the direction of the venous drainage is multidirectional[3,5].
The clinical features of CCFs are related to their size, exact location, duration, adequacy and route of venous drainage and the presence of arterial/venous collaterals[6]. The symptoms and signs that may be associated with CCF are listed in Table 1.
| Symptoms and signs |
| Exophthalmos, proptosis |
| Cephalic bruit |
| Conjunctival chemosis, ‘’red eye’’ |
| Pain, headache |
| Trigeminal nerve dysfunction |
| Elevated intraocular pressure, secondary glaucoma |
| Diminished visual acuity, visual loss |
| Subconjunctival hemorrhages |
| Corneal damage |
| Intracranial hemorrhage |
| Otorrhagia |
| Epistaxis |
| Differential diagnosis |
| Vascular pathologies |
| Marginal sinus fistulas (with a restriction of venous drainage via the jugular bulb) |
| Anomalous intracranial venous drainage (sigmoid sinus hypoplasia/aplasia) |
| Cavernous sinus thrombosis |
| Intraorbital lesions |
| Fibrous dysplasia |
| Frontal sinus mucocele |
| Ocular neoplasms |
| Osteoma |
| Hemangioma |
| Inflammatory, allergic and infectious pathologies |
| Conjunctivitis |
| Endocrine pathologies |
| Thyroid ophthalmopathy |
| Indications for emergency treatment |
| Angiographic findings |
| Pseudoaneurysm |
| Large varix of the cavernous sinus |
| Venous drainage to cortical veins |
| Thrombosis of distant venous outflow pathways |
| Clinical signs and symptoms |
| Increased intracranial pressure |
| Rapidly progressive proptosis |
| Intracerebral, subarachnoid and external hemorrhage |
| Transient ischemic attack |
| Treatment modalities |
| Conservative management (manual compression therapy and medical therapy) |
| Surgical management |
| Stereotactic radiosurgery |
| Endovascular management |
| Direct fistula |
| Transarterial treatment (preferred approach for direct CCF) |
| Detachable balloon occlusion |
| Transarterial coil and material embolization |
| Covered stent graft placement (endovascular reconstruction of the parent artery) |
| Parent artery occlusion |
| Transvenous treatment |
| Transvenous detachable coil embolization |
| Liquid embolizing agents (n-BCA, Onyx) |
| Indirect fistula |
| Transvenous treatment (preferred approach for indirect CCF) |
| Transvenous detachable coil embolization |
| Liquid embolizing agents (n-BCA, Onyx) |
| Transarterial treatment |
| Transarterial coil and material embolization |
The classic presentation for a direct, high-flow CCF is the sudden development of Dandy’s triad: exophthalmos, bruit, and conjunctival chemosis. Complete clinical triad is not always found but most patients present with proptosis (90%), chemosis (90%), diplopia (50%), cephalic bruit (25%), pain (25%), trigeminal nerve dysfunction, elevated intraocular pressure, and visual loss (up to 50%)[4].
Elevated pressure in veins draining the orbit may produce orbital venous congestion, transudation of interstitial fluid into the orbit with resultant proptosis, increased intraocular pressure due to impaired drainage of the aqueous humor, and secondary glaucoma. Elevated venous pressure and intraocular pressure can compromise retinal perfusion and result in severely diminished visual acuity[6]. Visual loss is one of the most worrying complications of CCFs and warrants immediate treatment. Although minor deficits in visual acuity almost have complete resolution after closure of the fistula, severe visual loss with loss of light perception rarely improves even if the fistulous communication is obliterated[18]. Subconjuctival hemorrhages can be seen due to rupture of dilated arterialized veins and, in addition, increased exposure of the cornea may cause corneal damage. Intracranial hemorrhage develops in 5% of patients, probably due to revised venous drainage into the sphenoparietal sinus with occlusion of other drainage pathways, resulting in cerebral cortical venous hypertension[4,7].
External hemorrhage such as otorrhagia and epistaxis can be seen in nearly 3% of cases in CCF[19]. Between 1% and 2% of CCF cases manifest life-threatening massive epistaxis caused by rupture of a pseudoaneurysmal cavernous sinus varix[4,7,20]. Most CCF cases that present with epistaxis have a pseudoaneurysm or venous pouch that entered the sphenoid sinus via a communication through a basal skull fracture[19]. Bleeding from the veins draining the ear canal can lead to otorrhagia[19].
Although the clinical manifestations may overlap, indirect CCFs often do not demonstrate the classic triad of symptoms. An ocular bruit may or may not be present with these lesions. The onset of symptoms of indirect CCFs is not as drastic as in direct CCFs. The symptoms and signs of indirect CCFs progress insidiously and the majority of cases present with progressive glaucoma, proptosis or conjunctival injection (red eye)[4,7,15,21].
The natural evolution of indirect CCFs is variable and the literature reports spontaneous resolution without treatment occurs in 10%-60% of cases, possibly due to rethrombosis of the involved segment of the cavernous sinus[1,15]. Although these lesions have a tendency to resolve spontaneously, patients suffering from progressive vision loss and intractable glaucoma require interventional therapy. Spontaneous thrombosis of traumatic indirect fistulas is rare because of higher flow rates than with the spontaneous type[17].
Exacerbation and remission of signs and symptoms are the hallmark of dural CCFs, possibly due to cavernous sinus thromboses and rerouting of venous flow in various directions[4]. Therefore any change in the symptoms must be followed up accurately because it may suggest alterations in venous drainage, possibly transitioning to higher-risk patterns despite overt clinical improvement. The “white eye syndrome” defines the clinical remission of ocular symptoms due to spontaneous occlusion of venous drainage pathways to the orbit and potentially leaving only more dangerous venous drainage routes[15].
Noninvasive imaging [computed tomography (CT), magnetic resonance (MR), CT angiography, MR angiography, Doppler] often is used in the initial work-up of a possible CCF. CT scan of the orbit usually demonstrates proptosis of the affected globe, enlargement of the extraocular muscles, dilatation and tortuosity of the superior ophthalmic vein (SOV), and enlargement of the ipsilateral cavernous sinus. A noncontrast head CT scan also allows for careful examination of possible cranial injuries, such as bony fractures or intracranial hematomas. MR imaging findings in CCFs are similar to those seen on CT with the addition of orbital edema and abnormal flow voids in the affected cavernous sinus. In the setting of a high-flow fistula and retrograde cortical venous reflux, MR or CT studies may reveal dilatation of leptomeningeal and cortical veins. In patients who have cerebral venous congestion and elevated intracranial pressures, cerebral edema and/or hemorrhage may be encountered[4,5,7].
While CT angiography can be used as a first-line diagnostic tool in evaluating the presence of a CCF it has some limitations. Despite its ability to reliably delineate certain draining veins, CT angiography rarely depicts small feeding arteries in dural CCFs or the exact site of fistulous communication in direct CCFs. Moreover, this technique cannot provide information about the blood-flow characteristics within fistulas[3].
Color Doppler imaging can assist in diagnosis and follow-up of patients with CCFs. Increased velocity with reversal of the direction of blood flow, arterial pulsations, and dilatation of SOV are characteristic findings[22].
Cerebral angiography is the gold standard for the definitive diagnosis, classification, and planning of endovascular intervention in CCFs. The initial angiographic evaluation can be used to obtain the following information: size and location of the fistula, differentiation of direct from indirect lesions, presence of any associated cavernous carotid aneurysm, presence of complete or partial steal phenomena, assessment of the global cortical arterial circulation and collateral flow through the circle of Willis, identification of high-risk features (e.g., cortical venous drainage, pseudoaneurysm, cavernous sinus varix), venous drainage patterns, determination of therapeutic route, associated vascular injuries (e.g., traumatic pseudoaneurysm, arterial dissection), identification of any dangerous collateral pathways and evaluation of carotid bifurcation before compression therapy[4-7,11,15].
In the evaluation of direct CCFs, identifying the exact location of fistulous communication in the cavernous ICA can be challenging because of the high flow- related washout of intra-arterial contrast and instantaneous opacification of the cavernous sinus. Angiographic high-frame-rate imaging (> 5 frames/s) and rapid contrast injection rates (7 or 8 mL/s) may assist in evaluating the morphology of high-flow fistulas. High-flow CCFs may be difficult to capture in digital subtraction angiography, even with selective high frame rates. Specific maneuvers can be implemented to slow the fistula flow and facilitate image capture. The Mehringer-Hieshima maneuver consists of injecting the ipsilateral ICA and manual compression of the ipsilateral common carotid artery while filming at a slower frame rate. Use of this maneuver slows the rate of opacification of the fistula and thereby allows better delineation of the fistula site. Another maneuver consists of using of a double-lumen balloon catheter in the ipsilateral ICA with slow injection of contrast at 1 mL/s at one or two frames per second. Lastly, ipsilateral carotid compression during injection of the vertebral artery, called the Heuber maneuver, opacifies the fistula through a patent posterior communicating artery[4-7,11].
Tolerance for ICA occlusion should also be assessed to identify the appropriate therapeutic choices before embarking on a therapeutic intervention. Balloon test occlusion is the currently accepted technique for evaluation. After confirmation of ICA occlusion, detailed testing of mental status, speech, visual fields, facial animation, and motor power in all four extremities are performed. In the absence of significant deficit, the patient is observed for 15-20 min and re-examined. If the patient tolerates occlusion at normal blood pressure, nitroprusside infusion is initiated and titrated to achieve a mean arterial pressure two thirds of the patient’s baseline level. The patient is examined again and observed for 15-20 min. Single proton emission computed tomography (SPECT) may be used to rule out significant asymmetry in perfusion during balloon test occlusion (BTO). The SPECT evaluation can uncover the risk of suffering a major stroke after permanent carotid occlusion even in patients who seem to tolerate BTO during relative hypotensive challenge test[4].
The differential diagnosis of CCF includes a wide spectrum of pathologies (Table 1), so patients may be evaluated for endocrine, inflammatory, infectious and neoplastic etiologies before the presence of vascular pathology is recognized, especially in the early phase of the disease.
Intraorbital lesions (osteoma, hemangioma, fibrous dysplasia, frontal sinus mucocele, ocular neoplasms) may cause ocular pain, exophthalmos, and ophthalmoplegia although, bruits are not typically present[14].
Ocular findings of CCF may also mimic those associated with allergic/infectious conjunctivitis and thyroid ophthalmopathy. However, ocular involvement in hyperthyroidism typically occurs bilaterally[14,15].
Imaging techniques (e.g., MR, CT) displaying prominent vessels within the orbit can lead the clinician to consider vascular etiologies. Vascular pathologies that can result in SOV dilatation include marginal sinus fistulas with a restriction or obstruction of venous drainage via the jugular bulb[23], anomalous intracranial venous drainage such as sigmoid sinus hypoplasia/aplasia[24], and thrombosis of the cavernous sinus. Cerebral angiography must be performed to achieve a definitive diagnosis.
Halbach et al[19] reviewed angiographic and clinical data from 155 CCF patients to determine angiographic features associated with increased risk of morbidity and mortality. These features comprise the presence of a pseudoaneurysm, large varix of the cavernous sinus, venous drainage to cortical veins, and thrombosis of other venous outflow pathways distant from the fistula.
Varix of the cavernous sinus and pseudoaneurysm can both present with subarachnoid hemorrhage. Angiographic differentiation between a cavernous sinus varix and a pseudoaneurysm may be difficult or impossible. However, the clinical onset can be helpful to the differentiation process. The onset of the pseudoaneurysm is usually coincidental with trauma, as opposed to the delayed onset of the cavernous sinus varix, which develops after the occlusion of venous outflow pathways[19].
Massive cortical venous drainage can eventually result in hemorrhagic venous infarction and endovascular treatment should, therefore, be performed immediately[11].
Clinical signs and symptoms that are associated with poor prognosis include increased intracranial pressure; rapidly progressive proptosis, which may signify spontaneous thrombosis of venous outflow pathways to the orbit; diminished visual acuity; hemorrhage (e.g., intracerebral, subarachnoid and external hemorrhage; otorrhagia or epistaxis); and transient ischemic attacks; which may signify cerebral ischemia or impaired autoregulation in cerebral perfusion, secondary to the chronic steal phenomenon caused by the fistulous communication. The delineation of angiographic high-risk findings and recognition of the poor prognostic clinical features (Table 1) should warrant emergent and aggressive interventional treatment to improve outcome[4,15,19].
The treatment modalities include conservative management, which consists of medical management and manual compression therapy; surgical management; stereotactic radiosurgery and endovascular repair via a transarterial or transvenous route (Table 1).
A complete set of diagnostic angiographic evaluations is required for choosing the appropriate treatment modality. While higher risk fistulas deserve the most aggressive approach in order to eradicate the fistula, low-risk lesions with mild symptomatology may not require active intervention and can be managed conservatively. Patients with low-risk lesions can be given reassurance, educated regarding potential changes in symptoms, and allowed time for potential spontaneous closure of the fistula[11].
Spontaneous resolution of dural fistulas can occasionally occur within days to months after symptomatic presentation secondary to further thrombosis of the involved segment of the cavernous sinus. Therefore, an accepted practice is to treat the patient’s ocular symptoms medically with prism therapy or patching for diplopia, topical β-adrenergic blockers and acetazolamide for elevated intraocular pressure, lubrication for proptosis-related keratopathy, and/or systemic corticosteroids if needed[7].
Furthermore, manual external carotid-jugular compression therapy may be initiated as a noninvasive treatment for indirect CCFs. The patient is instructed to compress the carotid artery and jugular vein with the contralateral hand for a period of 10 s while sitting or lying down, four to six times each hour[7]. The aim of the compression therapy is the transient reduction of arteriovenous shunting by decreasing arterial inflow while simultaneously increasing the outlet venous pressure, thereby promoting spontaneous thrombosis within the fistula[25]. Use of the contralateral hand ensures that if ischemia develops, the symptomatic arm will fall away from the neck, thus allowing cortical revascularization immediately[5]. Intermittent self-administered manual carotid-jugular compression alone can result in a cure in 30% of patients with spontaneous CCFs[7,26]. However, this treatment is usually ineffective in the high-flow CCFs, which usually require endovascular intervention.
Cervical carotid bifurcation should be examined for atherosclerotic changes using color Doppler or angiography before initiation of compression therapy[26]. Contraindications to manual carotid-jugular compression are symptomatic bradycardia with carotid compression, significant cortical venous drainage which may result in venous infarction or hemorrhage during compression therapy as well as clinical features that can signify difficulty in tolerating transient occlusion of ipsilateral ICA such as atherosclerotic stenosis, ulceration of the carotid artery, history of cerebral ischemia[5,7].
The possible adverse effects of carotid compression may include hemodynamic or thromboembolic complications, vasovagal reactions, intracranial/retinal hemorrhage, clinical deterioration known as the “paradoxical worsening phenomenon”, vertebral artery occlusion, brachial plexus/supraclavicular nerve injury and temporary monocular blindness during carotid compression[26]. If the decision not to treat a CCF interventionally is made, the patient must be carefully followed for elevated intraocular pressure, progressive visual deterioration, neurological deficits and high-risk angiographic features[1,5,6].
Early treatments for CCF consisted of various surgical approaches such as ligation of the CCA, surgical trapping of the fistula, and surgical transvenous packing. Although surgical management can be useful for both direct and indirect CCFs, its role is limited because of associated morbidity from cranial nerve deficits and residual fistulous communications. Indications for surgical repair include compromised proximal arterial access that prevents endovascular repair or causes it to fail. Surgical management remains a consideration for salvage of failed endovascular treatments[4,7].
Complete angiographic documentation of the fistula and BTO should be performed during preoperative evaluation. The appearance and condition of the superficial temporal artery should also be noted because extracranial-to-intracranial bypass can be required to augment blood flow when surgical sacrifice is unavoidable[4].
Stereotactic radiosurgery has emerged as an alternative treatment option and has been investigated for the treatment of CCFs in several institutions. Gamma knife radiosurgery can be used either alone or as an adjunct therapy before/after endovascular intervention[27,28]. Although preliminary data show that radiosurgery is a safe and effective alternative treatment for indirect CCFs, the 22-mo average lag between treatment and complete symptom relief is a significant drawback[21]. Furthermore, an inability to manage emergencies and traumatic fistulae inhibit the usage of radiosurgery as a first line treatment[4].
Recent advances in endovascular technology have made available a number of different treatment options for CCFs. As a result of these advances, the endovascular approach has evolved as the primary treatment option in clinical emergencies and following the failure of conservative therapy. Although the clinical manifestations of direct and indirect fistulas may overlap, their natural history and method of endovascular treatment are often significantly different. The treatment choice is made according to the type, exact anatomy of the fistula, size of the arterial defect, and operator/institutional preferences.
Direct fistulas occur from a tear in the cavernous segment of the ICA or, less commonly, from the intracavernous rupture of an ICA aneurysm. The goal of treatment in direct CCFs is to occlude the tear between the ICA and the cavernous sinus while preserving the patency of the ICA. This goal can be accomplished by either transarterial obliteration of the fistula with a detachable balloon, transarterial or transvenous obliteration of the ipsilateral cavernous sinus with coils or other embolic material, or deployment of a covered stent across the fistula. Rarely, if the defect is large and cannot be repaired, the ICA may need to be sacrificed or trapped[7].
Indirect fistulas consist of small dural arteriovenous shunts between the meningeal branches of the ICA, the ECA, or both, and the cavernous sinus. The goal of treatment in this condition is to interrupt fistulous communications and decrease pressure in the cavernous sinus. This can be accomplished by occluding the arterial branches supplying the fistula (transarterial embolization) or, more commonly, by occluding the cavernous sinus that harbors the fistulous communications (transvenous embolization)[7]. The following sections provide a brief overview of the various endovascular options for the treatment of CCFs.
Detachable balloon occlusion: After Prolo and Hanberry described the use of a fixed balloon catheter to block a CCF in 1971, Serbinenko et al[29] reported the first case of successful embolization of a CCF from an endovascular approach using a detachable silicone balloon with preservation of the ICA[13]. The use of detachable balloon catheters has ushered a new age in the treatment of type A direct CCFs. Transarterial balloon detachment has been accepted as the endovascular treatment of choice for direct CCFs since the 1980s.
The small-diameter vessels that often make up dural fistulas usually do not allow the introduction of a balloon. However, the large carotid defect commonly present in type A CCFs frequently permits transarterial balloon occlusion of the fistula with preservation of the ICA[1,4].
The technique for detachable silicone balloon occlusion of a CCF involves transfemoral access to the proximal CCA with a 7-French guide catheter or long 6-French sheath. Next, the uninflated balloon is advanced to the distal end of the guide catheter; at this point, roadmap imaging is used for further balloon positioning. The balloon offers the advantage of being able to be flow-directed through the fistula and into the cavernous sinus. The balloon is inflated to a volume larger than the orifice of the fistula to prevent its retrograde prolapse into the ICA and then is detached[7]. Following successful balloon deployment, cerebral angiography is repeated to ensure closure of the fistula and patency of the ICA. A single silicone balloon is usually sufficient for most CCFs. However, multiple balloons may be required in the setting of a large tear in the ICA.
The advantage of balloon occlusion of a CCF is the ability to occlude the fistula rapidly with preservation of the ICA. However, technical difficulties can be encountered. The size of the cavernous sinus and the fistula may affect the success rate of detachable-balloon embolization of a CCF. The cavernous sinus must be large enough to accommodate the detachable balloon/balloons for embolization. The size of the fistula must be smaller than the inflated balloon, but large enough to allow access for a deflated or partly inflated balloon. However, the size of the fistula should not be too large, because the embolization balloon may retract to the ICA on inflation in the cavernous sinus[30]. To provide easier navigation of the balloon into the cavernous sinus and prevent protrusion of the inflated balloon through the fistula site to narrow the adjacent ICA lumen, Teng et al[30] developed a double-balloon technique.
Inadequate embolization may be seen due to early balloon detachment, deflation or rupture by contact with a bony fragment[4,5,7,31]. As a rare complication, the balloon can migrate to the venous side of the treated fistula resulting ophthalmoplegic signs due to mechanical compression of cranial nerves in close proximity to the cavernous sinus[32,33].
Coil and material embolization: Transarterial embolization with coils or other embolic material now is the mainstay of endovascular treatment for high-flow direct CCFs, given the limited availability of detachable balloons[7]. Transarterial CCF embolization can be performed with the same technique as aneurysmal embolization. Embolization can be achieved with detachable platinum coils, silk and liquid embolic agents such as n-butyl cyanoacrylate (n-BCA), and ethylene-vinyl alcohol copolymer (EVOH)[5]. The standard transarterial approach consists of placing a guiding catheter in the cervical ICA. Next, a microcatheter is superselectively advanced into the cavernous segment of the ICA and through the tear into the cavernous sinus. Through this microcatheter, embolic material is placed into the cavernous sinus[7].
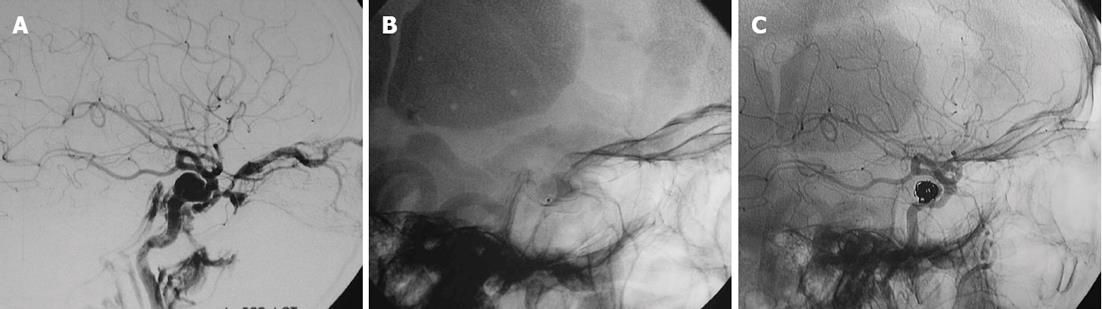
Detachable platinum coils are preferred because of their reliable and controlled deployment (Figure 1). The coils can be adjusted easily or removed if the placement is not optimal. Dense coil packing is performed using the same principles as aneurysm coiling. Thrombogenic, nonretrievable fibered microcoils can also be used but these are associated with the risk of coil deposition into the ICA if the microcatheter recoils into the parent artery during placement[6]. The advantages of coil occlusion of CCFs, when compared with balloon embolization, include ease of access and availability of a variety of sizes of the embolic device. Potential disadvantages include slower gradual occlusion of the fistula, which increases procedure time, and the risk of incomplete fistula occlusion with loss of transarterial access; a loss which would then require a second transvenous approach[4]. The transvenous approach is discussed more thoroughly under the heading “transvenous embolization”.
Complications of transarterial coil embolization include thromboembolus, ICA compromise by protruding coil mass, and ICA dissection[4]. For preventing the retrograde herniation of the embolic material into the parent artery and distal intracranial circulation, the assistance of a nondetachable balloon (balloon-assist technique) or a porous stent may be preferred especially in the setting of a large tear in the ICA[4,5]. Stents also allow initial reconstruction of the damaged segment of the ICA and increase the ability to successfully treat fistulas without parent artery sacrifice[34].
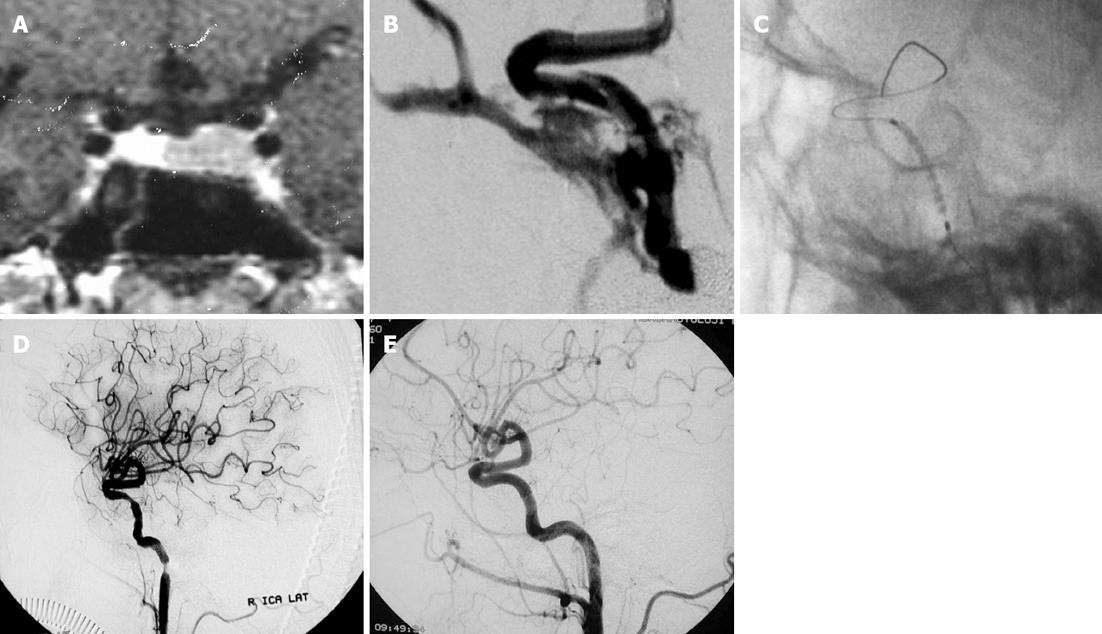
Covered stent graft placement: Recent advances in endovascular techniques such as placement of polyfluorotetraethylene-covered stents have created alternatives to ICA sacrifice in traumatic arterial damage, especially in the setting of an unsuccessful balloon test occlusion study. Covered stent grafts can be extremely useful for the immediate obliteration of a direct CCF, while preserving ICA patency (Figure 2). Additionally, they may decrease the risk of ischemic stroke by preserving the involved artery while simultaneously sealing the site of the fistula[5,7,35]. Covered stent grafts have the technical disadvantage of limited longitudinal flexibility, making it difficult to navigate them through the tortuosity of the intracranial vasculature. Furthermore, the irritation caused by the stiffness of covered stents may frequently lead to periprocedural vasospasms, especially at the ends of the stent (Figure 2). Intra-arterial nimodipine and papaverine infusion can be used for the prevention and resolution of these vasospasms[36,37].
The complications of this procedure include endoleak, coverage of vital perforators, dissection and rupture[7,35].
Although covered stent grafts offer a promising endovascular technique, their usage as a widely accepted method in traumatic CCF is limited due to lack of configurations compatible with intracranial use and long-term safety data.
Parent artery occlusion: Arterial sacrifice may be required as a life-saving emergency treatment when endovascular occlusion of a direct CCF with preservation of the ICA is not feasible due to extensive traumatic vessel-wall damage, active hemorrhage or a rapidly expanding hematoma of the soft tissues. Emergency surgical occlusion of ICA has been relegated to historical status with the advance of therapeutic endovascular techniques, which can be performed immediately after diagnostic therapy under local anesthesia, thereby allowing monitorization of the neurological status[5,13,35].
If a decision to perform endovascular arterial sacrifice is made, assessment of the collateral flow and patient’s ability to tolerate ICA occlusion is paramount. In cases of complete steal presenting without any ischemic symptom, the quality of collateral flow through circle of Willis is confirmed and a test occlusion may be unnecessary. If anterior and posterior communicating artery collaterals are found to be patent, the safety of parent artery occlusion is also high. Otherwise, balloon occlusion test is recommended to ensure distal perfusion from collateral circulation before permanent occlusion[4,7].
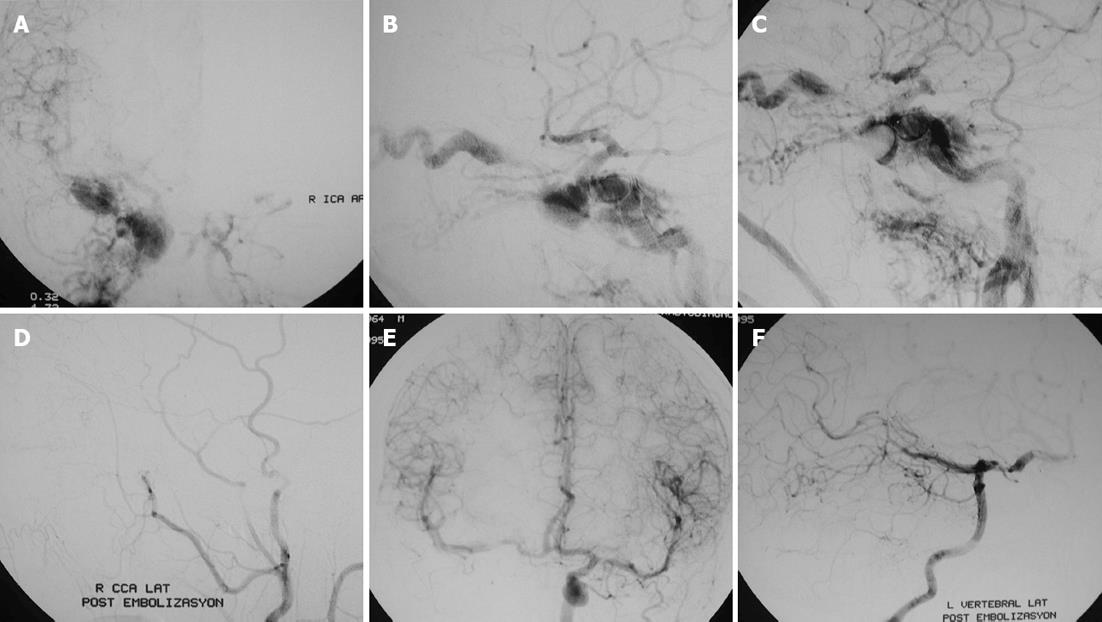
ICA occlusion can be performed with various endovascular techniques such as coil, balloon (Figure 3) and vascular plug embolization. Occlusion of the ipsilateral ICA is performed with coil embolization in a distal-to-proximal approach to prevent the retrograde arterial filling of the fistula from the supraclinoid ICA[5,7]. Recently, the Hydrocoil Embolization System (HES) has been introduced, which can achieve high rates of volumetric occlusion compared with that in platinum coils. The HES device consists of a carrier platinum coil coupled with an expandable hydrogel material that increases in volume on contact with blood and facilitates vessel sacrifice, decreasing the procedure and fluoroscopy times. Hydrogel-coated detachable coils can be utilized to achieve rapid, precise, targeted parent artery occlusion procedures, even in short segments of vessel[7,37].
Fistula entrapment can be performed with the aid of two ballons, which are positioned proximal and distal to the fistula. If it is impossible to navigate either a balloon or a microcatheter beyond the fistula to allow distal control, a microcatheter is navigated into the supraclinoid segment of the carotid artery via the anterior or posterior communicating arteries with the aid of marked retrograde flow to the fistula. Thereby, endovascular trapping of the traumatic CCF can be performed by a combination of proximal balloon occlusion and distal trapping with coils[38,39]. Deployment of a vascular plug is an alternative method but this device is limited to occlusions below the base of the skull because of the poor navigability in the distal ICA[40].
Transvenous embolization: Although transvenous embolization is an alternative technique in direct CCFs that cannot be treated by transarterial route, it is the preferred treatment for indirect CCFs. For indirect CCFs, transvenous techniques have precedence over transarterial methods because of their simplicity, lower ischemic risk, higher success rates and capability to cure the fistula in a single session. The aim of the transvenous approach is to catheterize the abnormal cavernous sinus superselectively and to occlude the fistula without rerouting venous drainage to cortical structures[4,5,7,21].
Navigation through the venous system and mechanical perforation are technical challenges in this procedure. Furthermore, usage of the transvenous approach in the acute fistula stages may be hazardous because the venous walls have not undergone wall thickening via arterialization[5].
Current microcatheter technology permits access to the cavernous sinus via multiple routes. The most commonly used venous pathway for cannulation of the cavernous sinuses is via the IPS. This transvenous route is usually used from a posterior direction through the internal jugular vein and the IPS up to the pathologic shunts of the cavernous sinus[5,7,15]. Anatomically, catheterization of the cavernous sinus through the IPS is feasible in the great majority (99%) of cases. However, accessibility of the cavernous sinus through the IPS can become technically difficult as the disease progresses. Difficulties may result from occlusion of the IPSs due to longstanding venous hypertension, prior embolization, or both[18,41]. Because the drainage of the pons and brainstem may be via the IPS, and any damage to these veins may result in fatal venous thrombosis, the catheterization of the IPS merits special attention[6].
Anterior approach through the SOV via the facial vein provides a convenient alternative pathway for transvenous embolization of dural CCFs when cannulation of the IPS is not successful, thereby increasing the technical success rate[18].
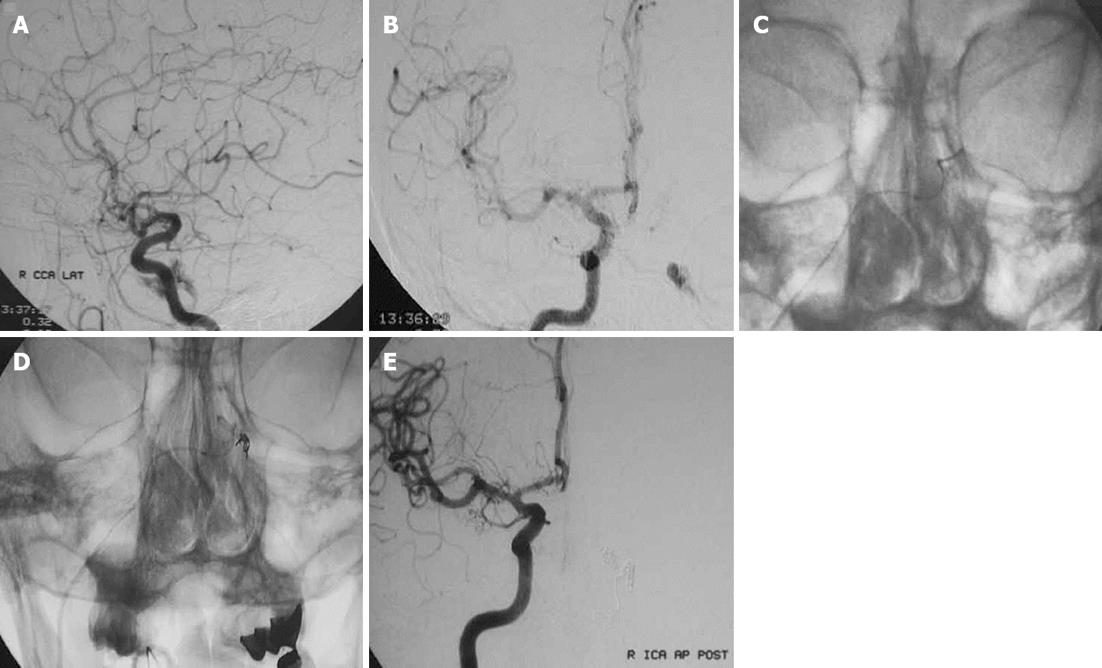
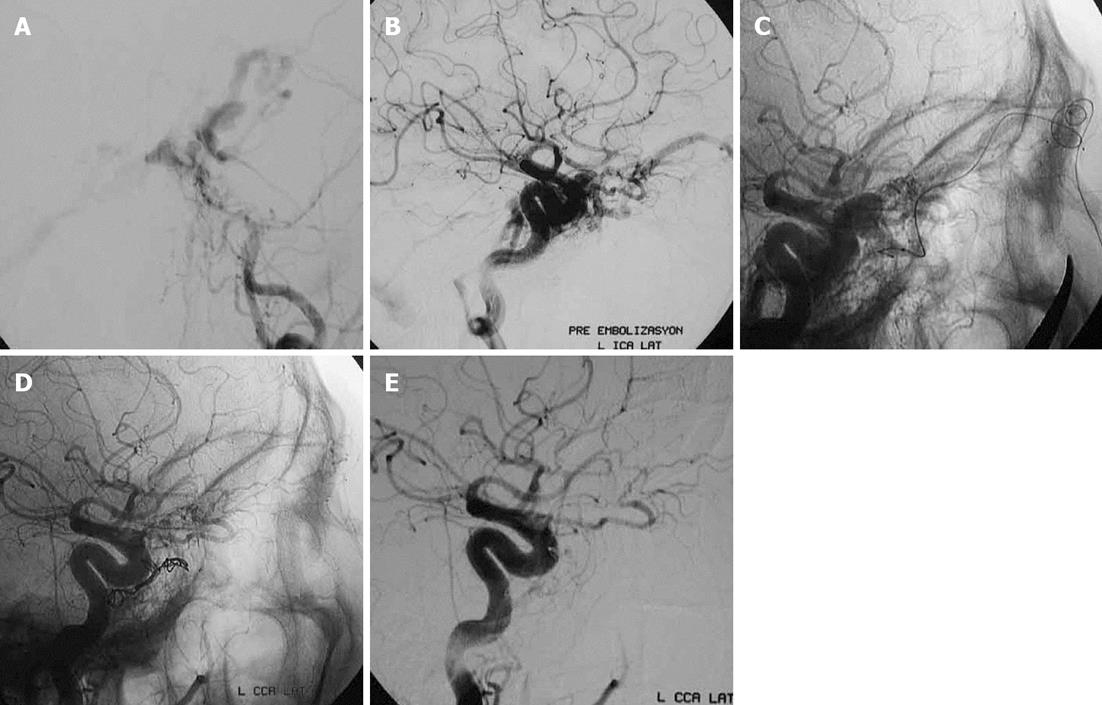
Less commonly used transvenous approaches are through the lateral pterygoid plexus, superior petrosal sinus, cortical veins, the inferior ophthalmic vein or the contralateral IPS (Figure 4) or SOV with access into the ipsilateral cavernous sinus through the circular sinus[5,7,15].
Alternatively, in extremely difficult cases of venous occlusion, stenosis, or marked tortuosity, access to the cavernous sinus can be provided by combined surgical and endovascular approaches (Figure 5)[7].
Direct transorbital puncture or indirect puncture through the superior or inferior ophthalmic vein allows straightforward access to the cavernous sinus[42]. Surgical access also may be obtained into the SOV, superficial middle cerebral vein, or sphenoparietal sinus leading to the cavernous sinus. Quiñones et al[21] proposed surgical exposure of the SOV and retrograde venous catheterization in indirect CCF patients who present with decreased visual acuity and predominant anterior venous drainage. Surgical exposure permits direct visibility and immobilization of the SOV, with less risk of rupture of the arterialized vein than with direct puncture. This approach also allows immediate control of possible orbital hemorrhage. The potential risks of the direct puncture or surgical exposure are orbital hemorrhage, nerve damage and laceration of the ICA resulting in direct CCF, globe puncture, and infection[21,42].
Sonographically guided direct percutaneous access through the facial vein can be performed to eliminate the risk of complications, such as intra- or retro-orbital hemorrhage, cranial nerve damage, subarachnoid or intracranial hemorrhage, and arterial damage, which may result from direct puncture of the SOV or the cavernous sinus. Reduction in radiation exposure and ease of manual compression of the puncture site after the procedure are additional advantages of this procedure although the small diameter of the facial vein may cause technical limitations[41]. Following successful catheterization of the cavernous sinus, various embolic materials such as coils, n-BCA, and EVOH, can be used either alone or in combination.
The advantages of coils include their radiopacity, ease of use, and the ability to redeploy or remove the devices if the initial placement is not optimal. However, there may be difficulty in achieving adequate volumetric packing or complete occlusion, especially in septated cavernous sinuses. Moreover, the reported rates of cranial nerve paresis are higher with coil embolization, probably because of their mass effect[7]. Consequently, transvenous liquid embolic agents are being used increasingly, either alone or in combination with platinum coils. EVOH has the capability of mechanical occlusion without vessel wall adhesion. Its nonadhesive nature decreases the risk of microcatheter retention and allows a slow single injection of embolic agent with concomitant angiogram checks. It must be remembered that EVOH has a propensity for retrograde filling of arterial feeders and must be used cautiously in order to prevent retrograde reflux into the ICA and ECA branches[7,43].
n-BCA has the advantages of rapid polymerization and permanent occlusion of the injected feeders. However, in contrast to EVOH, catheter repositioning during embolization and the reflux-hold-reinjection technique cannot be performed in embolization with n-BCA. Prolonged injections are not possible and as they may risk gluing the catheter because of the adhesive nature of n-BCA[7,43]. The polymerization of n-BCA is accompanied by heat production, which may lead to a degree of angionecrosis[6].
Transarterial embolization: Transarterial embolization of indirect low-flow CCFs generally is cumbersome because of the small size, complex anatomy, and multiplicity of arterial feeders. Multiple staged sessions may be necessary because of technical difficulties associated with superselective distal access into small-sized arterial feeders. Additionally, potential complications (e.g., thromboembolic stroke, cranial nerve palsies) restrain the choice of the transarterial approach as the mainstay treatment of spontaneous indirect CCFs. Therefore, transarterial embolization is typically used only to reduce arterial inflow before transvenous occlusion for high-flow indirect CCFs and as a viable alternative after failure of transvenous attempts[4,7].
The management strategy for traumatic indirect CCFs differs from that for spontaneous indirect CCFs. For traumatic lesions, transarterial embolization may be preferred because the single arterial supply is large enough to provide access to the feeder and cavernous sinus by microcatheter. The transvenous approach is reserved for cases in which failure or recurrence of the fistula is observed and arterial access to the site of the fistula is not feasible[17].
Transarterial techniques involve distal catheterization of the small meningeal branches supplying the fistula. Ideally, placement of the superselective microcatheter is performed with the microcatheter tip as close as possible to the point of fistulous communication. Once a satisfactory microcatheter position is achieved, liquid embolic agents (n-BCA, EVOH) are injected under fluoroscopic control with the goal of occluding the fistulous connections and penetrating the cavernous sinus. Although coils and particulate agents have been used, these agents used alone cannot provide permanent occlusion of the fistula[7].
While ocular symptoms resolve rapidly following successful treatment, patients may become transiently more symptomatic due to propagation of thrombus throughout the cavernous sinus and extending into the SOV. This clinical deterioration is called the “paradoxical worsening phenomenon” and can be observed in patients after transarterial embolization, gamma knife radiosurgery or conservative treatment. Although disconcerting to the patient, such symptoms usually resolve spontaneously over time. A brief course of corticosteroids may help to reduce inflammation associated with sinus thrombosis[15,26].
Severe progression of the ocular manifestations in the early postoperative period and recurrence of symptoms may suggest recurrent CCF. In cases where recurrent CCF is suspected, control cerebral angiography should be performed and possible re-treatment should be planned. After complete resolution of the ocular manifestations, additional control imaging and follow-up is not required.
In patients who were treated by the placement of a covered stent, stent-graft patency should be followed carefully as long-term safety data are lacking[35,44]. Long-term follow-up is necessary in CCF cases treated by parent artery occlusion to monitor the possible development of hemodynamic aneurysm in the anterior communicating artery, due to increased flow and alteration in hemodynamics.
Patients who have fistulas demonstrating cortical venous rerouting or partially treated CCFs may show clinical deterioration. In such cases, urgent examination should be performed and treatment should be planned if required. Acute onset of focal neurological deficit deserves immediate clinical evaluation and control imaging.
With advances in catheter design, embolic agents, and fluoroscopic imaging equipment, interventional neuroendovascular techniques have become the preferred treatment modality for carotid cavernous fistulas and favorable long-term outcomes have been achieved. The endovascular approach should be tailored to individual cases according to the type, exact anatomy, and extent of each fistula. With increasing knowledge about novitious endovascular techniques, such as placement of covered stent grafts, higher success rates can be achieved with preservation of the ICA even in urgent cases.
P- Reviewer Karmy-Jones R S- Editor Gou SX L- Editor Hughed D E- Editor Xiong L
| 1. | Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 735] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Ernst RJ, Tomsick TA. Classification and angiography of carotid cavernous fistulas. Carotid cavernous sinus fistula. Cincinnati: Digital Education Publishing 1997; 13-22. |
| 3. | Coskun O, Hamon M, Catroux G, Gosme L, Courthéoux P, Théron J. Carotid-cavernous fistulas: diagnosis with spiral CT angiography. AJNR Am J Neuroradiol. 2000;21:712-716. [PubMed] |
| 4. | Ringer AJ, Salud L, Tomsick TA. Carotid cavernous fistulas: anatomy, classification, and treatment. Neurosurg Clin N Am. 2005;16:279-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Tjoumakaris SI, Jabbour PM, Rosenwasser RH. Neuroendovascular management of carotid cavernous fistulae. Neurosurg Clin N Am. 2009;20:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Connors JJ, Wojak JC. Interventional Neuroradiology: Strategies and Practical Techniques. Philadelphia: WB Saunders Co 1999; 215-226. |
| 7. | Gemmete JJ, Ansari SA, Gandhi DM. Endovascular techniques for treatment of carotid-cavernous fistula. J Neuroophthalmol. 2009;29:62-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Tomsick TA. Type A (direct) CCF: etiology, prevalence, and natural history. Carotid cavernous sinus fistula. Cincinnati: Digital Education Publishing 1997; 35-38. |
| 9. | Wanke I, Doerfler A, Stolke D, Forsting M. Carotid cavernous fistula due to a ruptured intracavernous aneurysm of the internal carotid artery: treatment with selective endovascular occlusion of the aneurysm. J Neurol Neurosurg Psychiatry. 2001;71:784-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Germain DP, Herrera-Guzman Y. Vascular Ehlers-Danlos syndrome. Ann Genet. 2004;47:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Debrun GM. Angiographic workup of a carotid cavernous sinus fistula (CCF) or what information does the interventionalist need for treatment? Surg Neurol. 1995;44:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Lewis AI, Tomsick TA, Tew JM. Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995;36:239-44; discussion 244-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 203] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Higashida RT, Halbach VV, Tsai FY, Norman D, Pribram HF, Mehringer CM, Hieshima GB. Interventional neurovascular treatment of traumatic carotid and vertebral artery lesions: results in 234 cases. AJR Am J Roentgenol. 1989;153:577-582. [PubMed] |
| 14. | Ferrera PC. Traumatic carotid-cavernous sinus fistula with spontaneous resolution. Am J Emerg Med. 1997;15:386-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Meyers PM, Halbach VV, Dowd CF, Lempert TE, Malek AM, Phatouros CC, Lefler JE, Higashida RT. Dural carotid cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol. 2002;134:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Pang D, Kerber C, Biglan AW, Ahn HS. External carotid-cavernous fistula in infancy: case report and review of the literature. Neurosurgery. 1981;8:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Luo CB, Teng MM, Chang FC, Chang CY. Traumatic indirect carotid cavernous fistulas: angioarchitectures and results of transarterial embolization by liquid adhesives in 11 patients. Surg Neurol. 2009;71:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Yu SC, Cheng HK, Wong GK, Chan CM, Cheung JY, Poon WS. Transvenous embolization of dural carotid-cavernous fistulae with transfacial catheterization through the superior ophthalmic vein. Neurosurgery. 2007;60:1032-1037; discussion 1037-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Halbach VV, Hieshima GB, Higashida RT, Reicher M. Carotid cavernous fistulae: indications for urgent treatment. AJR Am J Roentgenol. 1987;149:587-593. [PubMed] |
| 20. | Tomsick TA. Type B,C, and D (dural) CCF: etiology, prevalence, and natural history. Carotid cavernous sinus fistula. Cincinnati: Digital Education Publishing 1997; 59-73. |
| 21. | Quiñones D, Duckwiler G, Gobin PY, Goldberg RA, Viñuela F. Embolization of dural cavernous fistulas via superior ophthalmic vein approach. AJNR Am J Neuroradiol. 1997;18:921-928. [PubMed] |
| 22. | Belden CJ, Abbitt PL, Beadles KA. Color Doppler US of the orbit. Radiographics. 1995;15:589-608. [PubMed] |
| 23. | Turner RD, Gonugunta V, Kelly ME, Masaryk TJ, Fiorella DJ. Marginal sinus arteriovenous fistulas mimicking carotid cavernous fistulas: diagnostic and therapeutic considerations. AJNR Am J Neuroradiol. 2007;28:1915-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Tech KE, Becker CJ, Lazo A, Slovis TL, Rabinowicz IM. Anomalous intracranial venous drainage mimicking orbital or cavernous arteriovenous fistula. AJNR Am J Neuroradiol. 1995;16:171-174. [PubMed] |
| 25. | McConnell KA, Tjoumakaris SI, Allen J, Shapiro M, Bescke T, Jabbour PM, Rosenwasser RH, Nelson PK. Neuroendovascular management of dural arteriovenous malformations. Neurosurg Clin N Am. 2009;20:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Komiyama M, Nakajima H, Nishikawa M, Yasui T. Brachial plexus and supraclavicular nerve injury caused by manual carotid compression for spontaneous carotid-cavernous sinus fistula. Surg Neurol. 1999;52:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Link MJ, Coffey RJ, Nichols DA, Gorman DA. The role of radiosurgery and particulate embolization in the treatment of dural arteriovenous fistulas. J Neurosurg. 1996;84:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 88] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Koebbe CJ, Singhal D, Sheehan J, Flickinger JC, Horowitz M, Kondziolka D, Lunsford LD. Radiosurgery for dural arteriovenous fistulas. Surg Neurol. 2005;64:392-38; discussion 392-38;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41:125-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 524] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Teng MM, Chang CY, Chiang JH, Lirng JF, Luo CB, Chen SS, Chang FC, Guo WY. Double-balloon technique for embolization of carotid cavernous fistulas. AJNR Am J Neuroradiol. 2000;21:1753-1756. [PubMed] |
| 31. | Lasjaunias P, Berenstein A, Ter Brugge KG. Surgical neuroangiography. 2nd ed. Vol 2, Clinical and endovascular treatment. Berlin, Heidelberg: Springer-Verlag 2004; . |
| 32. | Sencer S, Minareci O, Poyanli A. Management of a rare complication of endovascular treatment of direct carotid cavernous fistula. AJNR Am J Neuroradiol. 1999;20:1465-1466. [PubMed] |
| 33. | Klisch J, Schipper J, Husstedt H, Laszig R, Schumacher M. Transsphenoidal computer-navigation-assisted deflation of a balloon after endovascular occlusion of a direct carotid cavernous sinus fistula. AJNR Am J Neuroradiol. 2001;22:537-540. [PubMed] |
| 34. | Morón FE, Klucznik RP, Mawad ME, Strother CM. Endovascular treatment of high-flow carotid cavernous fistulas by stent-assisted coil placement. AJNR Am J Neuroradiol. 2005;26:1399-1404. [PubMed] |
| 35. | Kocer N, Kizilkilic O, Albayram S, Adaletli I, Kantarci F, Islak C. Treatment of iatrogenic internal carotid artery laceration and carotid cavernous fistula with endovascular stent-graft placement. AJNR Am J Neuroradiol. 2002;23:442-446. [PubMed] |
| 36. | Gomez F, Escobar W, Gomez AM, Gomez JF, Anaya CA. Treatment of carotid cavernous fistulas using covered stents: midterm results in seven patients. AJNR Am J Neuroradiol. 2007;28:1762-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Kallmes DF, Cloft HJ. The use of hydrocoil for parent artery occlusion. AJNR Am J Neuroradiol. 2004;25:1409-1410. [PubMed] |
| 38. | Uysal E, Kizilkiliç O, Ulusay M, Basak M. Endovascular trapping of direct carotid-cavernous fistula. J Clin Neurosci. 2010;17:392-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Coley SC, Pandya H, Hodgson TJ, Jeffree MA, Deasy NP. Endovascular trapping of traumatic carotid-cavernous fistulae. AJNR Am J Neuroradiol. 2003;24:1785-1788. [PubMed] |
| 40. | Ross IB, Buciuc R. The vascular plug: a new device for parent artery occlusion. AJNR Am J Neuroradiol. 2007;28:385-386. [PubMed] |
| 41. | Berkmen T, Troffkin NA, Wakhloo AK. Transvenous sonographically guided percutaneous access for treatment of an indirect carotid cavernous fistula. AJNR Am J Neuroradiol. 2003;24:1548-1551. [PubMed] |
| 42. | White JB, Layton KF, Evans AJ, Tong FC, Jensen ME, Kallmes DF, Dion JE, Cloft HJ. Transorbital puncture for the treatment of cavernous sinus dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2007;28:1415-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Suzuki S, Lee DW, Jahan R, Duckwiler GR, Viñuela F. Transvenous treatment of spontaneous dural carotid-cavernous fistulas using a combination of detachable coils and Onyx. AJNR Am J Neuroradiol. 2006;27:1346-1349. [PubMed] |
| 44. | Archondakis E, Pero G, Valvassori L, Boccardi E, Scialfa G. Angiographic follow-up of traumatic carotid cavernous fistulas treated with endovascular stent graft placement. AJNR Am J Neuroradiol. 2007;28:342-347. [PubMed] |