Revised: August 30, 2012
Accepted: January 31, 2013
Published online: February 28, 2013
AIM: To investigate the frequency, typical and atypical locations and patterns of melanoma metastases identifiable by computed tomography (CT) in the abdomen and pelvis.
METHODS: We performed a retrospective review of index CT examinations of the abdomen and pelvis in patients with melanoma and recorded all findings suggestive of metastatic disease.
RESULTS: Metastases were present on 36% (181/508) of the index examinations and most commonly involved the liver (47%) and pelvic lymph nodes (27%). Lower extremity primaries had the highest rate of metastasis (52%). Ocular and head and neck melanomas have a predilection to metastasize to the liver (hepatic involvement in 70% and 63%, respectively, of patients with metastatic disease) and metastases from lower extremity primaries most commonly involve pelvic lymph nodes (54% of patients with metastatic disease). Metastases to atypical locations were present in 14% of patients and most commonly occurred in the subcutaneous tissue and spleen. Primary tumors of the lower extremity, back and head and neck were most commonly associated with atypical metastases. Pelvic metastases are more common with lower extremity primaries (accounting for 70% of cases with pelvic metastases) but 5% of patients with supraumbilical primaries also had pelvic metastases.
CONCLUSION: The distribution of metastatic melanoma in the abdomen and pelvis that we have defined should help guide the interpretation of CT exams in these patients.
- Citation: Trout AT, Rabinowitz RS, Platt JF, Elsayes KM. Melanoma metastases in the abdomen and pelvis: Frequency and patterns of spread. World J Radiol 2013; 5(2): 25-32
- URL: https://www.wjgnet.com/1949-8470/full/v5/i2/25.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i2.25
Melanoma is known for its ability to metastasize to any site in the body making evaluation for metastatic disease time consuming and fraught with potential missed diagnoses. To minimize the risk of missed findings, the interpreting radiologist must have a defined search pattern and should take advantage of the tools at their disposal including: window and level adjustments, comparison examinations and multiplanar reformatting. Additionally, knowledge of the common sites of metastatic disease and patterns of spread unique to primary tumors in specific locations can help guide the radiologist in their search. Series in the literature detailing the distribution of metastatic disease are primarily surgical and autopsy series with only small radiologic series published to date. While surgical and autopsy series represent a gold standard of sorts, these do not necessarily reflect what is visible to the radiologist. Metastases identified in surgical and pathologic series may be micrometastases or may be indistinguishable from background tissue by computed tomography (CT). Thus, there is value in reviewing the CT examinations of melanoma patients to identify typical distributions of radiologically visible disease.
Our goal was to define the common locations of abdominal and pelvic melanoma metastases that are visualized by CT and to determine if there are patterns in the distribution of metastases based upon the location of the primary tumor.
This study was performed in compliance with the health insurance portability and accountability act (United States). Institutional review board approval was obtained for the review of subjects’ medical records. Due to the retrospective nature of the investigation, patient informed consent was not required.
We performed a search of the Radiology Informatics System over the period of June 1995 - September 2010 for CT examinations with a clinical indication referring to melanoma (e.g., “melanoma - evaluate for metastases” or “melanoma - staging exam”). CTs performed for evaluation of metastatic melanoma at our institution typically employ intravenous and oral contrast media and are reconstructed at 5 mm axial intervals. Retrieved CT reports were reviewed to identify the first (index) examination for each patient and duplicate or follow up examinations were discarded. This review was limited to index examinations as the goal of this research was to assess the typical pattern of disease spread rather than the pattern of disease progression over time.
CT reports for the index examination for each patient were reviewed for findings which suggested the presence of metastatic disease. Findings considered consistent with evidence of metastatic disease included: solid organ implants, mesenteric implants, focal bowel wall thickening and/or nodularity, and enlarged (generally > 1 cm, > 1.5 cm in groin) lymph nodes. Sites of findings suspicious for metastatic disease were recorded for each patient. Using our institution’s electronic data repository, the medical records of the patients were reviewed to identify demographic information, the location of the primary tumor, and date of diagnosis.
Five hundred and eight patient exams were identified which met our inclusion criteria. Two hundred and eighty-two (55.5%) of the patients were male and 226 were female (44.5%). The mean age of the population was 55.1 ± 16 years (range: 18-89 years). The mean duration of time between diagnosis and the index examination was 811.8 ± 1248 d (median = 335.5 d). The back (n = 114) and lower extremity (n = 103) were the most common primary tumor locations in this population (22.4% and 20.3% respectively). Table 1 details the distribution of primary tumors in this population. Metastases were present on 181/508 (35.6%) of the index exams and involved the liver most commonly (n = 85, 16.7% of all cases, 47% of cases with metastases). Table 2 details the distribution of metastases in this population.
| Primary tumor location | Cases |
| Back | 114 (22.4) |
| Lower extremity | 103 (20.3) |
| Head/neck | 88 (17.3) |
| Ocular | 72 (14.2) |
| Anterior chest wall | 33 (6.5) |
| Upper extremity | 29 (5.7) |
| Pelvis | 15 (3.0) |
| Anorectal | 13 (2.6) |
| Sinonasal | 10 (2.0) |
| Anterior abdominal wall | 8 (1.6) |
| Labia/vagina | 4 (0.8) |
| Oral mucosa | 3 (0.6) |
| Enteric | 1 (0.2) |
| Unknown | 15 (3.0) |
| Location of metastasis | Number of cases | % of cases (n = 508) | % of cases with metastases (n = 181) |
| Liver | 85 | 16.7% | 47.0% |
| Pelvic nodes | 48 | 9.4% | 26.5% |
| Adrenal | 28 | 5.5% | 15.5% |
| Subcutaneous implants | 27 | 5.3% | 14.9% |
| Spleen | 22 | 4.3% | 12.2% |
| Retroperitoneal nodes | 20 | 3.9% | 11.0% |
| Intraperitoneal nodes | 18 | 3.5% | 9.9% |
| Mesenteric/peritoneal implants | 18 | 3.5% | 9.9% |
| Bone | 11 | 2.2% | 6.1% |
| Kidney | 10 | 2.0% | 5.5% |
| Small bowel | 10 | 2.0% | 5.5% |
| Retroperitoneal implants | 7 | 1.4% | 3.9% |
| Pancreas | 3 | 0.6% | 1.7% |
| Gastric | 2 | 0.4% | 1.1% |
| Large bowel | 1 | 0.2% | 0.6% |
| Ovary | 1 | 0.2% | 0.6% |
| Appendicular skeletal muscle | 1 | 0.2% | 0.6% |
| Diaphragm | 1 | 0.2% | 0.6% |
The distribution of metastases varies based on the location of the primary tumor. The rate of metastases by primary site and the most common location of metastases are detailed in Table 3. Briefly, metastases from lower extremity primaries most commonly involve pelvic (including inguinal) lymph nodes (53.7%) and metastases from ocular and head and neck melanoma have a strong predilection for involvement of the liver (70% and 62.5%, respectively).
| Location of primary tumor | Cases with metastases | Most common sites of metastatic disease | |||
| n | % | ||||
| Lower extremity | 54/103 | 52.4% | Pelvic lymph node 53.7% | Liver 29.6% | Retroperitoneal lymph node 20% |
| Back | 40/114 | 35.1% | Liver 45% | Adrenal 25% | Spleen 22.5% |
| Head/neck | 24/88 | 27.3% | Liver 62.5% | ||
| Ocular | 20/72 | 27.8% | Liver 70% | Abdominal lymph node 15% | |
| Anterior chest wall | 12/33 | 36.4% | Liver 41.7% | Adrenal 16.7% | Pelvic lymph node 16.7% |
| Upper extremity | 8/29 | 27.6% | 1 | 1 | 1 |
| Pelvis | 5/15 | 33.3% | 1 | 1 | 1 |
| Anorectal | 3/13 | 23.1% | 1 | 1 | 1 |
| Sinonasal | 3/10 | 30% | 1 | 1 | 1 |
| Anterior abdominal wall | 1/8 | 12.5% | 1 | 1 | 1 |
| Labia/vagina | 1/4 | 25% | 1 | 1 | 1 |
| Oral mucosa | 0/3 | 0% | 1 | 1 | 1 |
| Enteric | 0/1 | 0% | 1 | 1 | 1 |
| Unknown | 10/15 | 66.7% | 1 | 1 | 1 |
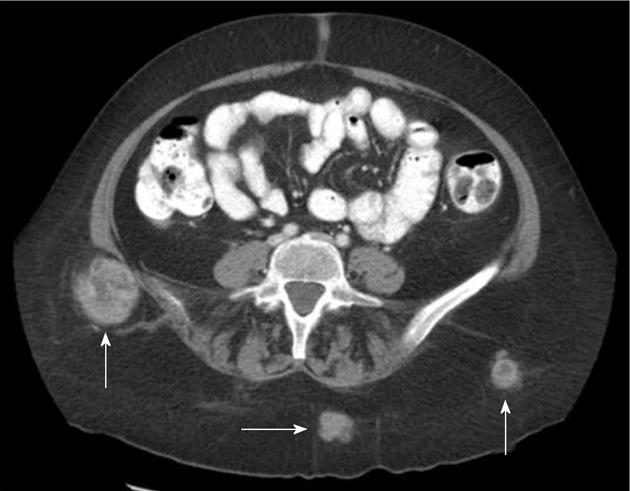
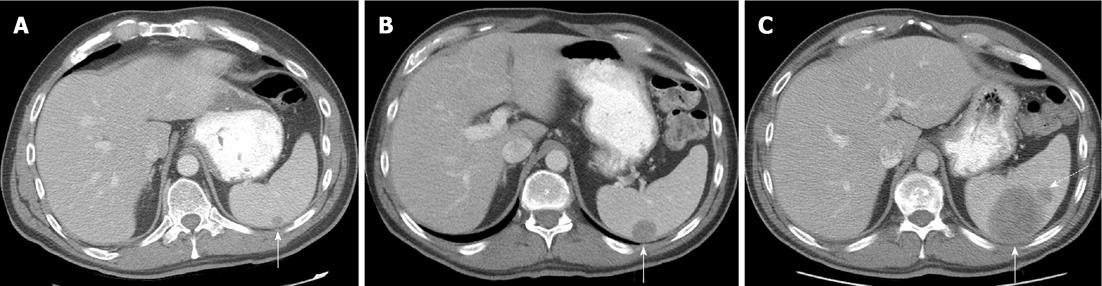
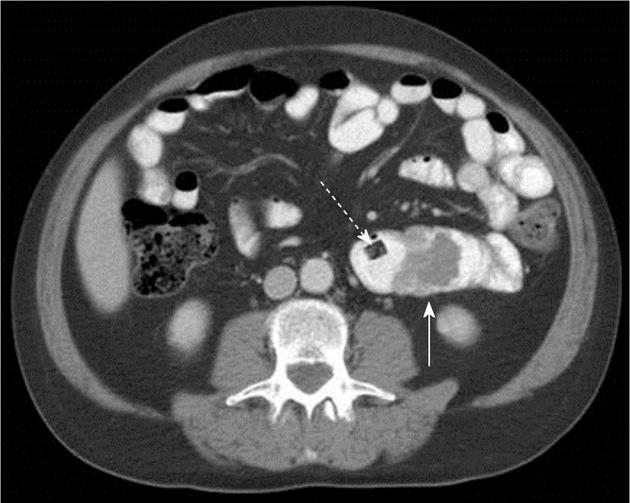
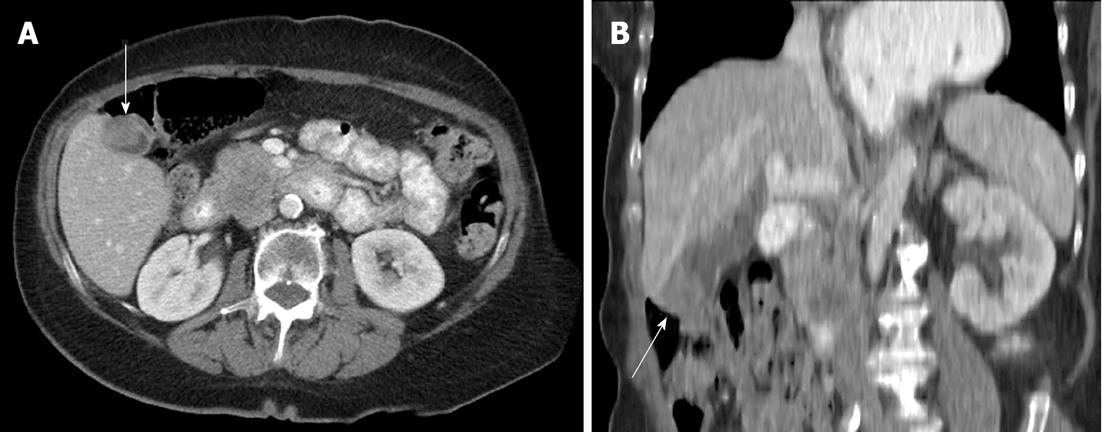
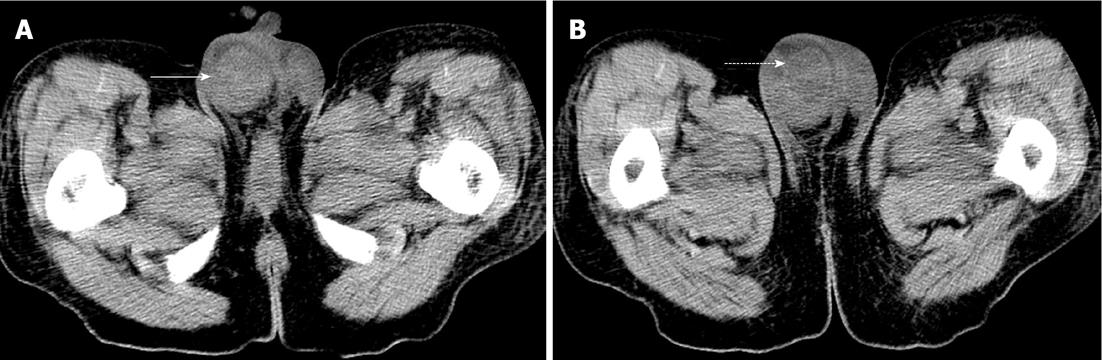
Atypical metastases (defined as those not involving the liver, adrenal gland, bones or lymph nodes - typical sites for metastases in the abdomen and pelvis) were present in 71 (39.2%) of the 181 patients with metastatic disease (14% of all patients) and 21/181 patients (11.6%) had metastases only to atypical locations. Subcutaneous implants (Figure 1) were the most common atypical metastases (n = 27/181, 14.9% of patients with metastatic disease) followed by splenic metastases (n = 22/181, 12.2% of patients with metastatic disease) (Figure 2). In patients with only atypical metastases, subcutaneous tissue (n = 9/21, 42.9%), spleen (n = 4/21, 19%) and small bowel (n = 3/21, 14.3%) (Figure 3) were the most common sites of involvement (Table 4). Primary tumors located in the lower extremity, back and head and neck accounted for the most cases of isolated atypical metastases (28.6%, 23.8% and 19.0%, respectively) (Table 5).
| Location of atypical metastases | |
| Subcutaneous implant | 9 (42.90) |
| Enteric | 5 (23.80) |
| Small bowel | 3 (14.30) |
| Large bowel | 1 (4.80) |
| Gastric | 1 (4.80) |
| Spleen | 4 (19.00) |
| Peritoneal implant | 1 (4.80) |
| Kidney | 1 (4.80) |
| Multiple sites (subcutaneous, mesenteric, retroperitoneal, diaphragm) | 1 (4.80) |
| Primary site | Cases with atypical metastases n (% of cases with metastases) | Cases with only atypical mets | |||
| n | % of cases of that primary | % of cases of that primary with metastases | % of cases with only atypical metastases | ||
| Head/neck | 14/24 (58.3) | 4 | 4.5% | 16.7% | 19.0% |
| Back | 20/40 (50) | 5 | 4.4% | 12.5% | 23.8% |
| Lower extremity | 15/54 (27.8) | 6 | 5.8% | 11.1% | 28.6% |
| Anterior chest wall | 5/12 (41.7) | 2 | 6.1% | 16.7% | 9.5% |
| Ocular | 5/20 (25) | 0 | 0% | 0% | 0% |
| Upper extremity | 4/8 (50) | 1 | 3.4% | 12.5% | 4.8% |
| Pelvis | 2/5 (40) | 1 | 6.7% | 20% | 4.8% |
| Anorectal | 1/3 (33.3) | 0 | 0% | 0% | 0% |
| Sinonasal | 1/3 (33.3) | 0 | 0% | 0% | 0% |
| Anterior abdominal wall | 0/1 (0) | 0 | 0% | 0% | 0% |
| Labia/vagina | 0/1 (0) | 0 | 0% | 0% | 0% |
| Oral mucosa | 0/0 (0) | 0 | 0% | 0% | 0% |
| Enteric | 0/0 (0) | 0 | 0% | 0% | 0% |
| Unknown | 4/10 (40) | 2 | 13.3% | 20% | 0% |
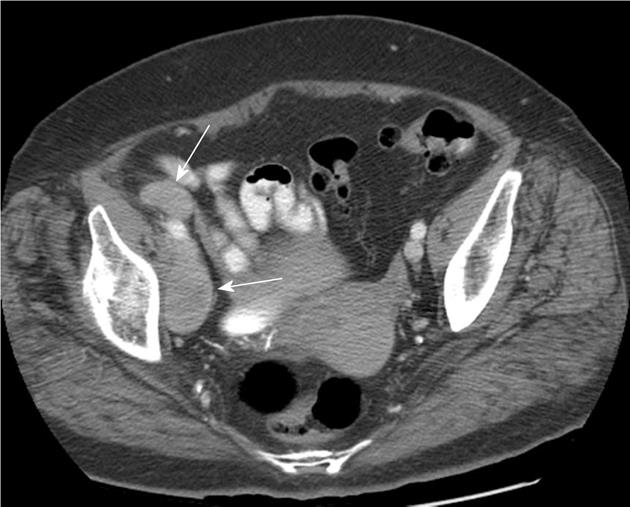
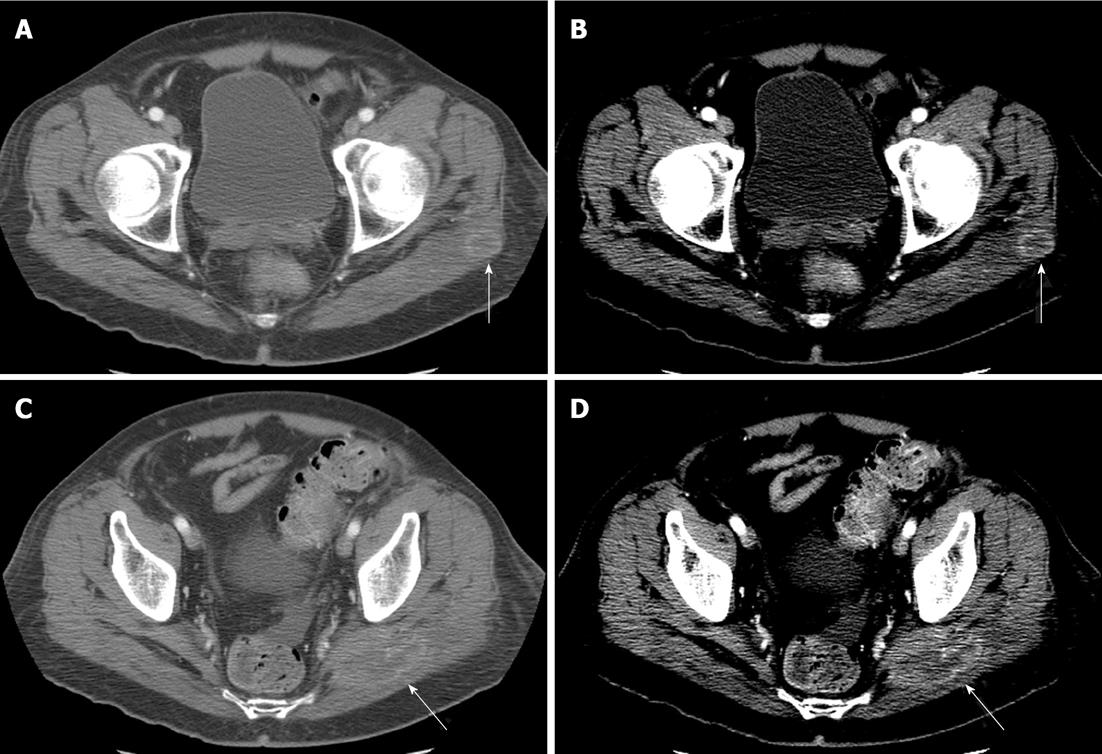
The pelvis was scanned in 79% (n = 405) of patients (abdomen only in the remaining 21%) with identification of findings suspicious for pelvic metastases (Figure 4) in 15.5% (n = 54/405). Patients with primary tumors of the pelvis and lower extremity (infraumbilical primaries) represented 70.4% (n = 38/54) of the patients with pelvic metastases. Of those patients with supraumbilical primary tumors, 14/270 (5.2%) had pelvic metastases.
A predilection for atypical metastases (non-liver, adrenal, bone, or lymph node) is one of the characteristics which separates melanoma from other primary malignancies and makes interpreting abdomen and pelvic CTs in this population difficult. It is impossible, however, to avoid these examinations as CT remains one of the imaging modalities of choice in the evaluation of abdominal and pelvic metastatic disease in melanoma patients[1-5]. Kostrubiak et al[6] showed that when properly employed, CT of the abdomen and pelvis changes the extent of disease in 53% of cases and alters therapy in 28% of cases.
The goal of this study was to build upon data in the literature detailing the distribution of metastases in the melanoma patient with emphasis on those metastases which can be identified by CT. Clinical and pathologic series have identified the following locations as the most common sites for melanoma metastases[7]:
Clinical: Lymph nodes (42%-59%), liver (14%-20%), bone (11%-17%), GI (1%-7%).
Pathologic/Autopsy: Liver (54%-77%), lymph nodes (50%-75%), pancreas (38%-53%), adrenal (36%-54%), kidney (35%-48%), GI (26%-58%), bone (23%-49%).
In our population, metastases were identified in approximately 36% of patients, a rate that is mildly higher than that previously reported by Johnson et al[8] (27% in their series) but lower than the 60% rate of metastases reported by Shirkhoda et al[9]. Both our series and the series by Johnson et al[8] reviewed only the first exam after diagnosis while Shirkhoda et al[9] reviewed serial examinations in patients over time, possibly accounting for the higher observed rate of metastasis.
Our series identifies liver (16.7%) and pelvic lymph nodes (9.4%) as the most common location of metastases identifiable by CT. While the actual rate of metastatic deposits is lower in our series than in the clinical or pathologic series described above, the more common sites mirror those identified in both series.
Comparisons between series describing the distribution of metastases in a population are difficult due to population differences. Autopsy series represent the most advanced disease - generally patients who have succumbed to their disease and thus likely have the most extensive disease burden. Clinical series may be based on populations with symptomatic disease; e.g., palpable lymph nodes, pain, GI upset or bleeding. Metastases so small as to not be palpable and those in locations which do not produce identifiable symptoms may not be catalogued in these series. Consequently, differences in the distribution and frequency of metastatic foci between our population and those previously described is not surprising. When directly compared with one of the larger radiologic series, the higher overall rate of metastases in our population relative to that described by Johnson et al[8] may relate to the fact that the study by Johnson et al[8] represented routine screening exams within 2 mo of diagnosis. Our population includes patients who are years from their initial diagnosis and many patients with clinical findings suspicious for metastatic disease - a population with a higher pre-test probability of having metastatic disease.
Among patients with metastatic disease in our population, there is variability in the frequency and distribution of metastases which depends on the location of the primary tumor. Knowledge of such differences/predilections can help to focus the abdominal imager’s search pattern in these cases. In our population, the site of primary melanoma which was associated with the highest rate of metastatic disease (52.4%) was the lower extremity. While this was the most common primary tumor to metastasize, metastases were generally confined to pelvic lymph nodes. Primary tumors of the anterior chest wall and back had the highest rate of intraabdominal metastases (36.4% and 35.1%, respectively). This is somewhat different from data in the literature which identifies head and neck and ocular melanoma as the primary tumor locations with the highest rates of intraabdominal metastases[9,10]. In our population, head and neck and ocular melanomas did have high rates of intraabdominal metastases (27.3% and 27.8%, respectively) but these were lower than those for anterior chest wall and back primaries. Ocular and head and neck primaries are unique, however, in their high rates of metastases to the liver (70% and 62.5%, respectively) despite their distance from the abdomen.
Previous authors have also emphasized the significance of the location of the primary tumor in terms of frequency and location of metastases[11]. In a series of 127 patients, Johnson et al[8] observed that pelvic metastases only occurred in patients with lower extremity primaries and concluded that CT of the pelvis was not indicated unless the primary tumor was located in the lower extremity. While our data demonstrate that most (70.4%) pelvic metastases occur in patients with lower extremity primaries, 25.9% of pelvic metastases occur in patients that have a supraumbilical primary and 5.2% (n = 14/270) of patients with a supraumbilical primary have pelvic metastases. Based on our data it is clear that in patients with lower extremity primary melanomas, scanning the pelvis is essential and the radiologist should pay particular attention to pelvic lymph nodes as possible sites of metastases in these patients. However, our data do not support scanning only the abdomen in patients with primary tumors outside of the lower extremity as a very real percentage of these patients had metastases to the pelvis.
Metastases to atypical locations are those which could be easiest to miss, particularly if the radiologist’s search pattern is limited to the typical solid organs (liver, adrenal) which are involved by metastatic disease from other primary tumors. Melanoma is a tumor which is known to widely metastasize, involving organs which are not commonly involved by other tumors[12-14]. For example, melanoma accounts for more than 50% of metastases to the gallbladder (Figure 5) and is the second most common metastasis to the spleen and the third most common to the testicle (Figure 6)[15-19]. In our population, 39% of patients with metastatic disease had metastases to atypical locations and 11.6% of patients had metastases only in atypical locations. There are, however, some locations in which primary melanoma appears to be associated with a higher rate of atypical metastases. These include head and neck, back and lower extremity primaries which have a 58.3%, 50% and 27.8% rate of atypical metastases respectively. These are the primary tumors which are also associated with the highest overall rate of metastases but are in a somewhat different order of frequency (overall highest rate of metastases: lower extremity > back > head and neck). Knowing that tumors in these locations are associated with higher rates of atypical metastases should prompt the radiologist to be particularly vigilant in these cases. In the hunt for atypical metastases, it pays to focus on the subcutaneous tissues, the bowel and spleen (Figures 1-3) as these are the most common sites for atypical metastases.
In order to best approach CTs for metastatic melanoma, it pays to understand the patterns of metastatic disease distribution in these patients. Data in the literature have described the pathologic and surgical distribution of metastases but there is little data concerning the distribution of disease by CT. Our data reveal the following patterns: (1) The liver is the most common site of metastases in the abdomen and pelvis; (2) The distribution of metastases varies by the location of the primary tumor. Lower extremity primaries tend to metastasize to the pelvis and ocular and head and neck melanoma have a particular predilection to metastasize to the liver; (3) Metastases to atypical locations are common (39%) with a high rate of isolated atypical metastases (11.6%). Lower extremity, back and head and neck primary tumors have the highest rate of atypical metastases and subcutaneous deposits, bowel and splenic metastases are the most common atypical metastases; and (4) Pelvic metastases are uncommon except in the setting of an infraumbilical primary tumor.
A defined search pattern is important in these complex cases and knowledge of these facts can help to guide imaging protocols and shape the radiologist’s search pattern. For example, a large display field of view should be used to include all of the subcutaneous tissue as this is the most common site of atypical metastases. Liberal use of windowing and leveling is also important. In addition to routine windowing, the entire data set should be reviewed in narrow windows as this can help to identify musculoskeletal metastases by accentuating subtle differences in attenuation (Figure 7).
While this study represents one of the largest radiologic series evaluating the distribution of melanoma metastases, this study has several limitations including the retrospective design and the selection bias incurred by using our Radiology Informatics System to identify cases. By relying on the existing CT reports, we are likely underestimating the true extent of atypical disease as this is the most difficult to identify and may have been overlooked by the interpreting radiologist. Moreover, although we limited results to the index CT (first scan after diagnosis) for each patient, the time between diagnosis and CT was highly variable. This is not a limitation that is unique to this study but this factor would certainly be expected to impact the visible distribution of disease. A lack of pathologic confirmation of the distribution of disease also limits these data. While CT is an excellent screening tool (high negative predictive value), there are data which demonstrate that CT examinations to evaluate for metastatic disease result in a fair number of false positives and thus pathologic confirmation is important prior to altering therapy[8,20,21]. Unfortunately, such confirmation would be difficult to obtain as all metastases and identified lesions are not biopsied. Moreover, the goal of this study is not to replace biopsy or autopsy series but to detail the distribution of lesions as identified by CT.
Interpretation of CT examinations of the abdomen and pelvis for melanoma metastases requires attention and a defined search pattern. This search pattern can be guided by knowledge of the common distribution of disease which depends both on the location of the primary tumor and on its pattern of spread. By CT, liver and pelvic lymph nodes are the most common locations of metastatic disease and attention should be paid to these areas. However, given the propensity of melanoma to metastasize to uncommon locations, the radiologist must remain vigilant to avoid subtle metastases and use all of the tools available to them.
Melanoma is known for its ability to metastasize to any site in the body making evaluation for metastatic disease time consuming and fraught with potential missed diagnoses. Series in the literature detailing the distribution of metastatic disease are primarily surgical and autopsy series with only small radiologic series published to date.
The data demonstrate several patterns of metastatic spread of melanoma in the abdomen and pelvis: Liver is the most common site of metastatic disease but metastases to atypical locations are common, most frequently involving the subcutaneous tissue, bowel and spleen. The location of the primary tumor influences the spread of metastatic disease - lower extremity primaries tend to metastasize to the pelvis and ocular and head and neck melanoma tend to metastasize to the liver and lower extremity, back and head and neck primary tumors have the highest rate of atypical metastases.
This study represents one of the largest radiologic series evaluating the distribution of melanoma metastases in the abdomen and pelvis and further defines the distribution of abdominal and pelvic metastases of melanoma.
Interpretation of computed tomography (CT) examinations of the abdomen and pelvis for melanoma metastases requires attention and a defined search pattern which can be guided by knowledge of the common distribution of disease which depends both on the location of the primary tumor and on its pattern of spread as detailed in this manuscript. In general, liver and pelvic lymph nodes are the most common locations of metastatic disease identifiable by CT and particular attention should be paid to these areas.
CT is one of the imaging modalities of choice for assessment of metastatic disease in patients with melanoma. Evaluation/imaging of both the abdomen and pelvis is frequently performed with some authors recommending against routine imaging of the pelvis except in the setting of a primary tumor of the lower extremity.
The authors reported the patterns of melanoma metastases to abdomen and pelvis identified by the first CT scan after diagnosis. The idea is original. The method is valid. It is well written.
P- Reviewers Wang WB, Minami Y S- Editor Cheng JX L- Editor A E- Editor Xiong L
| 1. | Doiron MJ, Bernardino ME. A comparison of noninvasive imaging modalities in the melanoma patient. Cancer. 1981;47:2581-2584. [PubMed] |
| 2. | Huang CL, Provost N, Marghoob AA, Kopf AW, Levin L, Bart RS. Laboratory tests and imaging studies in patients with cutaneous malignant melanoma. J Am Acad Dermatol. 1998;39:451-463. [PubMed] |
| 3. | Patnana M, Bronstein Y, Szklaruk J, Bedi DG, Hwu WJ, Gershenwald JE, Prieto VG, Ng CS. Multimethod imaging, staging, and spectrum of manifestations of metastatic melanoma. Clin Radiol. 2011;66:224-236. [PubMed] |
| 4. | Forschner A, Eigentler TK, Pflugfelder A, Leiter U, Weide B, Held L, Meier F, Garbe C. Melanoma staging: facts and controversies. Clin Dermatol. 2010;28:275-280. [PubMed] |
| 5. | Ho Shon IA, Chung DK, Saw RP, Thompson JF. Imaging in cutaneous melanoma. Nucl Med Commun. 2008;29:847-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 6. | Kostrubiak I, Whitley NO, Aisner J, Goose P, DeLuca RR, Didolkar MS, Elias EG. The use of computed body tomography in malignant melanoma. JAMA. 1988;259:2896-2897. [PubMed] |
| 7. | Provost N, Marghoob AA, Kopf AW, DeDavid M, Wasti Q, Bart RS. Laboratory tests and imaging studies in patients with cutaneous malignant melanomas: a survey of experienced physicians. J Am Acad Dermatol. 1997;36:711-720. [PubMed] |
| 8. | Johnson TM, Fader DJ, Chang AE, Yahanda A, Smith JW, Hamlet KR, Sondak VK. Computed tomography in staging of patients with melanoma metastatic to the regional nodes. Ann Surg Oncol. 1997;4:396-402. [PubMed] |
| 9. | Shirkhoda A, Albin J. Malignant melanoma: correlating abdominal and pelvic CT with clinical staging. Radiology. 1987;165:75-78. [PubMed] |
| 10. | Gutman H, Hess KR, Kokotsakis JA, Ross MI, Guinee VF, Balch CM. Surgery for abdominal metastases of cutaneous melanoma. World J Surg. 2001;25:750-758. [PubMed] |
| 11. | Alvarado GC, Papadopoulos NE, Hwu WJ, Bedikian AY, Homsi J, Myers JN, Bronstein Y, Bassett RL, Hwu P, Kim KB. Pelvic computed tomography scans for surveillance in patients with primary melanoma in the head and neck. Melanoma Res. 2011;21:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Alvarez FA, Nicolás M, Goransky J, Vaccaro CA, Beskow A, Cavadas D. Ileocolic intussusception due to intestinal metastatic melanoma. Case report and review of the literature. Int J Surg Case Rep. 2011;2:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Surov A, Hainz M, Holzhausen HJ, Arnold D, Katzer M, Schmidt J, Spielmann RP, Behrmann C. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Bender GN, Maglinte DD, McLarney JH, Rex D, Kelvin FM. Malignant melanoma: patterns of metastasis to the small bowel, reliability of imaging studies, and clinical relevance. Am J Gastroenterol. 2001;96:2392-2400. [PubMed] |
| 15. | García-González R, Pinto J, Val-Bernal JF. Testicular metastases from solid tumors: an autopsy study. Ann Diagn Pathol. 2000;4:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Haupt HM, Mann RB, Trump DL, Abeloff MD. Metastatic carcinoma involving the testis. Clinical and pathologic distinction from primary testicular neoplasms. Cancer. 1984;54:709-714. [PubMed] |
| 17. | Guida M, Cramarossa A, Gentile A, Benvestito S, De Fazio M, Sanbiasi D, Crucitta E, De Lena M. Metastatic malignant melanoma of the gallbladder: a case report and review of the literature. Melanoma Res. 2002;12:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Crippa S, Bovo G, Romano F, Mussi C, Uggeri F. Melanoma metastatic to the gallbladder and small bowel: report of a case and review of the literature. Melanoma Res. 2004;14:427-430. [PubMed] |
| 19. | Kamel IR, Kruskal JB, Gramm HF. Imaging of abdominal manifestations of melanoma. Crit Rev Diagn Imaging. 1998;39:447-486. [PubMed] |
| 20. | Kuvshinoff BW, Kurtz C, Coit DG. Computed tomography in evaluation of patients with stage III melanoma. Ann Surg Oncol. 1997;4:252-258. [PubMed] |
| 21. | Buzaid AC, Tinoco L, Ross MI, Legha SS, Benjamin RS. Role of computed tomography in the staging of patients with local-regional metastases of melanoma. J Clin Oncol. 1995;13:2104-2108. [PubMed] |