INTRODUCTION
Interventional neuroradiology, the cornerstone of patient management in many neurovascular diseases currently, has known a recent period of groundbreaking milestones with the improvement of techniques devices available[1,2]. These devices and procedures have rendered possible the treatment of stroke, vascular malformations as aneurysms and AVMs with a degree of precision and security that was previously unthinkable. As interventional techniques are evolving, it is imperative to dispose of more and more accurate and powerful imaging techniques that will allow us to not just perform imaging but also assess any pathological changes[3]. Also, due to the fact that sometimes neurointerventional training does not always encompass a full training in some of these new techniques, it is required that the neurointerventionalist immerses himself somewhat more in them in order to fully comprehend them. These new imaging techniques have made possible by the recent parallel developments of techniques such as spiral scanning and multi-slice computed tomography (CT) scanners[4] as well as fast magnetic resonance (MR) techniques[5,6]. Indeed, this gives imaging a more and more important role whenever an intervention and/or operation are being planned. Indeed, the physician will need not just to obtain optimal 3D visualization of the lesion but also to assess any damage to the surrounding brain tissue. CT imaging is very often the examination of choice for the acute investigation of patients: indeed it is widely used in traumatology due to its superior capacity to image bone lesions but is also a technique that is more easily used for the detection of any intracranial blood. Indeed, the detection of blood is absolutely necessary if one is to treat for ischemic stroke since its presence will be a contra-indication, and also for any kind of treatable cerebral hemorrhage such as subarachnoid hemorrhage. Together with CT perfusion and CT angiography[7-11] these techniques have become easily available and very powerful tools for the neurodiagnostician and neurointerventionalist. MR on the other hand disposes of more sensitive techniques such as diffusion-weighted MR[12-17] or susceptibility-weighted MR[18,19] that are able to detect lesions that may not be seen on CT. In addition to perfusion, additional techniques such as diffusion tensor imaging, MR spectroscopy, and 3D imaging and more, makes this is superior technique when it comes to the non-urgent evaluation of patients where exact lesion characterization is necessary. Over the last decade there has been a continuous increase in the field strength of clinical MR scanners: this has led to significant improvements in imaging; on the one hand signal increases overall with magnetic field so that an increase in resolution and a decrease in scanning time has been achieved[20]. The aim of this review is to provide an overview of established and new neuroimaging techniques that are increasingly being used in the evaluation of patients undergoing interventional neuroradiology procedures.
CT
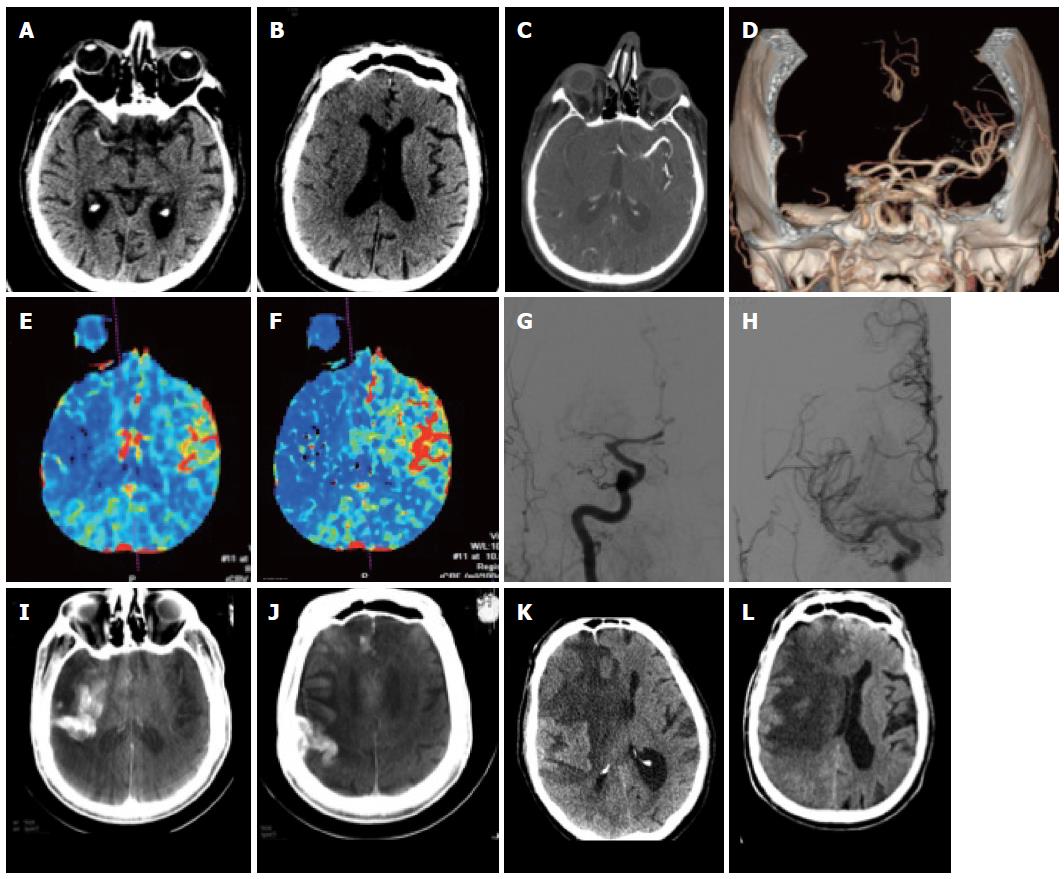
CT, since its introduction in the 1970’s remains the workhorse of neuroradiological diagnostic workup. While it has clearly remained the emergency radiology method of choice due to its intrinsic better bone detail, it has regained interest due to the development for spiral CT scanning and multi-slice scanners that allow obtaining additionally angiographic images as well as perfusion images of the brain. The plain unenhanced CT remains the workhorse for any vascular work-up since it will allow on the one hand todemonstrate or exclude easily hemorrhage. When considering stroke the early signs such as the dense artery, the disappearance of localized grey matter -white matter differentiation and hypodensity are subtle but reliable signs that can be used to detect ischemia (Figure 1).
Figure 1 Patient with acute stroke.
A: Unenhanced computed tomography (CT) at 3.5 h after onset shows hyperdense MCA on the right; B, C: There are subtle signs of grey-white dedifferentiation: the insula and the basal ganglia on the right are not distinguishable; the sulci on the right side are also slightly less visible; C-F: CT angiography shows an M1 occlusion on the right (C, D) with hypoperfusion with drops in the rCBV (E) and rCBF (F) maps in the right MCA territory; G, H: This patient underwent DSA that confirmed the right MCA occlusion (G) which was recanalized (H); I, J: On follow-up CT images reconstructed from the expert CT data set acquired with the flat panel angio unit there is initially contrast extravasation; K, L: Further follow-up CT showed marked midline shift due to almost complete right MCA infarction.
CT angiography
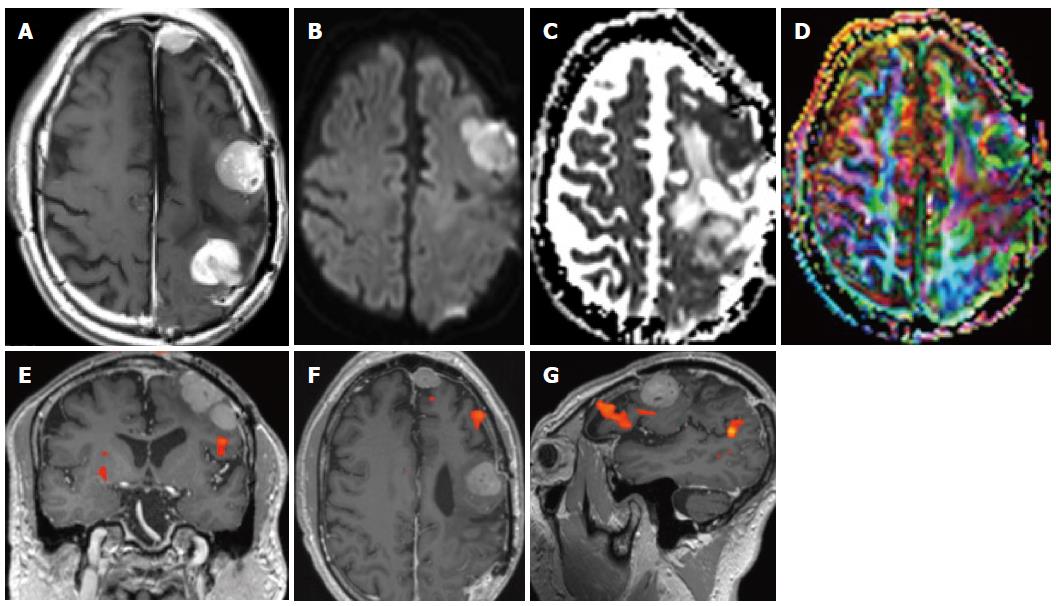
CT angiography now allows covering the vasculature from the aortic arch up to the circle of Willis in just a few seconds. Very often two imaging strategies are taken: for ischemic stroke, very often, the vasculature is done from the arch, even if sometimes this will cause venous superposition. For dedicated questions such as in patients with suspected aneurysms, it is very often decided to perform only and angio-CT at the level of the circle of Willis in order to have optimal aneurysmal filling; this has the disadvantage that if an endovascular approach is being discussed, the anatomy of the vessels as they arise from the arch is not known[21]. Also for the demonstration of vascular malformations, CT angiography can well demonstrate lesions that will be visible only well after contrast administration (Figure 2).
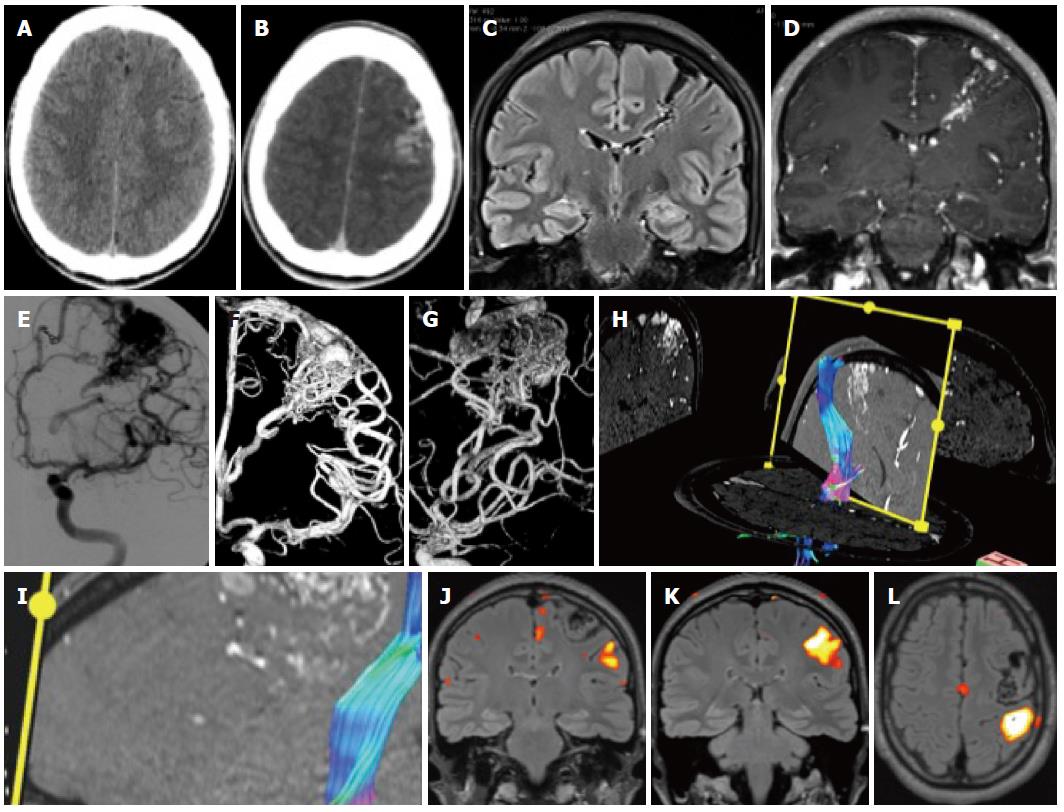
Figure 2 Patient with epilepsy.
A: Unenhanced computed tomography (CT) shows discrete hyperdensities in the left frontal white matter; B: After contrast administration, vascular structures are seen in the left frontal lobe, raising the suspicion of an AVM. On magnetic resonance imaging (MRI) one can see typical findings due to an Arteriovenous malformation; C, D: Long flow voids on the FLAIR images that run from the cortex to the paraventricular region in a triangular pattern; E-G: The Angiogram in same patient showing frontal AVM; H, I: Tractography shows the AVM to be close but separated from the corticospinal tract; J-L: Functional MRI was done with a motor paradigm that demonstrates cortical activation in the frontal motor cortex, the AVM is shown to be located anteriorly and superiorly to the activated area.
CT perfusion
CT perfusion techniques, which had been available for a long time have also become more widely accepted due to their capacity to detect ischemia very early on (i.e., at the same time as the initial emergency CT); while the techniques have somewhat suffered from not initially providing enough brain coverage in cases with extensive strokes or in cases with multiple areas being affected, more CT units are now providing coverage of almost or of the whole brain. Reconstruction algorithms are available that provide maps of cerebral perfusion parameters (CBV, CBF, MTT) that can help understand better the physiopathological processes and help determining tissue outcome[22] (Figures 1-3).
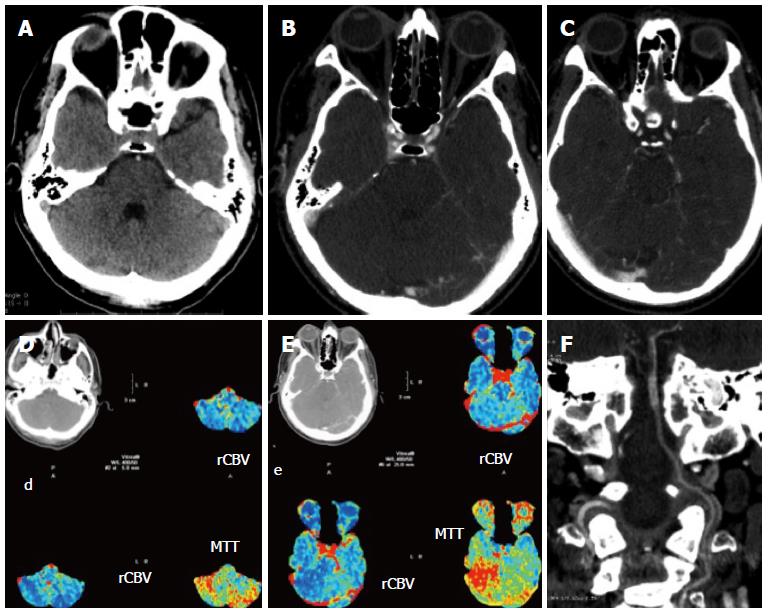
Figure 3 Patient with symptoms referable to the posterior fossa.
A-C: Unenhanced computed tomography (CT) shows a possible hyperdense artery sign of the basilar artery (A) which is confirmed by CT angiography: the basilar artery enhances less than the rest of the vessels (B, C); D, E: Perfusion imaging shows a drop in hemodynamics in the posterior fossa; F: The coronal reconstruction of the angio-CT shows well the length of the thrombus.
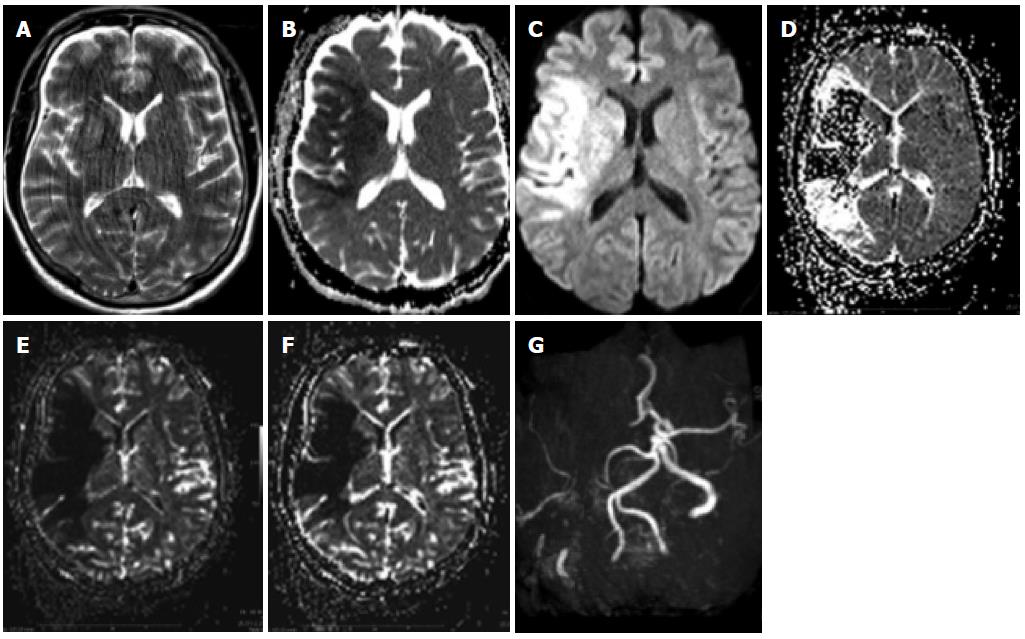
Dual energy CT
This is another rather recent development in CT technology that has quite some interesting applications for patients with neurovascular diseases. By using different kVp it is possible to visualize various tissue components that have different X ray absorption rates. It has been used to differentiate bone from other tissues and remove the skull as well as for removing vascular calcifications: More recently it has been used with success after thrombolysis to differentiate iodinated contrast extravasation from blood[23] or even after other endovascular procedures if a complication is suspected (Figure 4).
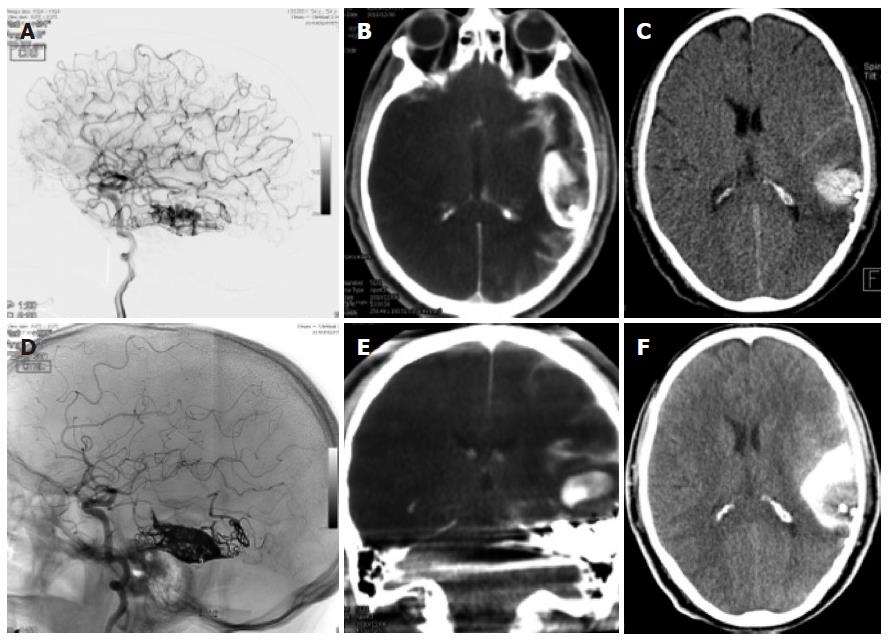
Figure 4 Dual energy computed tomography.
A, D: Patient having undergone angiography and embolization for a temporal left AVM; B, E: On the immediate expert computed tomography (CT) performed on the angio table, there was a large area of hyperdensity with probably contrast and blood but which were not distinguishable from one another: the Dual source CT shows that this is mainly due to contrast since there is sonly a small area of blood surrounding the embolized material; C, F: Again a normal C CT afterwards shows the more extensive hyperdensity in the subarachnoid regions due to contrast and blood.
MR IMAGING
MR imaging, due to its intrinsic improved tissular contrast with many sequences allowing performing tissue characterization is the method of choice for most situations that do not necessitate an emergency treatment. Indeed, despite the development of some really powerful MR techniques due to the implementation of both high field scanners as well as fast imaging, the technique is still somewhat more time-consuming and requires some more patient cooperation to be performed and has a few disadvantages (claustrophobia inducing, magnetic field). However due to the development of a multitude of various techniques, called sequences it is now possible to perform advanced tissue characterization in many cases. Also the development of high field scanners has been a recent important step in this direction.
CONVENTIONAL MR TECHNIQUES
MR imaging is based on the acquisition of image sequences; these image sequences provide images with different information regarding tissue protons according to their parameters. The classic imaging types are T1 and T2 with T1 images being additionally acquired after contrast administration.
DIFFUSION TECHNIQUES
Diffusion MR techniques are techniques in which the diffusion within tissues is being measured. One the one hand there is diffusion-weighted MR which simply shows normality or abnormality of water movement and on the other hand there is diffusion tensor imaging in which water movement is displayed with a force and a directionality. Diffusion MR, which was developed by Le Bihan, consists in a simple Stejskal Tanner modification in which two diffusion-sensitizing pulses are added to a simple spin echo sequence[24]. This allows the image to gain diffusion-weighting. Images can be acquired with different amounts of diffusion-weighting: this is done by applying a b value: at the lowest b value one obtains an image with little diffusion weighting and which corresponds to a T2-weighted image and the more one has a higher b value the more the image will be diffusion-weighted. For clinical purposes and with current MR units and settings, a b value of around 100 has been found to be optimal for stroke imaging[25]. Diffusion techniques were and are very sensitive to the orientation of water movement: this phenomenon, which is very present in tissues, is called anisotropy.
Diffusion-weighted imaging
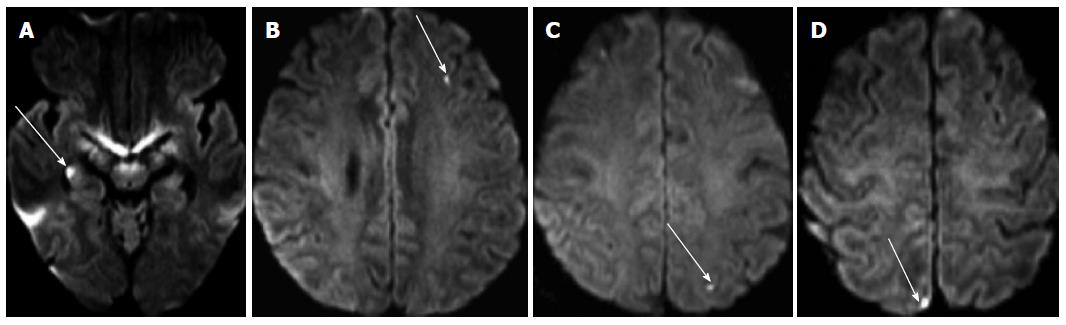
The classic situation where diffusion imaging has been used with success has been that of acute cerebral ischemia. While initially this was difficult clinically due to the inherent motion sensitivity of the sequence, its clinical implementation could be done with the implementation of clinical fast scanners of the echo-planar type in the early to mid- 1990’s. By performing diffusion images with multiple settings of b it is possible to obtain so-called maps of the apparent diffusion coefficient (ADC) where one is able not just to visualize motility and pathology thereof but to quantify it accordingly. This led to the first applications in imaging of cerebral ischemia: it was found that early on there was a decrease in water diffusion that led to a decrease of the ADC that led to an increase in signal on DW images with a maximum b value[26] (Figures 5 and 6); DW images are therefore used for detection of ischemia and ADC maps for its quantification. This early hypersignal on diffusion-weighted imaging (DWI) and hyposignal on ADC corresponds to an early decrease in diffusion in the tissues that is associated with the early cytotoxic edema. After this early decrease in the ADC there is a slow increase with time until the values become clearly positive. In animal models it was initially found that these diffusion changes would appear within minutes after occlusion; this has been confirmed in some select clinical human cases[27]. In order to reduce the influence of anisotropy artifacts, nowadays almost always so-called trace images are used at the maximum b value. The main advantage of DWI over CT is that it can detect very small lesions that may occur as a result of embolism and that may be less or even invisible on CT (Figure 5).
Figure 5 Diffusion images performed after catheter angiography demonstrating multiple small asymptomatic lesions in both brain hemispheres (arrows).
They are found in the hippocampus (A), the left frontal lobe (B), the parietal lobes (C, D).
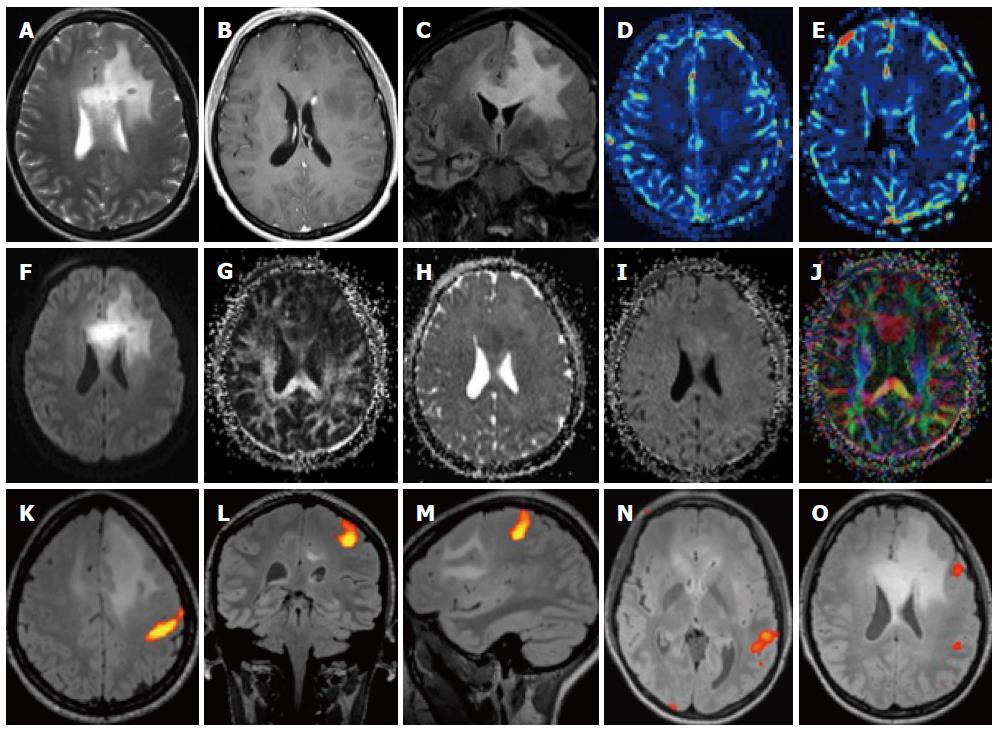
Figure 6 Patient with penumbra and mismatch.
A: A patient with an acute left-sided hemisyndrome was referred to our hospital: T2 images showed no clear signs of ischemia despite motion artifacts; B, C: The apparent diffusion coefficient (ADC) was lowered in the right MCA territory with a corresponding hyperintensity on the diffusion-weighted imaging (DWI) images with the maximum b value; D-F: There was additional hypoperfusion in a more extensive area on the MTT maps (D) as well as on the maps of rCBV (E) and RCB (F), which was sue to an occlusion of the MCA on the right.
Diffusion tensor imaging
A further improvement of diffusion MR techniques, diffusion tensor imaging uses the intrinsic capacity of diffusion imaging to detect differences of water force orientation by diffusion sequences[28]. While this was initially more of a problem and an artifact, it has been used to demonstrate changes in the so-called fractional anisotropy of the brain. These changes in anisotropy can also be found in pathological processes such as brain tumours (Figures 7-9). By extrapolating the changes one observes on diffusion images (Figure 7), it has been possible also to reconstruct images of the white matter tracts: this is called diffusion tensor tractography (Figure 8). While one must be aware that these images do not provide a direct view of the white matter tracts since they correspond to water movement along them, they have become a great help in evaluating both normal and abnormal situations involving the long white matter pathways.
Figure 7 Patient with left frontal glioma.
A-C: On T2 images there is a large area of high density in the frontal lobe which infiltrates the corpus callosum (A), there is a small deep paraventricular enhancement (B) and the involvement of the callosal white matter is better seen on the coronal FLAIR image (C); D-G: The lesion is hypoperfused (D, E) and there is a high signal on diffusion-weighted imaging (DWI) (F), a low FA (G) as well as a slightly lower ADC; K-O: Functional magnetic resonance (MR) with motor paradigms (K-M) as well as language paradigms was performed (N, O). The lesion is located in the frontal lobe, well demarcated from the motor cortex (K) but close to Broca’s area (O).
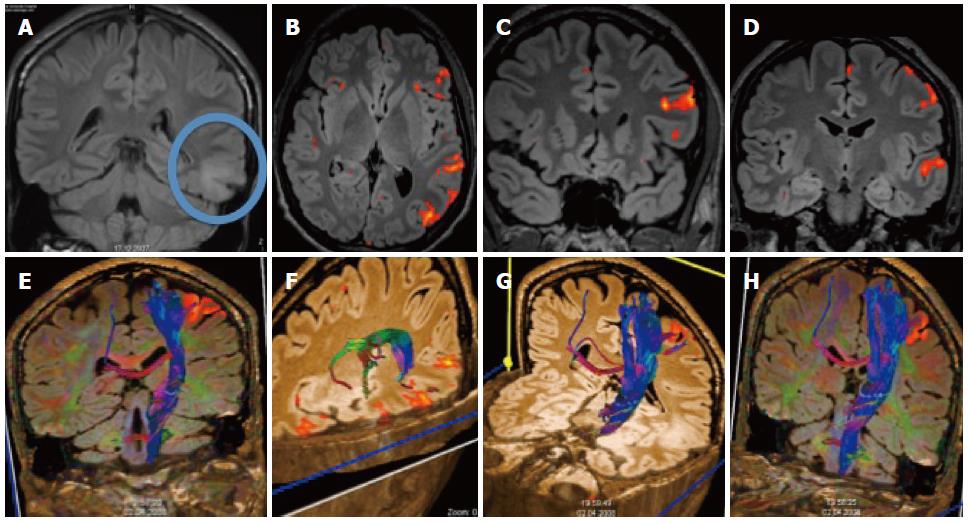
Figure 8 Patient with temporal glioma.
A: The cortical and subcortical lesionis best seen on the coronal FLAIR; B-D: Additional functional magnetic resonance imaging with language paradigms showed the tumor to be well demarcated from the Broca and Wernicke areas; E-H: Additional DTI shows the location of the long cortico-spinal tracts: the pyramidal tract is displayed in relation to the frontal cortical activation obtained with a motor paradigm.
Figure 9 Patient with meningiomatosis.
On conventional T1-weighted magnetic resonance (MR) with contrast multiple cortical nodules are seen (A) on diffusion the lesions are slightly hyperintense, corresponding to high cellularity (B, C) along with a reduced anisotropy (D).
PERFUSION TECHNIQUES
Perfusion is the measurement of the passage of blood over a time period; perfusion techniques with MR can be acquired with different techniques[29], both with T1[30] T2*[31,32] contrast-enhanced techniques[33] as well as Arterial Spin Labeling techniques that require no exogenous contrast[34-37]. The most commonly used ones rely on T2* imaging where the use of contrast induces a change in the local magnetic field which induces a signal drop from which the various perfusion parameters can be deduced. T1 perfusion imaging, which is much less frequently done but has some potential benefits, also requires the use of a contrast agent. Another perfusion technique is Arterial spin labeling where the inherent capacity of spins entering a slice to provide contrast is being used. This very promising technique was for a long time only available in single-slice mode but with the implementation of high field scanners was able to provide perfusion maps covering the whole brain. While the technique has the advantage of being repeatable without any contrast administration, it does not provide much signal and is prone to artifacts. The first widely accepted application for perfusion imaging with magnetic resonance imaging (MRI) was of course stroke[38] (Figure 6). The combined use of diffusion and perfusion allowed early stroke researchers to try and develop a working model of the penumbra where it was postulated that the initial diffusion lesion was the central core and the hypoperfused area beyond it the penumbra or tissue at risk for further infarction[39]. While this model does not really correspond to the traditional model of the penumbra from a physiopathological point of view (it being a hemodynamically based model and not an energetically-based one)[40], it was at least a model that worked in a certain number of cases. While it seems that not all acute cases of stroke may have the same typical mismatch[41] it seems that at least the central diffusion lesion does correspond to the core[42]. The use of ASL techniques could be of further interest since it seems able to better demonstrate reperfusion and collateral flow than contrast techniques[43]. Perfusion techniques have also found progressive acceptance in the grading of brain tumours: together with spectroscopy they can help to grade brain tumours in a superior way[44].
MR ANGIOGRAPHY TECHNIQUES
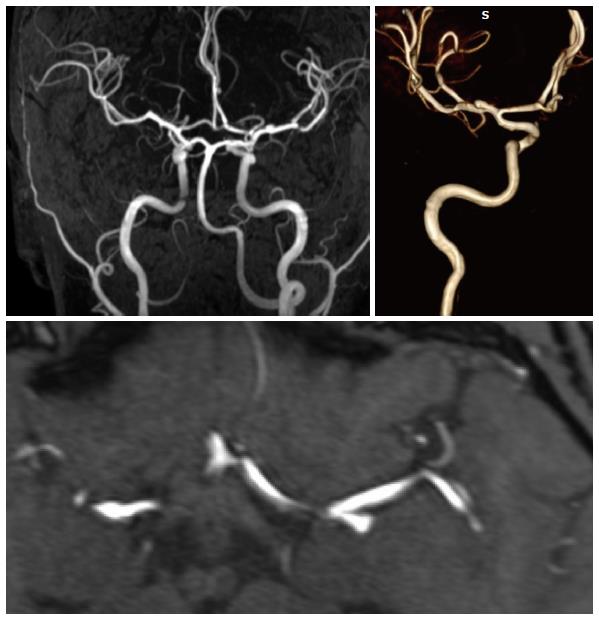
Most MR techniques are very sensitive intrinsically to flow so a natural development was that of MR angiographic techniques. Many various techniques have been developed that allow reconstructing angiographic images, based on time-of flight effects, or phase-contrast images or even contrast-enhanced images. Brain MRA are done predominantly by a time of flight technique at the level of the brain[45]; the TOF techniques can be also done with contrast, especially when imaging aneurysms in the follow-up since it improves visualization of any aneurysms remnant. Due to the presence of flow-related signal changes inherent to MR imaging, one does not currently advocate the use of MR sequences for the diagnosis of aneurysms (Figure 10) but more for their follow-up.
Figure 10 High resolution MRA of an MCA bifurcation aneurysm: A long vascular coverage is possible from the cervical region to the distal MCA and ACA branches.
Contrast-enhanced techniques are also used at the level of the neck for the carotid arteries where imaging has now been improved so much that coverage is provided that goes from the aortic arch to the circle of Willis[46]. Phlebographic techniques are also possible by using either time-of-flight techniques or also contrast-enhanced techniques[47].
SUSCEPTIBILITY-WEIGHTED MR IMAGING
This is also a technique that has been available for some time but which became implementable clinically only with the use of high-field scanners. This is a technique that provides very high T2 and T2* contrast images that have very little anatomic detail but very high contrast. This makes it a sequence that will be of high use to detect calcifications or hemorrhagic lesions.
FUNCTIONAL MR IMAGING
Functional MR imaging is a technique that relies on the capacity of T2* images to detect changes in local blood oxygenation[48]. It can be done on any kind of clinical MR imager, which operates at 1.5 T or more. It requires cooperation of the patient: the patient has to be able to lie with his head still for a period that will be longer than a routine examination while at the same moment undergoing a task. The tasks or paradigms that are tested can test any system in the nervous system. However it is indispensable that the patient understands and can cooperated with the examination: for example a patient with decreased consciousness is not going to be examinable and an aphasic patient will not be able to undergo language paradigms a well as a patient with a hemiparesis will not really be examinable by motor tasks on the affected side. The principal application has been in the localization of motor brain functions[49]. Clinically this will be applied to brain tumors or even arterio-venous malformations of the brain[50]. Stroke, while a potential application has shown to be more difficult to investigate[51].
MR SPECTROSCOPY
MR spectroscopy consists in a number of MR based techniques wherein the concentration of metabolites can be measured and or imaged. It is mostly done for follow-up of brain tumors[52] or in order to elucidate unclear cerebral processes that cannot be classified visually. Traditionally it is one by selecting one voxel in the brain at a time. In order to obtain a representative vision of metabolites in the brain it may require the acquisition of multiple voxels, thus significantly prolonging imaging time. Spectroscopy as with other techniques, is highly dependent on the strength of the magnetic field: with an increase in magnetic field there is a signal increase that will improve measurements.
PLANNING OF NEUROVASCULAR INTERVENTIONS
When planning and intervention, there are multiple complex questions that need to be answered: what is the type of the lesion, where is it located, what structures are nearby and how is the hemodynamic profile of the lesion and the surrounding structures. These questions can be addressed both by CT and MR techniques. Preferably this will be done with MRI especially in a non-emergent situation, since MR offers the possibility of multi-planar imaging with 3D imaging as well as the direct acquisition of multiple imaging planes.
MONITORING OF NEUROVASCULAR INTERVENTIONS
Diffusion-weighted MR has established itself as a sensitive tool to detect small ischemic lesions that may occur after interventional procedures. Thus very often it is performed at least afterwards but more importantly before and after procedures. Authors have established that a number of events occur even during cerebral angiography (Figure 5). In cases where for example stenting is performed, DWI before and after can assess the presence or absence of new lesions and determine safety of the procedure.
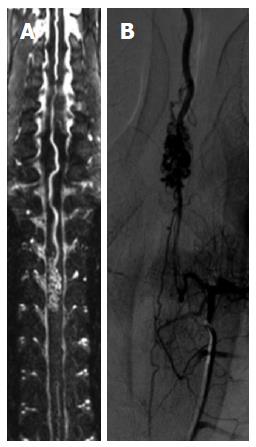
At the level of the spine, catheter angiography tends to be avoided or at least reserved for cases where an intervention is foreseen; this is evident due to the inherent sensitivity of the spinal vessels to any manipulation that could occlude them. In that sense it is obvious that spinal MRA would come and be a natural technique; however due to limitations of a technical nature, this has only been possible recently[53] (Figure 11).
Figure 11 Spinal MRA.
A spinal magnetic resonance (MR) angiography (A) with a long field of view demonstrates an AVM of the anterior spinal artery that is also shown by spinal DSA (B).
CONCLUSION
Modern diagnostic neuroimaging techniques offer very powerful means for the investigation of patients with diseases of the central nervous system. This has become indispensable nowadays since the physician who is confronted with a patient harboring a disease of the nervous system will need to not just use these techniques but to comprehend them intimately. This is true not just for any clinical neuroscientist, but is especially true for interventional neuroradiologists. One the one hand these imaging techniques have become intimately bound to the daily interventional routine since they allow diagnosis, staging as well as therapeutic planning and follow-up in a way that was entirely impossible until recently. Also, since what we consider neuroradiological interventions may sometimes be performed by physicians coming from other fields but who have a comparable training, it is necessary for a thorough understanding of these techniques to become a full part of the training curriculum. CT and MR are two well-established techniques that can in part perform quite similar evaluations of patients who require interventions but that are quite complementary. CT will be very powerful in an acute setting such as after a stroke or whenever there is a bleeding process intracranially; in acute stroke both techniques seem to perform equivalently[54]. CT techniques have the advantage of being able to detect blood in a superior way as well as to propose bone algorithms that are quite superior to MRI. In the 1990’s both techniques were relatively equally primitive; however with advances in knowledge and treatment of many diseases in medicine it was obvious that diagnostic techniques would have to follow. This then led to almost parallel developments in imaging speed and quality in both CT and MRI. MR technology was perhaps the first to benefit from these advances due to echo-planar imaging: diffusion, perfusion and angio-MR became tools for clinical routine; CT would know the same but with a slight delay: while techniques to perform both perfusion and angiography with CT had been developed almost since the beginning of the technique, it was only with the advent of multi-slice technology that one would see its wide distribution. CT while having increased drastically in quality and power still has the major drawback of potential major radiation exposure to the patient population; this is why, while it may be necessary for the initial examination due to its better luminographic capacities, in patients where follow-up is necessary MRI is going to be performed. Magnetic Resonance techniques however will also allow obtaining a much better image characterization. This has been further been improved with higher fields even if this is not without drawbacks due to a higher occurrence of artifacts due to the higher magnetic field[55].
P- Reviewers: Hiwatashi A, Leonardi M, Sener RN S- Editor: Song XX L- Editor: A E- Editor: Liu XM