Revised: September 13, 2012
Accepted: January 31, 2013
Published online: March 28, 2013
Computed tomography (CT) colonoscopy is a well-established technique for evaluation of colorectal cancer. Significant advances have been made in the technique of CT colonoscopy since its inception. Excellent results can be achieved in detecting both colorectal cancer and significant sized polyps as long as a meticulous technique is adopted while performing CT colonoscopy. Furthermore, it is important to realize that there is a learning curve involved in interpreting these studies and adequate experience is essential to achieve high sensitivity and specificity with this technique. Indications, contraindications, technique and interpretation, including potential pitfalls in CT colonoscopy imaging, are reviewed in this article. Recent advances and the current role of CT colonoscopy in colorectal cancer screening are also discussed.
- Citation: Ganeshan D, Elsayes KM, Vining D. Virtual colonoscopy: Utility, impact and overview. World J Radiol 2013; 5(3): 61-67
- URL: https://www.wjgnet.com/1949-8470/full/v5/i3/61.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i3.61
Colorectal cancer is a common malignancy associated with significant morbidity and mortality. It is the third most common cancer diagnosed in both sexes in the United States and it is estimated that 101 700 new cases of colon cancer and 39 510 new cases of rectal cancer will be diagnosed in 2011. The lifetime risk for developing colorectal cancer is about 5%. Furthermore, colorectal cancer is the second leading cause of cancer-related deaths in the United States and is expected to cause about 49 380 deaths during 2011[1].
However, the encouraging fact is that the death rates from colorectal cancer have been dropping in both men and women for more than two decades. Apart from major advances in treatment, the two key reasons contributing to this decline in death rates from colorectal cancer are early detection and treatment of polyps before they have become malignant, as well as detection of malignant tumors at earlier stages, which has a significantly better prognosis compared to later stages. The 5-year survival rate of stage 1 colorectal cancer is 74%, whereas for stage 4, it drops to 6%[2].
The principle of colorectal cancer screening is based on the fact that most colorectal adenocarcinomas develop from pre-existing adenomas via numerous molecular and genetic steps, the adenoma-to-carcinoma sequence theory[3-7]. Fortunately, it is estimated that there is a long time interval of 10 to 15 years for the development of colorectal carcinoma from normal colon and almost 5 years for the development of adenoma from normal colon[3]. Hence, by diagnosing and removing the polyps before they become malignant, it may be possible to potentially prevent invasive colorectal cancers. It has been reported that colonoscopy and polypectomy decrease the incidence of colorectal cancer by 76%-90%[4].
Apart from histology (villous has greater malignancy potential compared to tubular adenomas), polyp size is a crucial factor in determining the risk of malignancy[5]. Only about 1% of adenomas less than 1 cm in diameter turn malignant, whereas this risk rises to 10% in adenomas measuring 1-2 cm in diameter and more than 40% in those greater than 2 cm[6]. However, since there is a small risk of high grade dysplasia (up to 4.8%) in polyps of 6-9 mm, other authors have suggested that polypectomy be performed for polyps of all size in order to reduce the risk of developing colorectal carcinoma.
Hence, the success of any diagnostic test as a colorectal cancer screening tool lies in its ability to accurately diagnose polyps measuring 1 cm and more as well as detect early colorectal cancers.
Optical colonoscopy has been the standard test for evaluation of the colon, with an excellent sensitivity and specificity for diagnosis of colorectal cancer and polyps. However, it is an invasive test and carries the risk of significant complications, such as perforation. Apart from its invasive nature, other issues such as cost, availability and patients’ experience led to the introduction and development of diagnostic imaging methods for evaluating the colon and rectum.
Traditionally, double contrast barium enema (BE) has been the workhorse for imaging the colon and rectum in patients with suspected colorectal cancer. As colorectal cancers were more common in the rectum and sigmoid colon, it was common practice to perform a flexible sigmoidoscopy (which is quicker, safer and technically easier than colonoscopy) and the rest of the colon was evaluated by a BE. However, the sensitivity of a BE was shown to be low, even for polyps greater than 1 cm[7-9]. In prospective studies, BE was reported to have a sensitivity of 48% for polyps greater than or equal to 1 cm and this fell even lower to 41% for polyps between 6 to 9 mm[8,9]. The ACR pushed DCBE for a decade and its use did not impact the low compliance of the US population to ACS colorectal cancer screening guidelines. Improving CRC screening compliance and the limited use and declining volume of BE necessitated a more robust imaging technique for colorectal cancer evaluation
Computed tomographic colonography (CTC) was first introduced by Vining[10] in 1994 as an alternative imaging method for evaluation of the colon. In this technique, helical computed tomography (CT) data is used to produce three dimensional images and hence simulates a virtual endoluminal view; hence, also called virtual colonoscopy. High sensitivity rates for colorectal cancer can be obtained by this method. In a recent meta-analysis, Pickhardt et al[11] reported that the pooled sensitivity of CT colonography for colorectal cancer was about 96% compared to 95% seen in optical colonoscopy. It has to be noted that this excellent sensitivity for colorectal cancer was achieved in spite of including studies from five different continents with considerable variability in CT colonography techniques and technologies [using both single and multidetector CT (MDCT) scanners][11,12]. This underlines the excellent potential of CT colonoscopy as a screening tool for colorectal cancer on a universal basis[12].
With regards to colonic polyps, the sensitivity of CTC is dependent on the size of the polyps. The sensitivity and specificity for CTC in diagnosing polyps larger than 1 cm is 93% and 97% respectively. However, both the sensitivity and specificity reduces to 86% for polyps measuring between 6 to 9 mm[11,13]. Although its sensitivity is reduced in small sized polyps, the risk of malignancy in a polyp less than 1 cm in diameter is less than 1%[14].
Besides being an excellent screening tool for colorectal cancer, it is minimally invasive, less time consuming and does not require sedation. Furthermore, it offers staging information, which is not possible with either optical colonoscopy or a BE. Complications of colonic tumors, such as obstruction, perforation and fistula, can be readily visualized with CTC. Finally, it also highlights the presence of any significant extra colonic pathology, which may significantly alter the management[15-17].
CTC is an established test for screening asymptomatic individuals for early detection of colorectal cancer. Both the American College of Radiology and American Cancer Society have approved CTC for colorectal cancer screening in patients older than 50 years old, those with a positive fecal occult blood test or individuals at moderate risk with a personal history of adenoma or colorectal cancer or with family history of adenoma or cancer in a first degree relative[18-20]. High risk individuals with hereditary non polyposis colorectal cancer are better evaluated with OC as there is a high pretest probability of identifying polyps/tumors in this group and hence biopsy or polypectomy may be performed at the same sitting.
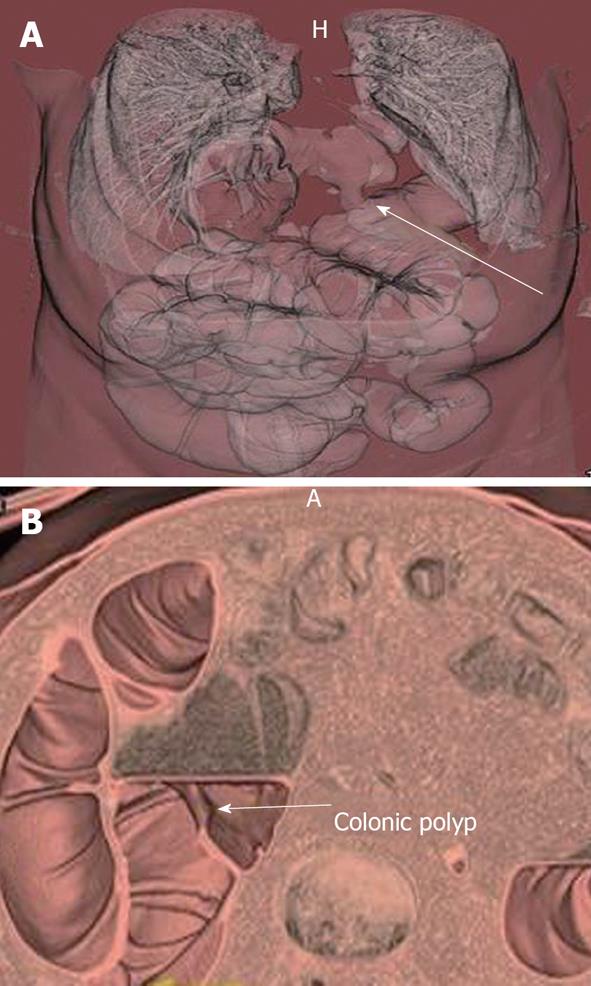
Patients with failed or incomplete colonoscopy (5%-10%), those who are reluctant to undergo OC or those who may be at an increased risk with OC can be evaluated by CTC (Figures 1 and 2)[21,22]. CTC has been used in the evaluation of patients presenting with obstructing colonic neoplasms and rarely in inflammatory bowel disease[23-25]. However, CTC is contraindicated in the presence of any acute inflammatory condition of the bowel, such as acute inflammatory bowel disease or diverticulitis, due to the increased risk of perforation following insufflation of colon[26-28].
CT colonography is based on a helical, thin-section CT of the cleansed and distended colon. In general, a MDCT scanner is superior to single detector row CT for performing CTC as it allows acquisition of multiple thin sections, superior multiplanar reconstructions and faster imaging time[14]. A 16 slice CT scanner or higher is recommended for CTC, but it can be performed even with an 8 slice scanner, although this will be of lesser quality. It is important to keep the radiation dose to as low as reasonably possible, while maintaining diagnostic quality.
Based on a recent survey involving 34 different institutions, Liedenbaum[29] reported that the median effective radiation dose per institution was 5.7 mSv for screening protocols and 9.1 mSv for daily practice (CTC in non-screening population). It has been estimated that a typical CTC may be associated with a 0.14% life time cancer risk for a 50 years old, but this risk can be significantly reduced by a factor of 5-10 times by optimizing the CT protocol[30]. Indeed, low dose CTC can be successfully performed by reducing the milli-ampere-seconds level and using automated dose reduction software[31-33].
Apart from proper CT protocol, other factors critical for performing a successful CTC study includes achieving a clean, well distended bowel and achieving adequate experience in interpreting the imaging datasets obtained, which have been well described before[34,35].
Numerous cathartic agents, including magnesium citrate, sodium phosphate, picolax and polyethylene glycol, have been used for cleaning the bowel in CTC[36-38]. Fecal tagging with oral contrast agents such as barium or iodinated contrast is commonly used in CTC to overcome the disadvantage of residual fluid and feces, which may occur in spite of an adequate bowel preparation regimen[39,40]. The contrast tags the feces, allowing it to be differentiated from polyps. Furthermore, it also tags residual fluid, which helps to detect the polyps submerged in fluid as filling defects against the high attenuation background of contrast tagged residual fluid. It can also coat the polyps, especially the villous ones. Furthermore, the high density of the tagged fluid permits electronic subtraction software to be effective.
CTC without using laxatives has also been used by some authors wherein the bowel cleansing is performed digitally, using advanced software[39-45]. Although this offers a great option, especially for the frail patients, this has to be balanced against the greater diagnostic accuracy that is likely to be achieved with a more rigorous bowel preparation. Further research on its diagnostic accuracy compared to CTC performed with cathartic bowel preparation is required before this can become a routine practice.
It is vital that either the radiologist or the radiology technician ensures that the bowel is adequately distended when the study is being performed. This way, it may be possible to perform any additional sequences deemed necessary to ensure a complete CTC study, whilst the patient is still on the table[46]. It is important to realize that rectal catheters may hide lesions in the lower rectum or anus and hence it may be better to remove the catheter or at least deflate the balloon after the supine scan and then do the prone scan.
Bowel insufflation is usually achieved either by carbon dioxide (CO2) introduced through automated delivery systems or room air insufflation[47,48]. CO2 causes less discomfort than compared to room air and the automated system allows greater control in terms of flow rate, total volume administered and maintaining intracolonic pressure[47,48]. In general, about 2-4 L of gas is insufflated but this is highly variable and hence monitoring intracolonic pressure (maximum of 25 mmHg) is a better option.
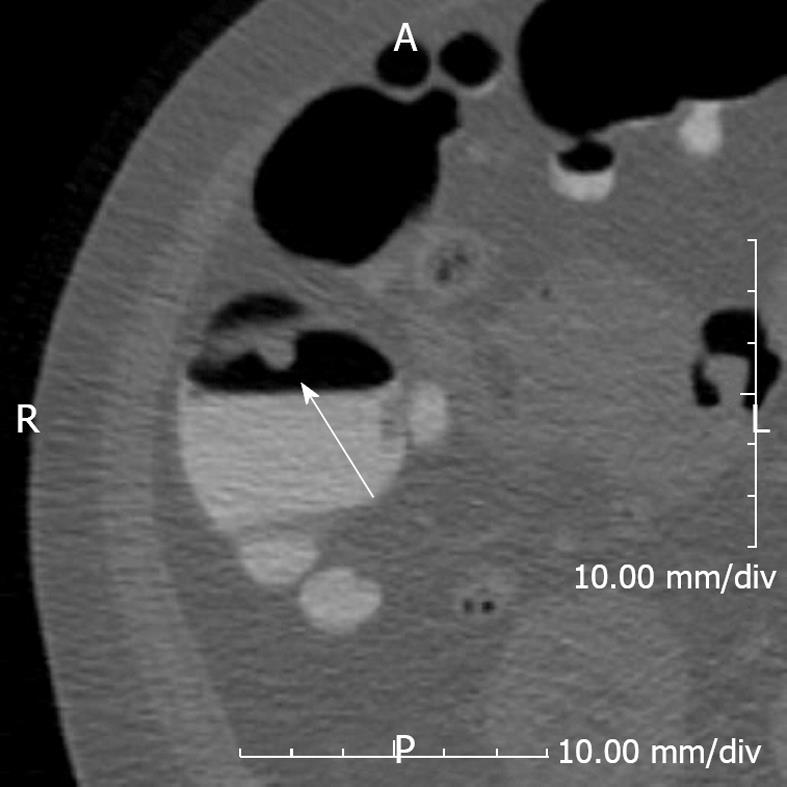
CTC is usually performed with the patient scanned in the supine and prone position, but in some patients, decubitus scans may be required[46,49]. It is vital to get a scout view prior to each scan to ensure that adequate colonic distension is maintained. Obtaining scans with the patient in different positions has a few advantages - Thirdly, it is useful to differentiate feces (which will be mobile) from true polyps (which are usually fixed). There are two exceptions to this rule: polyps with long stalks may still be mobile and feces may be adherent to bowel wall and appear as a fixed lesion but usually these can be identified by careful attention to 2D and 3D images. Also, change in posture helps to displace residual fluid so that the entire colon can be assessed using different data sets (Figure 3). Thirdly, obtaining multiple datasets provides an opportunity to obtain all segments of the colon well distended at least on one or more sequences.
It is usually performed without using an intravenous contrast media but this practice is variable across institutions[50-55]. However, it is still reasonable to do non contrast enhanced CTC in a screening population as the chances of identifying significant extracolonic pathology in them is low[14].
CTC is a safer technique than optical colonoscopy. Bassett et al reported just a single perforation out of 5306 CTCs performed at the National Naval Medical Center. Similarly, other authors have reported a very low incidence of colonic perforation following CTC (0.05%-0.06%)[50-55]. This complication is much lower than optical colonoscopy which is reported to have a risk of perforation in 0.1%-0.2%[53].
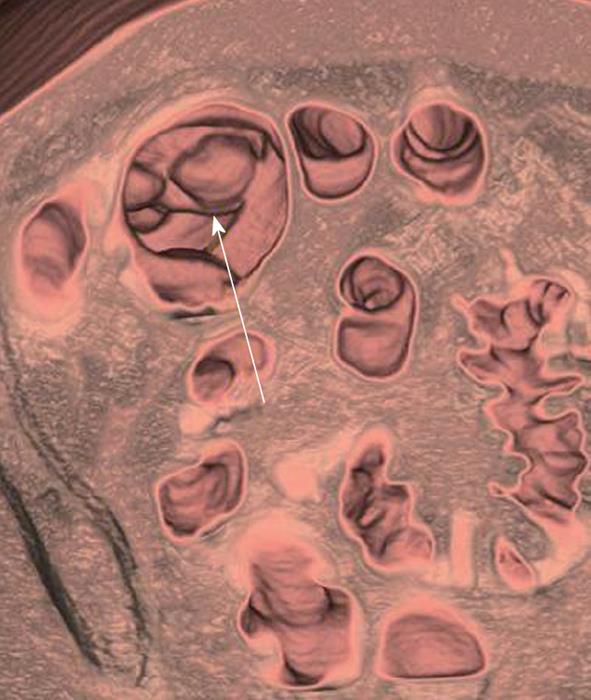
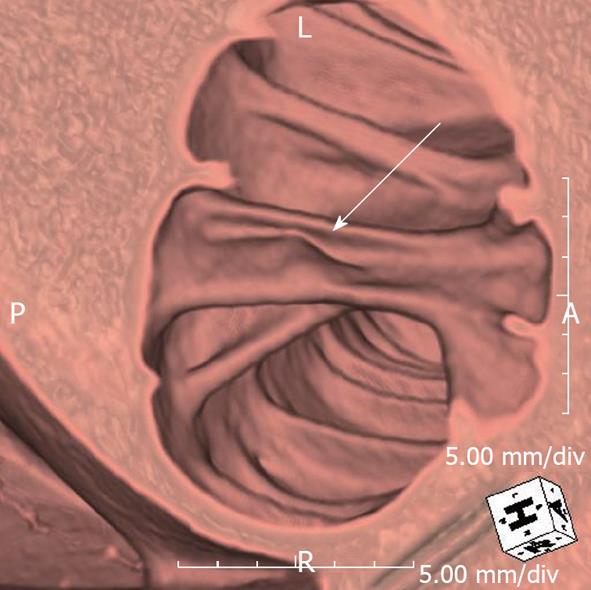
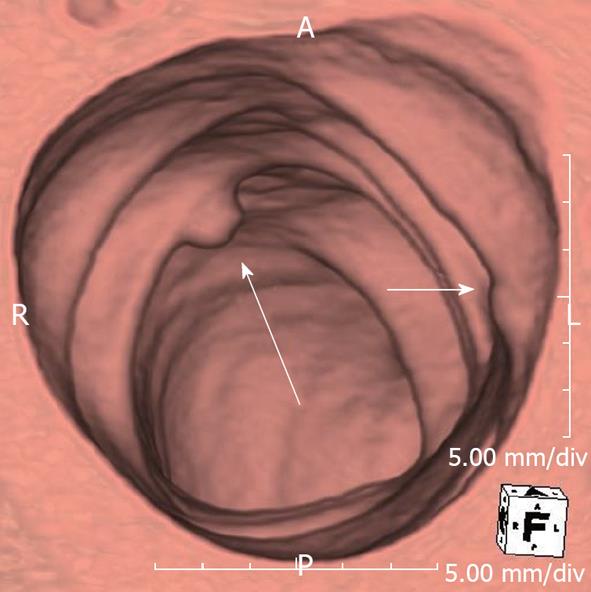
2D and 3D imaging in CTC complement each other[54-57]. 3D interpretation is useful for detecting polyps, particularly those on the folds (Figure 4)[54-57]. On the other hand, 2D interpretation helps to confirm whether the lesion identified on a 3D image is a true polyp or just a lipoma, adherent feces or a prominent fold (Figure 5). Also, measuring polyp size, which is crucial for management, can sometimes be challenging and in most cases it would be useful to measure it both on 2D and 3D views to get a true size estimate or even volume measurement[58,59].
However, various pitfalls have been described whilst interpreting 2D and 3D imaging of CTC studies[51,56,60]. Polyps on a fold, as well as flat and carpet lesions can be missed on 2D interpretations. Similarly, annular masses may be mistaken for under distension or even completely missed on 3D images by the inexperienced reader. Furthermore, extrinsic lesions and impacted diverticula may lead to pseudo lesions on 3D evaluation but these can be easily confirmed on 2D imaging[56]. With increasing experience, one may then become a primary 2D or 3D reader, using the other data set to solve problems.
Also, When using oral fecal tagging, it is important to realize that sometimes the contrast (be it barium or iodinated contrast) may be too dense such that polyps submerged in tagged residual fluid may still not be visible unless the window width and level is adjusted accordingly.
Recent advances in 3D visualization have resulted in new 3D tools, such as virtual dissection, panoramic views, unfolded, cube projections and translucency rendering[61-64]. These have been designed with an idea to reduce time (eliminating need for bidirectional study as in virtual dissection) and increase specificity by assigning different colors for tissues of different attenuations such as feces and polyps (Translucency rendering).
However, recent studies indicate that use of advanced techniques such as virtual dissection can allow for short learning curves and improve detection rates, even for inexperienced readers[65].
It is also possible to track the visualized 3D endoluminal surface so that any areas which may have been missed initially can be re-interrogated, thereby reducing potential misses[66]. Computer-aided detection (CAD) systems are currently available in most CTC workstations. It can be helpful to increase the sensitivity of the radiologists, especially when they are relatively new to this technique[67].
Following a standardized, structured reporting for CTC is useful in many ways. It helps patients and physicians to decide a management plan and also ensures consistency while comparing CTC studies and reports performed at different institutions, an increasingly common phenomenon. Furthermore, it helps when it becomes necessary to evaluate or audit the quality of the CTC study, the quality of interpretation and to calculate cost-effectiveness with regards to patient outcome. Finally, this can help create an excellent database which would be an invaluable tool for the education of radiologists worldwide.
In 2005, a standardized reporting scheme, “C-RADS-CT Colonography Reporting and Data System”, was put forward by the working group on virtual colonoscopy[68]. They proposed that the report should include lesion size, number, morphology, location, attenuation and recommendations for lesion surveillance.
It would also be very useful to create and save a 3D map and multiplanar reconstructed digital images highlighting the polyp locations in each segment such that the endoscopist can use it as a road map while performing the optical colonoscopy.
Every diagnostic imaging tool is only as good as the radiologist who interprets it. Clearly, the sensitivity and specificity of CTC in the hands of experts are significantly higher than those who are less trained in this procedure. Hence, it is important to ensure high standards in the interpretation of CTC and to meet this goal several training and accreditation course are advocated by various expert panels, including the American College of Radiology[69,70].
Similarly, it would be very useful to educate CT technicians to look for issues such as collapsed segments of bowel at the time of scan so that additional images in decubitus views may be obtained in the same sitting, which can help to obviate the need for a future repeat study or optical colonoscopy[71]. Likewise, it may be useful to teach technicians to identify obvious colorectal tumors so that a staging scan of the chest can also be performed at the same time.
CTC is an excellent diagnostic tool for a comprehensive evaluation of the entire colon. Recent studies have confirmed that when CTC is properly performed and evaluated, a high diagnostic accuracy can be achieved for clinically significant polyps and colorectal cancer. This has resulted in CTC being approved for colorectal cancer screening and surveillance by the American Cancer Society, the American College of Radiology and the US Multi-Society Task Force on colorectal cancer[18-20,71,72]. However, CTC is not currently reimbursed for routine colorectal cancer screening in the United States and this has undeniably reduced its widespread use in this part of the world.
P- Reviewers Oto A, Cerwenka HR S- Editor Cheng JX L- Editor Roemmele A E- Editor Zheng XM
| 1. |
|
| 2. | Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z. SEER Cancer Statistics Review, 1975-2008. National Cancer Institute. Bethesda, MD. Available from: http://seer.cancer.gov/csr/1975_2008/. Based on November 2010 SEER data submission, posted to the SEER web site, 2011. |
| 3. | Day DW, Morson BC. The adenoma-carcinoma sequence. Major Probl Pathol. 1978;10:58-71. [PubMed] |
| 4. | Morson BC. The evolution of colorectal carcinoma. Clin Radiol. 1984;35:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1379] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 6. | Toribara NW, Sleisenger MH. Screening for colorectal cancer. N Engl J Med. 1995;332:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4464] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 8. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3126] [Article Influence: 97.7] [Reference Citation Analysis (1)] |
| 9. | Rockey DC, Paulson E, Niedzwiecki D, Davis W, Bosworth HB, Sanders L, Yee J, Henderson J, Hatten P, Burdick S. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 11. | Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection--systematic review and meta-analysis. Radiology. 2011;259:393-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 303] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 12. | Mang TCP, Lefere P. International implementation. Atlas of Virtual Colonoscopy. New York: Springer 2010; 9-48. |
| 13. | Halligan S, Altman DG, Taylor SA, Mallett S, Deeks JJ, Bartram CI, Atkin W. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology. 2005;237:893-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 242] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 14. | Hermanek P. Dysplasia-carcinoma sequence, types of adenomas and early colo-rectal carcinoma. Eur J Surg Oncol. 1987;13:141-143. [PubMed] |
| 15. | Kimberly JR, Phillips KC, Santago P, Perumpillichira J, Bechtold R, Pineau B, Vining D, Bloomfeld RS. Extracolonic findings at virtual colonoscopy: an important consideration in asymptomatic colorectal cancer screening. J Gen Intern Med. 2009;24:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Morrin MM, Farrell RJ, Kruskal JB, Reynolds K, McGee JB, Raptopoulos V. Utility of intravenously administered contrast material at CT colonography. Radiology. 2000;217:765-771. [PubMed] |
| 17. | Rajapaksa RC, Macari M, Bini EJ. Prevalence and impact of extracolonic findings in patients undergoing CT colonography. J Clin Gastroenterol. 2004;38:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1423] [Cited by in RCA: 1457] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 19. | McFarland EG, Fletcher JG, Pickhardt P, Dachman A, Yee J, McCollough CH, Macari M, Knechtges P, Zalis M, Barish M. ACR Colon Cancer Committee white paper: status of CT colonography 2009. J Am Coll Radiol. 2009;6:756-772.e4. [PubMed] |
| 20. | McFarland EG, Levin B, Lieberman DA, Pickhardt PJ, Johnson CD, Glick SN, Brooks D, Smith RA. Revised colorectal screening guidelines: joint effort of the American Cancer Society, U.S. Multisociety Task Force on Colorectal Cancer, and American College of Radiology. Radiology. 2008;248:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Neerincx M, Terhaar sive Droste JS, Mulder CJ, Räkers M, Bartelsman JF, Loffeld RJ, Tuynman HA, Brohet RM, van der Hulst RW. Colonic work-up after incomplete colonoscopy: significant new findings during follow-up. Endoscopy. 2010;42:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Macari M, Berman P, Dicker M, Milano A, Megibow AJ. Usefulness of CT colonography in patients with incomplete colonoscopy. AJR Am J Roentgenol. 1999;173:561-564. [PubMed] |
| 23. | Cha EY, Park SH, Lee SS, Kim JC, Yu CS, Lim SB, Yoon SN, Shin YM, Kim AY, Ha HK. CT colonography after metallic stent placement for acute malignant colonic obstruction. Radiology. 2010;254:774-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Coccetta M, Migliaccio C, La Mura F, Farinella E, Galanou I, Delmonaco P, Spizzirri A, Napolitano V, Cattorini L, Milani D. Virtual colonoscopy in stenosing colorectal cancer. Ann Surg Innov Res. 2009;3:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Ota Y, Matsui T, Ono H, Uno H, Matake H, Tsuda S, Sakurai T, Yao T. Value of virtual computed tomographic colonography for Crohn’s colitis: comparison with endoscopy and barium enema. Abdom Imaging. 2003;28:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Hanly P, Skally M, Fenlon H, Sharp L. Cost-effectiveness of computed tomography colonography in colorectal cancer screening: a systematic review. Int J Technol Assess Health Care. 2012;28:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Pickhardt PJ. Incidence of colonic perforation at CT colonography: review of existing data and implications for screening of asymptomatic adults. Radiology. 2006;239:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Boone D, Halligan S, Taylor SA. Evidence review and status update on computed tomography colonography. Curr Gastroenterol Rep. 2011;13:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Liedenbaum MH. CT colonography in faecal occult blood test positives. Available from: http://dareuvanl/document/165063. Amsterdam: AMC-UvA, 2010. |
| 30. | Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern. Gastroenterology. 2005;129:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Iannaccone R, Laghi A, Catalano C, Mangiapane F, Piacentini F, Passariello R. Feasibility of ultra-low-dose multislice CT colonography for the detection of colorectal lesions: preliminary experience. Eur Radiol. 2003;13:1297-1302. [PubMed] |
| 32. | Jensch S, van Gelder RE, Venema HW, Reitsma JB, Bossuyt PM, Laméris JS, Stoker J. Effective radiation doses in CT colonography: results of an inventory among research institutions. Eur Radiol. 2006;16:981-987. [PubMed] |
| 33. | Macari M, Bini EJ, Xue X, Milano A, Katz SS, Resnick D, Chandarana H, Krinsky G, Klingenbeck K, Marshall CH. Colorectal neoplasms: prospective comparison of thin-section low-dose multi-detector row CT colonography and conventional colonoscopy for detection. Radiology. 2002;224:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Mang T, Graser A, Schima W, Maier A. CT colonography: techniques, indications, findings. Eur J Radiol. 2007;61:388-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Philip AK, Lubner MG, Harms B. Computed tomographic colonography. Surg Clin North Am. 2011;91:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Keedy AW, Yee J, Aslam R, Weinstein S, Landeras LA, Shah JN, McQuaid KR, Yeh BM. Reduced cathartic bowel preparation for CT colonography: prospective comparison of 2-L polyethylene glycol and magnesium citrate. Radiology. 2011;261:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Macari M, Lavelle M, Pedrosa I, Milano A, Dicker M, Megibow AJ, Xue X. Effect of different bowel preparations on residual fluid at CT colonography. Radiology. 2001;218:274-277. [PubMed] |
| 38. | Taylor SA, Halligan S, Goh V, Morley S, Atkin W, Bartram CI. Optimizing bowel preparation for multidetector row CT colonography: effect of Citramag and Picolax. Clin Radiol. 2003;58:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Buccicardi D, Grosso M, Caviglia I, Gastaldo A, Carbone S, Neri E, Bartolozzi C, Quadri P. CT colonography: patient tolerance of laxative free fecal tagging regimen versus traditional cathartic cleansing. Abdom Imaging. 2011;36:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Liedenbaum MH, Denters MJ, Zijta FM, van Ravesteijn VF, Bipat S, Vos FM, Dekker E, Stoker J. Reducing the oral contrast dose in CT colonography: evaluation of faecal tagging quality and patient acceptance. Clin Radiol. 2011;66:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Callstrom MR, Johnson CD, Fletcher JG, Reed JE, Ahlquist DA, Harmsen WS, Tait K, Wilson LA, Corcoran KE. CT colonography without cathartic preparation: feasibility study. Radiology. 2001;219:693-698. [PubMed] |
| 42. | Mahgerefteh S, Fraifeld S, Blachar A, Sosna J. CT colonography with decreased purgation: balancing preparation, performance, and patient acceptance. AJR Am J Roentgenol. 2009;193:1531-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Pickhardt PJ. CT colonography without catharsis: the ultimate study or useful additional option. Gastroenterology. 2005;128:521-52; author reply 521-52;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Zalis ME, Hahn PF. Digital subtraction bowel cleansing in CT colonography. AJR Am J Roentgenol. 2001;176:646-648. [PubMed] |
| 45. | Zalis ME, Perumpillichira J, Del Frate C, Hahn PF. CT colonography: digital subtraction bowel cleansing with mucosal reconstruction initial observations. Radiology. 2003;226:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Buchach CM, Kim DH, Pickhardt PJ. Performing an additional decubitus series at CT colonography. Abdom Imaging. 2011;36:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Burling D, Taylor SA, Halligan S, Gartner L, Paliwalla M, Peiris C, Singh L, Bassett P, Bartram C. Automated insufflation of carbon dioxide for MDCT colonography: distension and patient experience compared with manual insufflation. AJR Am J Roentgenol. 2006;186:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH. Patient-controlled room air insufflation versus automated carbon dioxide delivery for CT colonography. AJR Am J Roentgenol. 2006;186:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Yee J, Kumar NN, Hung RK, Akerkar GA, Kumar PR, Wall SD. Comparison of supine and prone scanning separately and in combination at CT colonography. Radiology. 2003;226:653-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 50. | Burling D, Halligan S, Slater A, Noakes MJ, Taylor SA. Potentially serious adverse events at CT colonography in symptomatic patients: national survey of the United Kingdom. Radiology. 2006;239:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Pickhardt PJ. Differential diagnosis of polypoid lesions seen at CT colonography (virtual colonoscopy). Radiographics. 2004;24:1535-1556; discussion 1557-1559. [PubMed] |
| 52. | Sosna J, Blachar A, Amitai M, Barmeir E, Peled N, Goldberg SN, Bar-Ziv J. Colonic perforation at CT colonography: assessment of risk in a multicenter large cohort. Radiology. 2006;239:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 53. | Anderson ML, Pasha TM, Leighton JA. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol. 2000;95:3418-3422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 54. | Dachman AH, Kuniyoshi JK, Boyle CM, Samara Y, Hoffmann KR, Rubin DT, Hanan I. CT colonography with three-dimensional problem solving for detection of colonic polyps. AJR Am J Roentgenol. 1998;171:989-995. [PubMed] |
| 55. | Macari M, Milano A, Lavelle M, Berman P, Megibow AJ. Comparison of time-efficient CT colonography with two- and three-dimensional colonic evaluation for detecting colorectal polyps. AJR Am J Roentgenol. 2000;174:1543-1549. [PubMed] |
| 56. | Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol. 2007;189:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Royster AP, Fenlon HM, Clarke PD, Nunes DP, Ferrucci JT. CT colonoscopy of colorectal neoplasms: two-dimensional and three-dimensional virtual-reality techniques with colonoscopic correlation. AJR Am J Roentgenol. 1997;169:1237-1242. [PubMed] |
| 58. | Pickhardt PJ, Lee AD, McFarland EG, Taylor AJ. Linear polyp measurement at CT colonography: in vitro and in vivo comparison of two-dimensional and three-dimensional displays. Radiology. 2005;236:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Pickhardt PJ, Lehman VT, Winter TC, Taylor AJ. Polyp volume versus linear size measurements at CT colonography: implications for noninvasive surveillance of unresected colorectal lesions. AJR Am J Roentgenol. 2006;186:1605-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Macari M, Bini EJ, Jacobs SL, Lange N, Lui YW. Filling defects at CT colonography: pseudo- and diminutive lesions (the good), polyps (the bad), flat lesions, masses, and carcinomas (the ugly). Radiographics. 2003;23:1073-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Hoppe H, Quattropani C, Spreng A, Mattich J, Netzer P, Dinkel HP. Virtual colon dissection with CT colonography compared with axial interpretation and conventional colonoscopy: preliminary results. AJR Am J Roentgenol. 2004;182:1151-1158. [PubMed] |
| 62. | Juchems MS, Fleiter TR, Pauls S, Schmidt SA, Brambs HJ, Aschoff AJ. CT colonography: comparison of a colon dissection display versus 3D endoluminal view for the detection of polyps. Eur Radiol. 2006;16:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Silva AC, Wellnitz CV, Hara AK. Three-dimensional virtual dissection at CT colonography: unraveling the colon to search for lesions. Radiographics. 2006;26:1669-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Vos FM, van Gelder RE, Serlie IW, Florie J, Nio CY, Glas AS, Post FH, Truyen R, Gerritsen FA, Stoker J. Three-dimensional display modes for CT colonography: conventional 3D virtual colonoscopy versus unfolded cube projection. Radiology. 2003;228:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Hock D, Ouhadi R, Materne R, Aouchria AS, Mancini I, Broussaud T, Magotteaux P, Nchimi A. Virtual dissection CT colonography: evaluation of learning curves and reading times with and without computer-aided detection. Radiology. 2008;248:860-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Pickhardt PJ, Taylor AJ, Gopal DV. Surface visualization at 3D endoluminal CT colonography: degree of coverage and implications for polyp detection. Gastroenterology. 2006;130:1582-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 67. | Taylor SA, Halligan S, Burling D, Roddie ME, Honeyfield L, McQuillan J, Amin H, Dehmeshki J. Computer-assisted reader software versus expert reviewers for polyp detection on CT colonography. AJR Am J Roentgenol. 2006;186:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Zalis ME, Barish MA, Choi JR, Dachman AH, Fenlon HM, Ferrucci JT, Glick SN, Laghi A, Macari M, McFarland EG. CT colonography reporting and data system: a consensus proposal. Radiology. 2005;236:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 422] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 69. | Burling D. CT colonography standards. Clin Radiol. 2010;65:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Fletcher JG, Chen MH, Herman BA, Johnson CD, Toledano A, Dachman AH, Hara AK, Fidler JL, Menias CO, Coakley KJ. Can radiologist training and testing ensure high performance in CT colonography Lessons From the National CT Colonography Trial. AJR Am J Roentgenol. 2010;195:117-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Haycock A, Burling D, Wylie P, Muckian J, Ilangovan R, Thomas-Gibson S. CT colonography training for radiographers--a formal evaluation. Clin Radiol. 2010;65:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1202] [Article Influence: 70.7] [Reference Citation Analysis (0)] |