Published online Nov 28, 2013. doi: 10.4329/wjr.v5.i11.436
Revised: August 19, 2013
Accepted: August 28, 2013
Published online: November 28, 2013
Processing time: 151 Days and 8.2 Hours
The purpose of this pictorial essay is to review the common and uncommon sites of renal cell carcinoma recurrence throughout the body by examining their appearances on computerized tomography (CT). CT imaging protocols will be discussed. The sites of recurrence have been categorized into 4 groups: chest and mediastinum, abdomen and pelvis, musculoskeletal, and neurological. For each site of recurrence, a representative CT image correlate with discussion is provided. The unique CT appearance of renal cell carcinoma recurrence and how it can be used in lesion detection will be discussed. Renal cell carcinoma recurrences are hypervascular like the primary tumor, which can aid in not only lesion detection but also in some cases, differentiation from other primary tumors. Through CT case review of various sites of recurrence, lesions are shown to be easily seen on arterial phase while sometimes being nearly inconspicuous on venous or delayed phases. Coronal and sagittal reconstructions can also improve diagnostic sensitivity. CT is the most commonly used imaging tool for surveillance of renal cell carcinoma recurrence after nephrectomy. Knowledge of sites of recurrence as well as the utility of arterial phase imaging and multiplanar reconstructions will aid in optimizing detection of disease recurrence.
Core tip: The computerized tomography (CT) imaging appearance of renal cell carcinoma recurrence mimics the appearance of the primary tumor. Just as a majority of renal cell carcinomas are hypervascular, recurrences tend to be hypervascular and are best seen on arterial phase. This paper demonstrates this tendency by giving case examples which review both common and uncommon sites of recurrence. Knowledge of their appearances on CT will help the radiologist in lesion detection and diagnosis, limiting delays in treatment and avoiding unnecessary biopsies.
- Citation: Coquia SF, Johnson PT, Ahmed S, Fishman EK. MDCT imaging following nephrectomy for renal cell carcinoma: Protocol optimization and patterns of tumor recurrence. World J Radiol 2013; 5(11): 436-445
- URL: https://www.wjgnet.com/1949-8470/full/v5/i11/436.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i11.436
According to the National Cancer Institute, over 60,000 new cases of kidney cancer are diagnosed annually.[1] Management of such cases will depend on the tumor stage as well as the patient’s functional status. Treatment options available include nephrectomy, partial nephrectomy, cryoablation or radiofrequency ablation, or active surveillance. While nephrectomy has been the traditional management of renal cell carcinomas, nephron sparing procedures are increasingly becoming more common.
After initial treatment, patients will continue to be monitored with imaging in order to detect recurrent or metastatic disease. Following nephrectomy, approximately 20%-40% of patients will develop recurrence. Most will develop recurrence within 2-4 years of nephrectomy[1-3]. One characteristic of renal cell carcinoma is the development of metastases many years after surgical treatment. According to one recent study, 6.4% of patients developed metastases 10 years after their initial surgery[1]. The rate of recurrence and the time to recurrence after nephrectomy is dependent on the stage of the tumor. The lower the stage of the tumor, the lower the rate of recurrence and the longer the time between initial treatment and recurrence[1,4].
The timing of the follow-up as well as the imaging included in the follow-up can vary by institution and specialty organization recommendations. In addition, the computerized tomography (CT) protocols used for surveillance imaging can vary across institutions. As renal cell carcinoma metastases typically follow the vascularity of the primary tumor and most renal cell carcinomas are hypervascular, arterial phase imaging is critical in lesion detection. One recent study reviewed the utility of the dual phase abdominal CT in the detection of renal cell carcinoma metastases. About 75% of the lesions were visualized in the arterial phase and in 9% of patients lesions were only visible in the arterial phase. These lesions were located in the liver, pancreas, and kidney[5]. In this paper, we will demonstrate the increased conspicuity of recurrent and metastatic lesions on arterial phase as compared to venous and delayed phases. We will also show how post processing techniques such as coronal and sagittal multiplanar reconstructions and 3D rendering can also aid in lesion detection. The importance of arterial phase imaging and the post processing techniques will be demonstrated through review of common and uncommon locations of recurrence. All of the images used in this paper were obtained from our institution.
At our institution, our CT surveillance protocols for patients with one remaining kidney are similar to the protocols used for the detection, characterization, and staging of renal masses, which was published previously by Sheth et al[6]. The protocol includes a multiphase scan after the injection of iodinated contrast (320 mg/mL iso-osmolar contrast with volume according to patient’s renal function, between 70-120 cc). With increasing contrast injection rates (now 5 cc/s) and faster scanners, the time delay between onset of contrast administration and imaging of arterial and venous phase has decreased. Arterial (corticomedullary) phase imaging of the abdomen and pelvis is obtained at 25-30 s, previously published at 30-40 s[6]. Venous (nephrographic) phase imaging is obtained of the kidney at 60 s, previously published at 80 s[6]. Delayed phase imaging of the abdomen and pelvis is obtained at approximately 5 min. Axial images are reconstructed in 3 mm thick slices every 3 mm. Sagittal and coronal multiplanar reconstructions are generated at the scanner. Interactive 2D and 3D rendering with volume rendering and maximum intensity projection (MIP) techniques is performed at a separate workstation by an experienced radiologist.
In this pictorial essay, we will demonstrate numerous sites of recurrent and metastatic disease. The sites of disease will be organized into the following anatomic categories: chest and mediastinum, abdomen and pelvis, musculoskeletal, and neurological (Table 1). Table 2 lists the incidence of recurrence after nephrectomy for the most common locations of recurrence.
| Anatomic Category | Location |
| Chest and Mediastinum | Lung |
| Lymph Nodes | |
| Thyroid | |
| Abdomen and Pelvis | Liver |
| Nephrectomy Site | |
| Contralateral Kidney | |
| Adrenal Gland | |
| Pancreas | |
| Spleen | |
| Stomach | |
| Intestine | |
| Ovary | |
| Musculoskeletal | Bone |
| Muscle | |
| Breast | |
| Neurological | Brain |
| Spine |
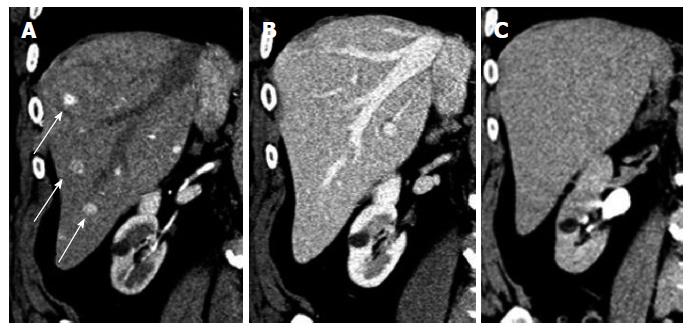
Lung: The lung is the most common site of recurrence and often the earliest site of recurrence. Chae et al[2] reported in their study of post-operative recurrence after nephrectomy that the lung was the first site of recurrence in 56% of patients. The appearance of the metastases may be well defined, rounded nodules of varying size or be irregular in shape. They may be singular or multiple. On soft tissue windows, these metastases may enhance as brightly as the pulmonary vasculature (Figure 1). If they are large enough, they may show central necrosis.
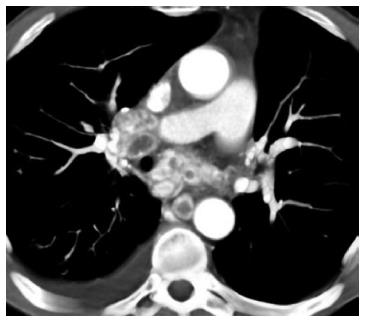
Lymph nodes: Lymph node recurrence rates have been reported in up to 63% of cases[2], and can be found in both the chest and abdomen/pelvis. The nodes are usually enlarged, enhance greater than expected for reactive lymph nodes, and often show central necrosis (Figure 2). Thoracic lymph node involvement can include the hilar lymph nodes, subcarinal lymph nodes, and paratracheal lymph nodes. If extensive, the mass effect from the lymph nodes can cause compromise of both the airways and the pulmonary vasculature such that it can be life threatening. Supraclavicular lymph node involvement can also be seen.
Thyroid: More than 150 cases of metastatic renal cell carcinoma to the thyroid (Figure 3) have been reported in the literature[7]. Like other sites of metastases, lesions can occur more than 20 years after nephrectomy. The thyroid’s rich blood supply may predispose it to metastatic lesions[7]. Patients may present with cough, as well as breathing and swallowing difficulties due to mass effect from the lesion. It is important to distinguish metastatic lesions from primary thyroid malignancies as they are treated differently. Fine needle aspiration of the nodule can be performed.
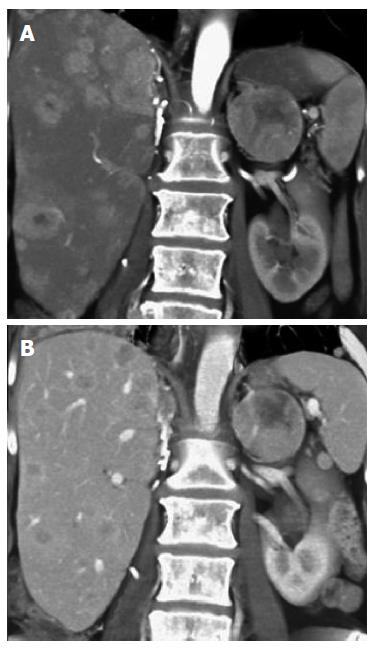
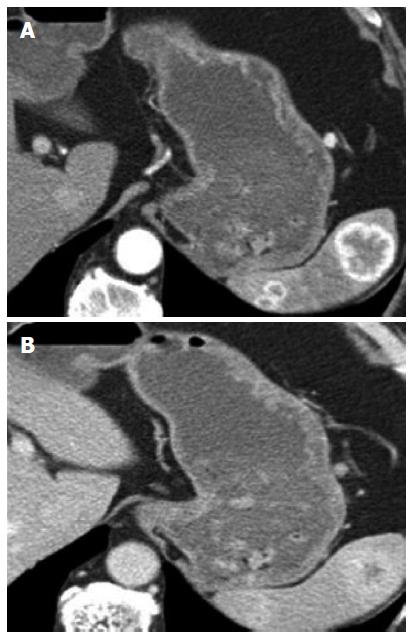
Liver: The incidence of liver metastasis after nephrectomy has been reported between 7%-23%[3]. Due to their predominately hypervascular nature, the lesions are best seen on arterial phase (Figures 4 and 5), washing out on venous and delayed acquisitions. The lesions may be inconspicuous on non-contrast, venous, or delayed phase images.
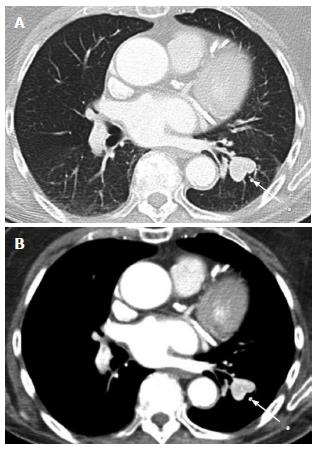
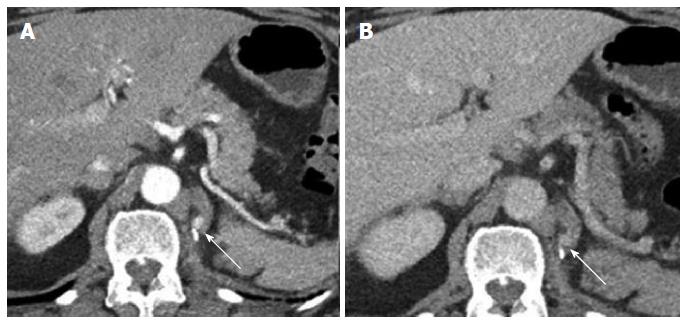
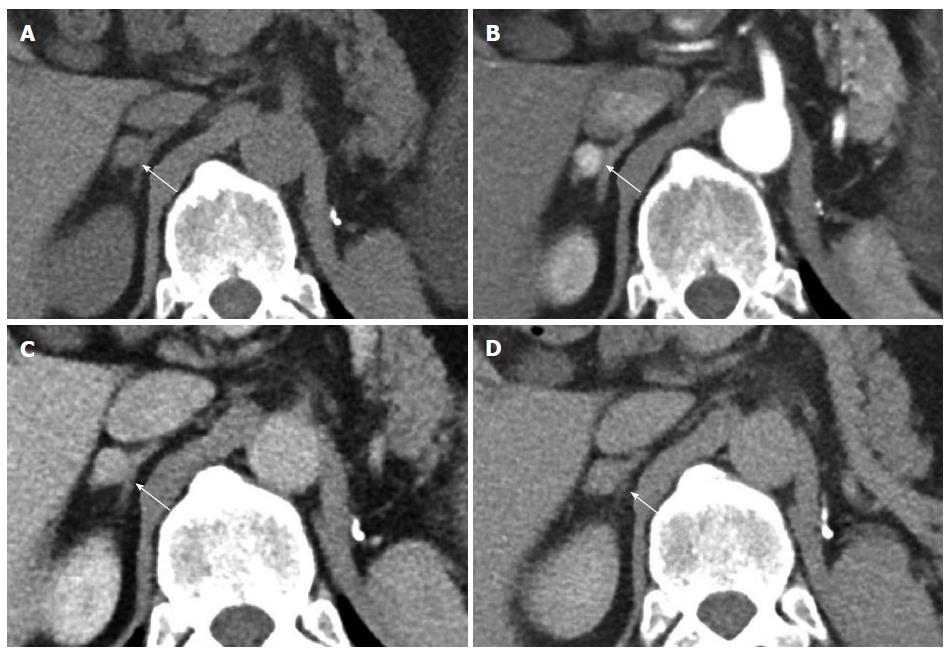
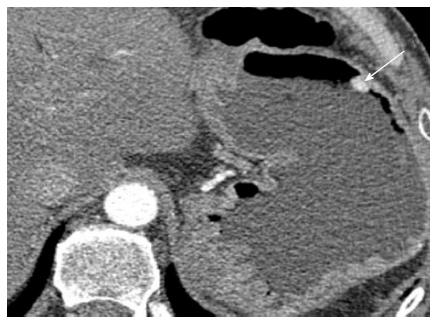
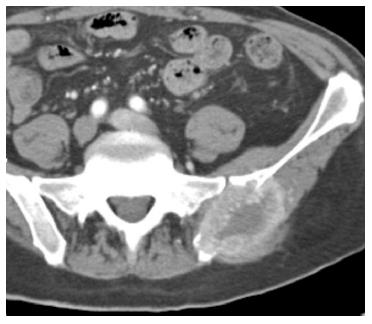
Nephrectomy site: The incidence of local recurrence (Figures 6 and 7) has been reported between 9%-10%[2,8]. The recurrence may also invade the IVC or the adjacent psoas (Figure 7) musculature. Scrutinization of the surgical bed and renal fossa is important in the detection of early recurrence. This may be challenging due to the presence of small bowel or pancreatic tail occupying the surgical bed. Evaluation for hypervascular lesions on arterial phase may help to distinguish tumor from post-surgical changes. Even subcentimeter hypervascular lesions (Figure 6) may be seen on arterial phase.
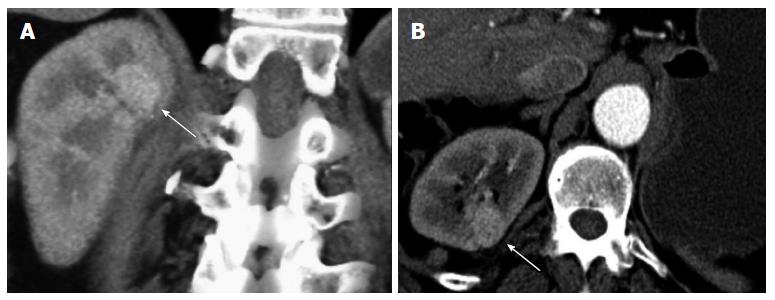
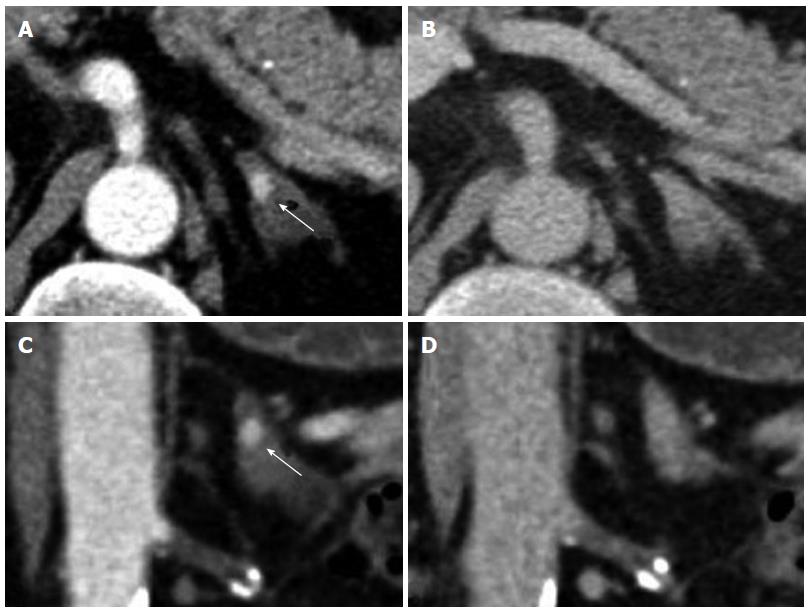
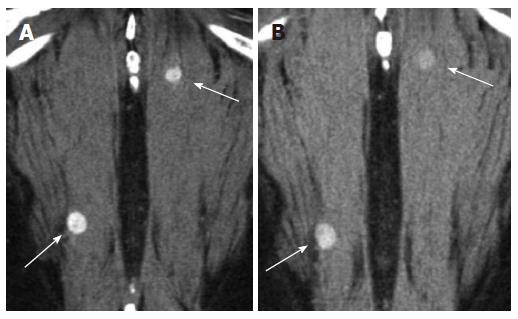
Contralateral kidney: In a study by Bani-Hani AH et al[9]. contralateral recurrence (Figure 8) occurred in 1.2% of 2352 patients undergoing surveillance after nephrectomy. The mean time to recurrence was 5.2 years. Positive surgical margins and multifocality of the tumor within the kidney were risk factors for developing contralateral recurrence. Coronal reconstructions may be helpful in lesion detection, especially for those recurrences in the upper and lower poles that may be not as obvious on axial imaging.
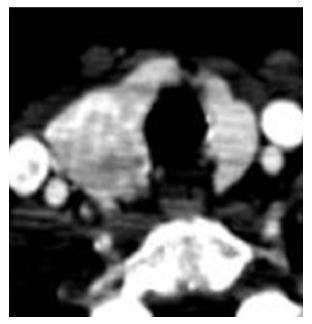
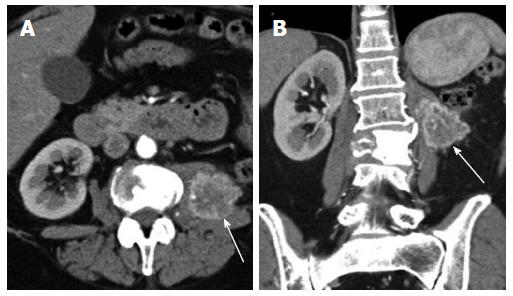
Adrenal gland: After nephrectomy, the incidence of adrenal metastasis (Figure 9) has been reported as high as 10%[3]. A study by Weight et al[10]. regarding the utility of ipsilateral adrenalectomy at the time of nephrectomy reported an incidence of 3.7% for asynchronous adrenal metastasis after surgery. A normal adrenal gland on CT has a 100% negative predictive value for tumor involvement[3]. As with local recurrence, adrenal metastases can be quite small and may only be seen on arterial phase (Figure 10). Owing to their vascularity, they can mimic adrenal adenomas in showing washout. A recent article by Choi evaluated absolute percentage washout (APW) and relative percentage washout (RPW) of known adrenal lesions in patients with RCC and found them to be similar to lipid poor adenomas. Using an APW threshold of 60% and an RPW threshold of 40%, 89% and 95% of known metastases were classified as adenomas[11]. However, the hypervascular nature of the lesion (arterial phase enhancement > 100 HU) is not characteristic of an adenoma. In an investigation comparing the dual phase CT enhancement patterns of adrenal adenoma and pheochromocytoma, arterial phase enhancement > 100 HU was only identified with pheochromocytoma[12], which enhances similar to metastatic hypervascular renal cell carcinoma. As an arterial phase acquisition is a routine part of the renal cell carcinoma CT protocol, review of the arterial phase attenuation should prove sufficient to distinguish a hypervascular renal cell metastasis from an adrenal adenoma.
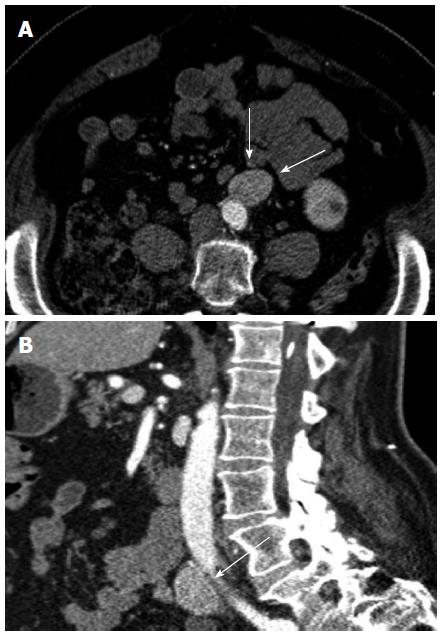
Lymph nodes: In the abdomen and pelvis, lymph node recurrence is most often in the retroperitoneum (Figure 11), up to 33% of recurrences[3]. Many will be enlarged and show conspicuous enhancement. It is difficult by CT to determine metastatic involvement to normal sized nodes; however, the incidence of microscopic lymph node invasion has been reported as low as 5%[3]. Mesenteric lymph nodes can also be involved.
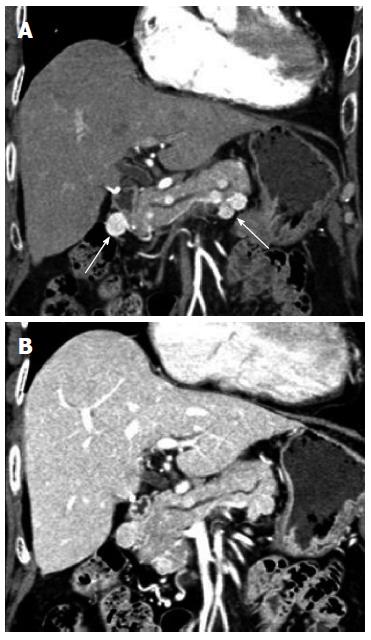
Pancreas: Metastatic lesions to the pancreas (Figure 12) are rare, but renal cell carcinoma’s predisposition for unusual sites of metastases also includes the pancreas. In an autopsy study of patients with renal cell carcinoma, less than 2% had metastasis to the pancreas. Nonetheless, renal cell carcinoma is the most common tumor to metastasize to pancreas[13]. Late recurrences more than 10 years after initial surgery have been reported[14]. Due to the hypervascular nature of the metastases, these lesions could be confused with pancreatic neuroendocrine tumors which are also typically vascular. Multiplicity of the pancreatic lesions, evidence of additional sites of recurrent or metastatic disease elsewhere in the body, and even biopsy may be useful in distinguish the two entities. If there is limited involvement within the pancreas, patients may go on to surgical resection, such as undergoing a Whipple procedure for lesions in the pancreatic head.
Spleen: While other cancers are more commonly known to metastasize to the spleen such as lung, melanoma, and breast cancer, renal cell carcinoma rarely spreads to the spleen (Figure 13). A review of the literature of splenic metastases from renal cell carcinoma by Moir et al[15]. produced 8 such cases. Despite this low number, autopsy studies of patients with renal cell carcinoma have reported an incidence of splenic metastases of 4.6%[15].
Stomach: Patients with metastases to the stomach (Figure 14) usually have other sites of metastases within the body. Patients may present with gastrointestinal bleeding, epigastric pain, or dyspepsia. Pollheimer et al[16]. reported a 0.2% incidence rate of metastases over a 22 year period. The study found that a gastric mass in a patient with a history of renal cell carcinoma is twice as likely to be a primary gastric tumor rather than a metastasis[16]. If this is the patient’s only site of disease, the patient may be treated with partial gastrectomy.
Intestine: Bianchi reported that large intestine metastases contributed 1.3% to the total number of metastases in renal cell carcinoma patients, and small intestine metastases (Figure 15) contributed 1.1%[17]. Patients may present with abdominal pain, gastrointestinal bleeding, or intussuception. Most are treated surgically followed by chemotherapy.
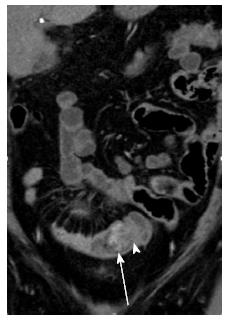
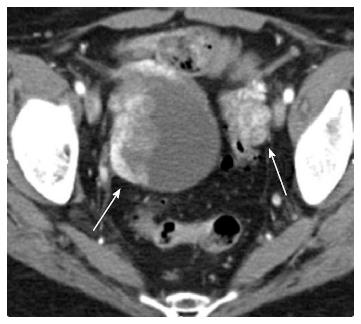
Ovary: Ovarian metastases are thought to occur through hematogenous spread via the renal vein to the ovarian veins, with a higher predilection for left sided renal tumors due to the retrograde flow via the gonadal vein. Autopsy studies have reported ovarian metastases (Figure 16) in 0.5% of patients with renal cell cancer. Only 21 cases have been reported in the literature[7].
Bone: The second most common site of recurrence is in the skeletal system. Patients are not screened for bone metastases unless they are symptomatic or have metabolic abnormalities suggestive of bone metastases, therefore such metastases may be found incidentally on surveillance CTs of the chest, abdomen, and pelvis. In the study by Chae et al[2], the bone was the initial site of recurrence in 29% of patients[2]. The incidence of bone metastases has been reported as high as 45% in T3 patients[4]. The lesions are typically lytic and can have an associated soft tissue component that enhances similar to the primary tumor (Figure 17).
Muscle: In a review of the literature, only a total of 35 case reports of muscle involvement (Figure 18) have been published. One of the cases reported involved multiple muscle metastases in a patient who had undergone radical nephrectomy 19 years prior[7]. Intramuscular metastases may be small and only well visualized during the arterial phase. Coronal and sagittal reconstructions may be helpful as they may emphasize the distortion of the muscle plane by the tumor.
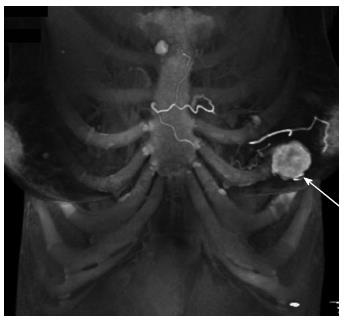
Breast: Metastases to the breast from extramammary malignancies are rare. The incidence of metastases in autopsy studies range from 5%-6%, and of those cases, 3% are attributable to a renal tumor. The most common malignancies to metastasize to the breast include melanoma, lymphoma, and lung cancer[18]. A review of the literature by Ganapathi et al[19] in 2008 reported 15 case reports of renal cell carcinoma metastases to the breast (Figure 19). The hypervascularity of the metastasis helps to distinguish it from a primary breast carcinoma and may help to avoid unnecessary biopsy.
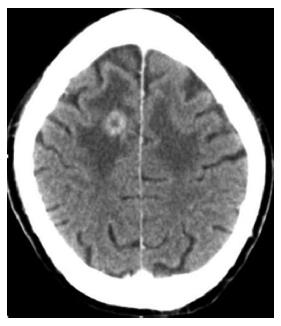
Brain: Brain metastases (Figure 20) usually occur in later stages of disease and their incidence has been reported between 2%-15%[3,4]. Patients are not typically screened from brain metastases. Typically patients are symptomatic, leading to their diagnosis. If a solitary lesion is present, this may be treated surgically.
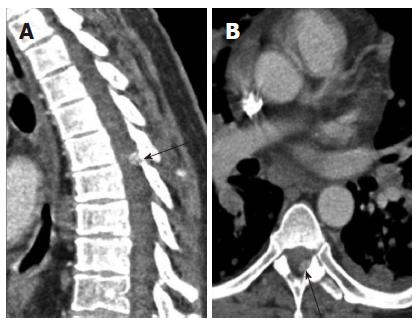
Spine: Most often the metastases to the spine are extramedullary (Figure 21) rather than intramedullary. Nevertheless, the presence of an intradural metastasis is a poor prognostic indicator and a sign of advanced disease. A review of the literature by Jost et al[20] in 2009 reported 32 cases in the literature of intradural metastases from renal cell carcinoma. Use of sagittal reconstructions to visualize the spinal canal longitudinally may help in lesion conspicuity.
In this pictorial essay we have demonstrated common and uncommon sites of metastatic recurrence in patients status post nephrectomy for renal cell carcinoma. A recent study by Jain et al[5] demonstrated the importance of arterial phase imaging in lesion detection within the liver, pancreas, and kidney. This case series confirms the utility of arterial phase imaging in detecting lesions elsewhere in the body by taking advantage of the metastases’ hypervascularity. Lesion hypervascularity may also help to distinguish a metastatic lesion from a second primary as in the cases of adrenal or breast metastases and thereby avoid unnecessary diagnostic procedures or therapies. Coronal and sagittal reconstructions are also helpful in lesion detection, specifically in evaluating for contralateral recurrences and spinal metastases, respectively.
P- Reviewers: Chen F, Kim CK S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Patel U, Sokhi H. Imaging in the follow-up of renal cell carcinoma. AJR Am J Roentgenol. 2012;198:1266-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Chae EJ, Kim JK, Kim SH, Bae SJ, Cho KS. Renal cell carcinoma: analysis of postoperative recurrence patterns. Radiology. 2005;234:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Griffin N, Gore ME, Sohaib SA. Imaging in metastatic renal cell carcinoma. AJR Am J Roentgenol. 2007;189:360-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Skolarikos A, Alivizatos G, Laguna P, de la Rosette J. A review on follow-up strategies for renal cell carcinoma after nephrectomy. Eur Urol. 2007;51:1490-1500; discussion 1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Jain Y, Liew S, Taylor MB, Bonington SC. Is dual-phase abdominal CT necessary for the optimal detection of metastases from renal cell carcinoma? Clin Radiol. 2011;66:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Sheth S, Scatarige JC, Horton KM, Corl FM, Fishman EK. Current concepts in the diagnosis and management of renal cell carcinoma: role of multidetector ct and three-dimensional CT. Radiographics. 2001;21 Spec No:S237-S254. [PubMed] |
| 7. | Sountoulides P, Metaxa L, Cindolo L. Atypical presentations and rare metastatic sites of renal cell carcinoma: a review of case reports. J Med Case Rep. 2011;5:429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Eggener SE, Yossepowitch O, Pettus JA, Snyder ME, Motzer RJ, Russo P. Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol. 2006;24:3101-3106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Bani-Hani AH, Leibovich BC, Lohse CM, Cheville JC, Zincke H, Blute ML. Associations with contralateral recurrence following nephrectomy for renal cell carcinoma using a cohort of 2,352 patients. J Urol. 2005;173:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Weight CJ, Kim SP, Lohse CM, Cheville JC, Thompson RH, Boorjian SA, Leibovich BC. Routine adrenalectomy in patients with locally advanced renal cell cancer does not offer oncologic benefit and places a significant portion of patients at risk for an asynchronous metastasis in a solitary adrenal gland. Eur Urol. 2011;60:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Choi YA, Kim CK, Park BK, Kim B. Evaluation of adrenal metastases from renal cell carcinoma and hepatocellular carcinoma: use of delayed contrast-enhanced CT. Radiology. 2013;266:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Northcutt B, Raman SP, Long C, Oshymyansky A, Siegelman SS, Fishman EK, Johnson PT. MDCT of Adrenal Masses: can dual-phase enhancement patterns be used to distinguish between adenoma and pheochromocytoma? Am J Roentgenol. 2013;201:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Ahmed S, Johnson PT, Hruban R, Fishman EK. Metastatic disease to the pancreas: pathologic spectrum and CT patterns. Abdom Imaging. 2013;38:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Wente MN, Kleeff J, Esposito I, Hartel M, Müller MW, Fröhlich BE, Büchler MW, Friess H. Renal cancer cell metastasis into the pancreas: a single-center experience and overview of the literature. Pancreas. 2005;30:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Moir JA, Sen G, Saif R, Haugk B, French JJ. Isolated splenic metastasis from renal cell carcinoma: case report and review. Case Rep Gastroenterol. 2011;5:166-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Pollheimer MJ, Hinterleitner TA, Pollheimer VS, Schlemmer A, Langner C. Renal cell carcinoma metastatic to the stomach: single-centre experience and literature review. BJU Int. 2008;102:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 481] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 18. | Alzaraa A, Vodovnik A, Montgomery H, Saeed M, Sharma N. Breast metastasis from a renal cell cancer. World J Surg Oncol. 2007;5:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Ganapathi S, Evans G, Hargest R. Bilateral breast metastases of a renal carcinoma: a case report and review of the literature. BMJ Case Rep. 2008;2008:bcr0620080239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Jost G, Zimmerer S, Frank S, Cordier D, Merlo A. Intradural spinal metastasis of renal cell cancer. Report of a case and review of 26 published cases. Acta Neurochir (Wien). 2009;151:815-821; discussion 821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |