Published online Oct 28, 2013. doi: 10.4329/wjr.v5.i10.372
Revised: August 29, 2013
Accepted: September 18, 2013
Published online: October 28, 2013
Processing time: 137 Days and 21.6 Hours
AIM: To evaluate the diagnostic accuracy of contrast-enhanced ultrasonography (CEUS) in the differential diagnosis between neoplastic and non-neoplastic peripheral pleuro-pulmonary lesions.
METHODS: One hundred patients with pleural or peripheral pulmonary lesions underwent thoracic CEUS. An 8 microliters/mL solution of sulfur hexafluoride microbubbles stabilized by a phospholipid shell (SonoVue®) was used as US contrast agent. The clips were stored and independently reviewed by two readers, who recorded the following parameters: presence/absence of arterial enhancement, time to enhancement (TE), extent of enhancement (EE), pattern of enhancement (PE), presence/absence of wash-out, time to wash-out, and extent of wash-out. After the final diagnosis (based on histopathologic findings or follow-up of at least 15 mo) was reached, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR) of each CEUS parameter in the differential diagnosis between neoplastic and non-neoplastic lesions were calculated. Furthermore, an arbitrary score based on the ratio between the PPVs of each CEUS parameter was calculated, to evaluate if some relationship could exist between overall CEUS behaviour and neoplastic or non-neoplastic nature of the lesions.
RESULTS: Five patients were lost at follow-up before a conclusive diagnosis was reached, 53 lesions resulted neoplastic and 42 non-neoplastic. Enhancement in the arterial phase was observed in 53/53 neoplastic lesions and 30/42 non-neoplastic lesions. On the whole, 40/42 non-neoplastic lesions showed absence of enhancement or early enhancement (95.2%) vs 3/53 neoplastic lesions (5.7%). EE was marked in 29/53 (54.7%) neoplastic lesions and 25/30 (83.3%) non-neoplastic lesions, moderate in 24/53 (45.5%) and 5/30 (16.7%), respectively. PE was homogeneous in 6/53 (11.3%) neoplastic lesions and 18/30 (60%) non-neoplastic lesions, inhomogeneous in 47/53 (88.7%) and 12/30 (40%), respectively. 19/30 (63.3%) non-neoplastic lesions enhancing in the arterial phase had no wash-out in the venous phase, 11/30 (36.7%) had late and mild wash-out. Wash-out was early in 26/53 (49%) neoplastic lesions, late in 26/53 (49%), absent in 1 (2%); marked in 16/53 (30.2%), and moderate in 36/53 (67.9%). The delayed enhancement in the arterial phase showed a sensitivity of 94.32%, specificity of 95.2%, PPV of 96.2%, NPV of 93%, PLR of 19.81, and NLR of 0.06 in identifying the neoplastic lesions. All other parameters individually considered showed unsatisfactory values of sensitivity, or specificity, or both, in differentiating neoplastic from non-neoplastic lesions. The median of the overall arbitrary score was 3 (range 0-14) in non-neoplastic lesions, and 16.5 (range 7.0-17.5) in neoplastic lesions (P < 0.001). The correlation between the diagnosis of neoplastic vs non-neoplastic lesion and the score value was statistically significant (r = 0.858, P < 0.001). Based on the score distribution, a cut-off of 7.5 enabled to reach a sensitivity of 98.1%, specificity of 95.1%, PPV 96.3%, NPV 97.5%, PVR 20.1 and NVR 0.02 in differentiating neoplastic from non-neoplastic lesions.
CONCLUSION: CEUS could be useful in the diagnostic workup of pleuropulmonary lesions. A delayed TE or a score ≥ 7.5 suggest the neoplastic nature of a lesion.
Core tip: Contrast-enhanced ultrasonography (CEUS) is widely used to characterize focal liver lesions. The lung has dual blood supply (pulmonary arteries and systemic bronchial arteries), and theoretically it should be suitable for CEUS evaluation of arterial vascularity. This study suggests that a delayed time to arterial enhancement or an arbitrary score based on the overall CEUS behaviour ≥ 7.5 have high sensitivity and specificity in suggesting the neoplastic nature of pleural or peripheral pulmonary lesions. CEUS could allow for discriminating between lesions which need invasive diagnostic procedures, and those which can be monitored with clinical and instrumental follow-up.
- Citation: Sartori S, Postorivo S, Vece FD, Ermili F, Tassinari D, Tombesi P. Contrast-enhanced ultrasonography in peripheral lung consolidations: What’s its actual role? World J Radiol 2013; 5(10): 372-380
- URL: https://www.wjgnet.com/1949-8470/full/v5/i10/372.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i10.372
Despite the limits caused by the sound reflection at the aerated lung surface, in the last years transthoracic ultrasonography (US) has gained increasing interest in the evaluation of peripheral pulmonary lesions abutting the pleura[1,2]. However, the correlation between the US pattern of lung consolidations and their specific pathology is quite poor, and transthoracic US is mainly used as an alternative to computed tomography (CT) to guide percutaneous needle biopsy of the lesions, when endoscopic biopsy fails in yielding adequate tissue specimens[3,4,5].
Contrast-enhanced ultrasonography (CEUS) with second generation US contrast agents is increasingly used in the diagnostic imaging of abdominal organs. In particular, in Europe, Eastern Countries, and Canada it is recommended as the first-line technique for the characterization of focal liver lesions[6-8]. The lung has dual blood supply (pulmonary arteries and systemic bronchial arteries), and this peculiarity could theoretically be exploited by CEUS to characterize and differentiate lesions with different arterial supply. However, at present only few preliminary studies on the role of CEUS in the characterization of peripheral lung masses have been reported in literature, and most of them were mainly descriptive and gave conflicting results[9-14]. This prospective pilot study was planned to evaluate the possible role and diagnostic accuracy of CEUS in the differential diagnosis between neoplastic and non-neoplastic pleural and peripheral pulmonary lesions.
From October 2009 to October 2011, 100 consecutive patients (56 males and 44 females, mean age 61 years, range 24-89 years) with pleural lesions or peripheral pulmonary consolidations abutting the pleura were enrolled into this prospective study. The study was approved by Ethics Committee of our hospital and informed consent was obtained from each patient. The inclusion criteria were the following: age > 18 years; presence of a pulmonary peripheral mass depicted by chest Rx or CT, and visible at transthoracic US; diagnostic course still running; absence of known hypersensitivity to sulfur hexafluoride, pulmonary hypertension, class IV New York Heart Association heart failure[15], unstable angina, acute or recent myocardial infarction, or adult respiratory distress syndrome.
A preliminary US evaluation of the lesions was performed using a real-time sonography system equipped with a 3.5 to 5.0 MHz convex transducer and a 5.0 to 7.5 MHz linear transducer (Mylab 70 XVG Gold, Esaote, Genova, Italy) to identify the optimal acoustic window for CEUS examination, according to the lesion location and patient’s body size[16-18]. CEUS was performed with a low mechanical index contrast-specific nonlinear technique (CnTI, Esaote, Genova, Italy) and an 8 microliters/mL solution of sulfur hexafluoride microbubbles stabilized by a phospholipid shell (SonoVue®, Bracco, Milan, Italy) was used as US contrast agent. Acoustic power was set at 40 kilo pascal for both high-frequency linear transducer and low-frequency convex transducer. All US and CEUS examinations were performed by one of two physicians (PT and SP) with at least 5 years of experience in CEUS examination of abdominal organs, and well experienced with US of the lung.
After iv administration of a bolus of 2.4 mL of Sonovue, the lesions were continuously examined for at least 180 s taking care to include a portion of normal surrounding parenchyma in the same US scan, in order to examine contemporaneously both the lesion and normal lung. If normal parenchyma could not be included in the same scan, or the mass was located at the basis of the lung, the surrounding chest wall, or the liver or spleen, were examined contemporaneously to the lesion. All CEUS examinations were recorded, stored and independently reviewed by the two operators.
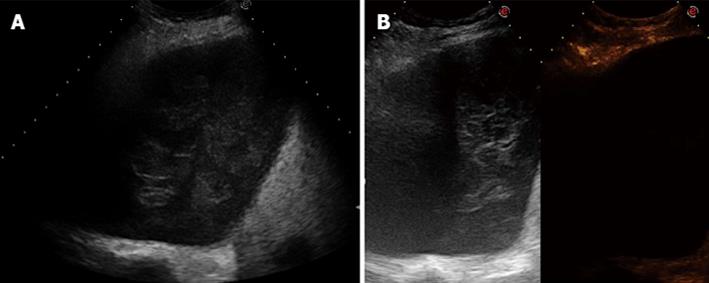
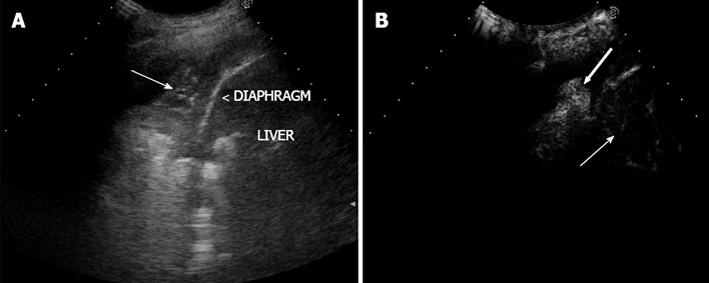
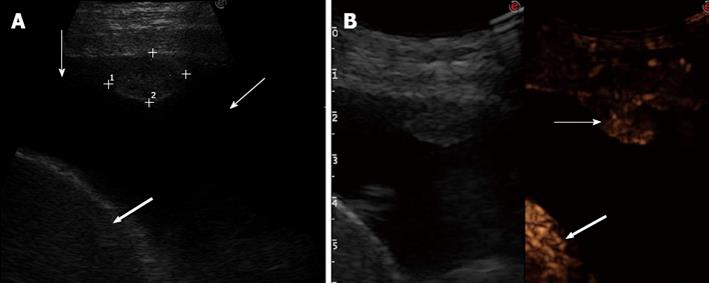
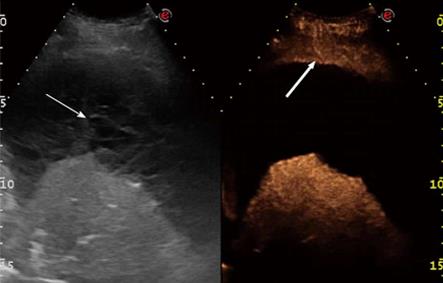
First, each lesion was assessed for the presence or the absence (Figure 1) of enhancement in the arterial phase, defined as the twenty s period following the appearance of the first microbubbles in the lesion or the surrounding parenchyma (or the chest wall, the liver, or spleen). If the lesion showed arterial enhancement, the following parameters were then recorded: time to enhancement (TE), extent of enhancement (EE), pattern of enhancement (PE), time to wash-out (TW), and extent of wash-out (EW). TE was defined as early if it was observed contemporaneously to the normal lung, or before the enhancement of the chest wall, liver or spleen (Figure 2); and as delayed if it was observed at least 2 s after the enhancement of the normal lung, or contemporaneously to the enhancement of the chest wall, liver or spleen (Figure 3). EE was defined marked (Figure 4) or moderate (Figure 3B) with respect to the EE of the normal lung, the chest wall, the liver or spleen. PE was classified as homogeneous (Figure 4) or inhomogeneous (Figure 3B). TW was defined as early if it occurred within 60 s after the injection of Sonovue, as late if it occurred later, and as absent if it was not observed within 180 s after the bolus. EW was classified as marked or mild according to its entity with respect to the entity of the arterial enhancement. The parameters examined are reported in Table 1. If some discordance in the evaluation of the CEUS parameters occurred between the two readers, the clip was reviewed and discussed with a third reader (SS) and the final decision was reached by consensus.
| TE | EE | PE | TW | EW | ||||||
| Enhancement present | Early (0-1 s with respect to normal lung) | Late (≥ 2 s later than normal lung) | Marked | Moderate | Homogeneous | - | - | - | - | - |
| Enhancement absent | Stop assessing CEUS parameters | - | - | - | - | - | ||||
| Wash-out | Early ( < 60 s) | Late (≥ 60 s) | Marked | Mild | Absent | |||||
The final diagnosis was based on histopathologic findings (by endoscopic or percutaneous imaging-guided biopsy, or surgical exploration), or clinical follow-up. Clinical proof of malignant lesion was accepted if the patient was treated for malignancy and the clinical course and response to therapy were appropriate. Clinical proof of benign lesion was accepted if the lesion disappeared spontaneously or after treatment other than antineoplastic chemotherapy, or no change in size was observed for more than 15 mo.
After the final diagnosis was reached, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative PLR of each CEUS parameter in the differential diagnosis between neoplastic and non-neoplastic lesions were calculated. First, the presence vs the absence of arterial enhancement was evaluated. If the lesion showed arterial enhancement, each distinctive feature of TE (i.e., early or delayed) was compared to the other one plus the absence of enhancement. The other parameters of arterial enhancement were analyzed as well (marked vs moderate enhancement, and homogeneous vs inhomogeneous enhancement). Finally, wash-out features were evaluated (presence vs absence, early vs late, and marked vs mild or absent) for the lesions that had enhancement in the arterial phase.
Furthermore, an arbitrary score based on the features of all CEUS parameters examined was conceived, in order to evaluate if some relationship could be found between the overall CEUS behaviour and the neoplastic or non-neoplastic nature of the lesions. The absence of arterial enhancement or wash-out (when arterial enhancement was present) was scored 0. For each parameter of enhancement or wash-out, the feature with lower PPV was scored 1 and the other one was scored according to the ratio between the two PPVs (for instance, homogeneous enhancement: PPV 25%, score 1; inhomogeneous enhancement: PPV 79.7%, score 3.2). The sum of the scores assigned to each CEUS parameter yielded the overall score, and the median values of the overall scores of neoplastic and non-neoplastic lesions were compared by using the non-parametric Mann Whitney test. Finally, the relationship between neoplastic or non-neoplastic nature of the lesions and overall CEUS score was analysed by using the non-parametric Spearman test. An alfa error < 5% was assumed as statistically significant in both analyses. All statistical analyses were performed with a statistical software program (Stata 11.0 for Windows, Stata Corp., College Station, TX, United States).
The quality of CEUS examination was good in all patients and no adverse reaction to Sonovue was observed. Both high-frequency linear transducer and low-frequency convex transducer yielded the same quality of CEUS examinations. There was no concordance between the readers in TE and EW evaluation in 5/100 cases and 4/100 cases, respectively (r = 0.899, and r = 0.9, respectively). In all these cases final consensus was reached after collegial review and discussion of the CEUS clips.
Mean diameter of the lesions was 3.5 cm (range 1-12 cm). Five patients were lost at follow-up before a conclusive diagnosis was reached. The final diagnosis was based on a median clinical follow-up of 17 mo (range 7-26 mo) in 23/95 cases (3 neoplastic and 20 non-neoplastic lesions), and histopathological findings (63 endoscopic or percutaneous biopsies, and 9 surgical explorations) in 72/95 cases (50 neoplastic and 22 non-neoplastic lesions). On the whole, fifty-three lesions resulted neoplastic (40 primary tumors, 13 metastatic tumors), and 42 resulted non-neoplastic. All the 22 biopsy diagnoses of non-neoplastic lesion were confirmed by the clinical follow-up. The final diagnoses are reported in detail in Table 2.
| Non-neoplastic lesions | n | Neoplastic lesions | Lost at follow-up (n) | |||
| Primary tumors | n | Metastatic tumors (origin) | n | |||
| Slow resolution pneumonia | 19 | Adenocarcinoma | 18 | Colon | 5 | 5 |
| BOOP | 5 | Squamous carcinoma | 5 | Stomach | 2 | |
| Abscess | 4 | Small-cell lung cancer | 3 | Testis (seminoma) | 2 | |
| Pulmonary infarction | 3 | Indifferentiate carcinoma | 6 | Liver (HCC) | 1 | |
| Organized pleural effusion | 4 | Sarcomatoid carcinoma | 2 | Breast | 1 | |
| Post-surgical fibrosis | 4 | Mesothelioma | 2 | Indeterminate | 2 | |
| Obstruction atelectasys | 2 | Schwannoma | 2 | |||
| Fibrotic plaque of the pleura | 1 | Plasmacytoma | 1 | |||
| Lymphoma | 1 | |||||
| 40 | 13 | |||||
| Total | 42 | 53 | 5 | |||
Enhancement in the arterial phase was observed in 53/53 neoplastic lesions and 30/42 non-neoplastic lesions. Arterial enhancement resulted absent in four organized pleural effusions, 1 abscess, 3 pulmonary infarctions, 3 post-surgical fibroses, and 1 fibrotic plaque of the pleura. TE was delayed in 50/53 neoplastic lesions (94.3%), and early in 28/30 non-neoplastic lesions (93.3%). On the whole, 40/42 non-neoplastic lesions showed absence of enhancement or early enhancement (95.2%) vs 3/53 neoplastic lesions (5.7%). EE was marked in 29/53 (54.7%) neoplastic lesions and 25/30 (83.3%) non-neoplastic lesions, moderate in 24/53 (45.5%) and 5/30 (16.7%), respectively. PE was homogeneous in 6/53 (11.3%) neoplastic lesions and 18/30 (60%) non-neoplastic lesions, inhomogeneous in 47/53 (88.7%) and 12/30 (40%), respectively. 19/30 (63.3%) non-neoplastic lesions with enhancement in the arterial phase had no wash-out in the venous phase, 11/30 (36.7%) had late and mild wash-out. Wash-out was early in 26/53 (49%) neoplastic lesions, late in 26/53 (49%), absent in 1 (2.0%); marked in 16/53 (30.2%), and moderate in 36/53 (67.9%). The results are summarized in Table 3.
| Enhancement | Wash-out | |||||||||||
| Early | Delayed | Absent | Marked | Moderate | Homogeneous | Inhomogeneous | Marked | Mild | Absent | Early | Late | |
| Non-neoplastic lesions | 28/42 | 2/42 | 12/42 | 25/30 | 5/30 | 18/30 | 12/30 | 0/30 | 11/30 | 19/30 | 0/30 | 11/30 |
| (66.7) | (4.8) | (28.6) | (83.3) | (16.7) | (60) | (40) | (0) | (36.7) | (63.3) | (0) | (36.7) | |
| Neoplastic lesions | 3/53 | 50/53 | 0/53 | 29/53 | 24/53 | 6/53 | 47/53 | 16/53 | 36/53 | 1/53 | 26/53 | 26/53 |
| (5.7) | (94.3) | (0) | (54.7) | (4.3) | (11.3) | (88.7) | (30.2) | (67.9) | (1.9) | (49) | (49) | |
The delayed enhancement in the arterial phase showed a sensitivity of 94.32%, specificity of 95.2%, PPV of 96.2%, NPV of 93%, PLR of 19.81, and NLR of 0.06 in identifying the neoplastic lesions. All the other parameters individually considered showed unsatisfactory values of sensitivity, or specificity, or both, in differentiating neoplastic from non-neoplastic lesions. All analyses are reported in detail in Table 4.
| Sensitivity | Specificity | PPV | NPV | PLR | NLR | ||
| 95%CI | 95%CI | 95%CI | 95%CI | 95%CI | 95%CI | ||
| Present | 100 | 28.6 | 63.9 | 100 | 1.4 | 0 | |
| 100-100 | 14.9-42.2 | 53.5-74.2 | 100-100 | 1.21-1.59 | -- | ||
| Early | 5.7 | 6.7 | 9.7 | 3.8 | 0.06 | 14.15 | |
| -0.719 | -17.9 | -20.8 | -10.5 | -2.24 | 12.8-15.5 | ||
| Delayed | 94.3 | 95.2 | 96.2 | 93 | 19.81 | 0.06 | |
| Enhancement | 88.1-100.6 | 88.8-101.7 | 90.9-101 | 85.4-100.6 | 18.4-21.2 | -2.2 | |
| Marked | 54.7 | 16.7 | 53.7 | 17.2 | 0.66 | 2.72 | |
| 41.3-68.1 | 3.3-30 | 40.4-67 | 3.5-31 | 0.36-0.95 | 1.86-3.57 | ||
| Moderate | 45.3 | 83.3 | 82.8 | 46.3 | 2.72 | 0.66 | |
| 31.9-58.7 | 70-96.7 | 69-96.5 | 33-59.6 | 1.86-3.57 | 0.36-0.95 | ||
| Homogeneous | 11.3 | 40 | 25 | 20.3 | 0.19 | 2.22 | |
| 2.8-19.9 | 22.5-57.5 | 7.7-42.3 | 10.1-30.6 | -1.62 | 1.77-2.67 | ||
| Inhomogeneous | 88.7 | 60 | 79.7 | 75 | 2.22 | 0.19 | |
| 80.1-97.2 | 42.5-77.5 | 69.4-89.9 | 57.7-92.3 | 1.77-2.67 | -1.62 | ||
| Marked | 30.8 | 100 | 100 | 23.4 | -- | 0.69 | |
| 18.2-43.3 | 100-100 | 100-100 | 11.3-35.5 | 0.5-0.87 | |||
| Wash-out | Mild | 69.2 | 0 | 76.6 | 0 | 0.69 | -- |
| 56.7-81.8 | 64.5-88.7 | 0.5-0.87 | |||||
| Absent | 98.1 | 63.3 | 82.5 | 95 | 2.68 | 0.03 | |
| 94.5-101.8 | 46.1-80.6 | 73.2-91.9 | 85-104.6 | 2.2-3.15 | -3.92 | ||
| Early | 50 | 100 | 100 | 29.7 | -- | 0.5 | |
| 36.4-63.6 | 100-100 | 100-100 | 15-44.5 | 0.23-0.77 | |||
| Late | 50 | 0 | 70.3 | 0 | 0.5 | -- | |
| 36.4-63.6 | 55.5-85 | 0.23-0.8 |
The correlation data between PPV and values of the arbitrary score are reported in Table 5. The risk score was calculated in all the patients, with overall median value of 15 (range 0-17.5). In non-neoplastic lesions the median score was 3 (range 0-14), in neoplastic lesions it was 16.5 (range 7.0-17.5) (P < 0.001). The correlation between the diagnosis of neoplastic vs non-neoplastic lesion and the score value was statistically significant (r = 0.858, P < 0.001). Based on the score distribution, we found that a cut-off of 7.5 enabled to reach a sensitivity of 98.1%, specificity of 95.1%, PPV 96.3%, NPV 97.5%, PVR 20.1 and NVR 0.02 in differentiating neoplastic from non-neoplastic lesions.
| PPV | Score | ||
| Enhancement | Absent | 63.9 | 0 |
| Early | 9.7 | 1 | |
| Delayed | 96.2 | 9.9 | |
| Marked | 53.7 | 1 | |
| Moderate | 82.8 | 1.5 | |
| Homogeneous | 25 | 1 | |
| Inhomogeneous | 79.7 | 3.2 | |
| Wash-out | Marked | 100 | 1.3 |
| Mild | 76.7 | 1 | |
| Absent | 69.3 | 0 | |
| Early | 100 | 1.4 | |
| Late | 70.3 | 1 | |
Thoracic US has become a well-established method to guide percutaneous biopsy of peripheral pulmonary lesions abutting the pleura, and is considered as effective as CT-guidance in terms of sample accuracy, with the advantages of lower cost, lack of exposition to ionizing radiation, shorter procedure time, and lower rates of post-procedural pneumothorax[19]. Despite the increasing applications of CEUS in the characterization of focal lesions of the liver and other abdominal organs[6-8,20], to date the use of second generation US contrast media in pleuropulmonary diseases is limited to improve the diagnostic yield of percutaneous biopsy[21,22]. The role of CEUS in the characterization of peripheral pulmonary lesions has been scarcely investigated, and the few preliminary studies published in literature were mainly descriptive and gave inconclusive and disappointing results[9-14,23]. The lung is characterized by dual blood supply: the bronchial arterial system, which provides nutrition for the bronchi, pulmonary vessels, alveoli, interstitial tissue, and visceral pleura; and the pulmonary arterial system, which is responsible for gas exchange[10]. Such a peculiarity could theoretically be exploited to differentiate pleural-based non-neoplastic lesions from neoplastic lesions, as the formers are supplied by both arterial systems, whereas tumor angiogenesis usually rises from bronchial arteries[12]. Therefore, a different TE should be seen in real time imaging, as the tissue enhancement resulting from the pulmonary arteries starts before the tissue enhancement resulting from the bronchial arteries. Moreover, a regular, dominant pulmonary arterial supply should lead to a more marked tissue enhancement than that observed in tissues only supplied by the bronchial arteries. CEUS represents the best imaging method to evaluate both the vascularity and transit time within an organ, as it enables the assessment of any time of enhancement during the arterial phase. Conversely, contrast-enhanced CT is an instant scanning modality, and can assess the vascularity only at a certain point in time during the arterial phase[24]
Although CEUS was shown to be as effective as CT in detecting peripheral lung cancer vascularisation[25] a prior study on 137 patients with pleural based lesions reported that CEUS did not allow to distinguish benign from malignant pulmonary consolidations[9]. However, 62% of malignant lesions had delayed TE, whereas 62% of inflammatory consolidations had early TE, and this observation was subsequently confirmed on larger and more recent series[10,11,26]. In our pilot study, delayed TE was observed in 94.3% of neoplastic lesions and in 4.8% of non-neoplastic lesions. A methodological defect could have biased the results of the above-mentioned studies. An arbitrary cut-off of six s after the iv bolus of the US contrast agent was used to define TE as early or delayed, because the time windows of pulmonary arterial vascularity and systemic bronchial arterial vascularity usually range from 1 to 5 s, and from 8 to 11 s, respectively[10,11]. However, a lot of physiological and pathological conditions, such as sitting or supine position of the patient, antiarrhythmic drugs, tachycardia, bradycardia, chronic heart failure, chronic pulmonary disease, hyperthyroidism and hypothyroidism, and so on, can modify the standard time window of both pulmonary and systemic bronchial arterial vascularities. Indeed, a wide variability in hepatic artery arrival time, ranging from 8 to 16 s, was observed even in healthy volunteers in a study investigating the hepatic transit time in healthy subjects and patients with liver metastases[27]. It follows that a cut-off based on the standard time windows of the pulmonary and systemic bronchial arteries can be misleading in the assessment of early or delayed TE. In our study, this potential bias was avoided defining TE as early if the enhancement in the lesion was contemporaneous to that of the normal lung, and as delayed if it appeared after the enhancement of the normal lung, or contemporaneously to that of the chest wall, liver, or spleen. In this way, a true early enhancement was observed in just three neoplastic lesions, probably due to a concomitant supply from pulmonary arteries, as described in some bronchioloalveolar carcinomas and adenocarcinomas[28]. Likewise, non-neoplastic lesions showed absence of arterial enhancement or early enhancement in all cases but two, in which the delayed enhancement was likely determined by a widespread vasoconstriction caused by hypoxia[10].
Conversely, and despite the theoretical rationale in favour of a more marked arterial enhancement in non-neoplastic lesions due to the dual blood supply, EE did not result useful to distinguish neoplastic from non-neoplastic lesions, as well as all the other CEUS parameters. The presence of enhancement and the absence of wash-out (when arterial enhancement was present) showed very good sensitivity in suggesting the neoplastic and the non-neoplastic nature of the lesions, respectively, but specificity of both parameters was quite poor; and the presence of early wash-out had a 100% specificity in predicting the neoplastic nature of the lesions, but an unacceptably low sensitivity. However, we analysed also the overall CEUS behaviour including all the CEUS parameters into an arbitrary score, either to limit the possible bias consequent to the subjective evaluation of a single parameter, or to explore the possibility of further improving the good performance yielded by the delayed TE. Indeed, the score was proven to be a reliable tool to differentiate the two populations, and the cut-off of 7.5 enabled to improve the sensitivity of the single CEUS parameter “delayed TE” in discriminating between neoplastic and non-neoplastic lesions.
Our study has several limits. First, US and even more CEUS are strictly operator-dependent techniques. However, an adequate learning curve can minimize the risk of high interobserver variability; indeed, in our study it resulted fairly low and limited to two CEUS parameters. Second, because of the low number of patients enrolled into this pilot study, benign neoplastic lesions were just 2/53, and both of them showed a CEUS behaviour comparable to that of malignant lesions. Consequently, our results suggest that CEUS can discriminate between neoplastic and non-neoplastic lesions, but it does not enable to distinguish benign from malignant neoplastic lesions. Finally, US and CEUS may represent useful tools to evaluate peripheral pulmonary masses, but they can not investigate central lung lesions.
Despite these limits, this prospective pilot study suggests that CEUS could play some role in the diagnostic work-up of peripheral lung lesions. If the results will be confirmed by wider series, a lesion with a CEUS score < 7.5 could undergo clinical and instrumental follow-up, whereas invasive diagnostic procedures could be reserved to lesions with delayed TE, or a score ≥ 7.5 if TE is not delayed.
The lung has dual blood supply (pulmonary arteries and systemic bronchial arteries), and this peculiarity could theoretically be exploited by contrast-enhanced ultrasonography (CEUS) to differentiate non-neoplastic from neoplastic lesions, as the formers are supplied by both arterial systems, whereas tumor angiogenesis usually rises from bronchial arteries.
CEUS represents the best imaging method to evaluate both the vascularity and transit time within an organ, as it enables the assessment of any time of enhancement during the arterial phase. Conversely, contrast-enhanced computed tomography is an instant scanning modality, and can assess the vascularity only at a certain point in time during the arterial phase
A different time to arterial enhancement (TE) should be seen in neoplastic and non-neoplastic lesions, as the enhancement resulting from the pulmonary arteries starts before that resulting from the bronchial arteries. However, the few preliminary studies investigating the role of CEUS in the characterization of lung consolidations gave inconclusive and disappointing results. In all these studies, an arbitrary cut-off of six s after the bolus of the US contrast agent was used to define TE as early or delayed, because the time windows of pulmonary arterial vascularity and bronchial arterial vascularity usually range from 1 to 5 s, and from 8 to 11 s, respectively. However, a lot of physiological and pathological conditions can modify the standard time window of both pulmonary and bronchial arterial vascularities. Authors avoided this potential bias defining TE as early if the enhancement in the lesion was contemporaneous to that of the normal lung, and as delayed if it appeared after the enhancement of the normal lung, or contemporaneously to that of the chest wall, liver, or spleen. In this way, a true early enhancement was observed in just 3/53 neoplastic lesions, and the delayed TE showed a very high diagnostic accuracy in identifying neoplastic lesions. Moreover, a cut-off value of 7.5 obtained by a cumulative arbitrary score based on the ratio between the positive predictive values of all CEUS parameters investigated, was shown to further improve the diagnostic performance of TE.
The study results suggest that CEUS could play some role in the diagnostic work-up of peripheral lung lesions. Lesions with a CEUS score < 7.5 could undergo clinical and instrumental follow-up, whereas invasive diagnostic procedures could be reserved to lesions with delayed TE, or a score ≥ 7.5 if TE is not delayed.
Low mechanical index CEUS with second generation ultrasonography contrast agents is a technique that allows for real-time depiction of tissue micro- and microvascularity, and in the last years it is increasingly used worldwide in abdominal imaging; in particular it is recommended as the first-line technique for the characterization of focal liver lesions in most western and eastern countries.
This is an interesting and somewhat unique study to identify neoplastic lesions in the lung using contrast-enhanced ultrasound.
P- Reviewers Alicioglu B, Maruyama H, Martins WP S- Editor Gou SX L- Editor A E- Editor Liu XM
| 1. | Sartori S, Tombesi P. Emerging roles for transthoracic ultrasonography in pleuropulmonary pathology. World J Radiol. 2010;2:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Sartori S, Tombesi P. Emerging roles for transthoracic ultrasonography in pulmonary diseases. World J Radiol. 2010;2:203-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Scisca C, Rizzo M, Maisano R, Monaco M, Ferrari M, Munaò S, Zavettieri M, Bonaffini O, Mare M, Rossi RT. The role of ultrasound-guided aspiration biopsy of peripheral pulmonary nodules: our experience. Anticancer Res. 2002;22:2521-2523. [PubMed] |
| 4. | Manhire A, Charig M, Clelland C, Gleeson F, Miller R, Moss H, Pointon K, Richardson C, Sawicka E. Guidelines for radiologically guided lung biopsy. Thorax. 2003;58:920-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 306] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Tombesi P, Nielsen I, Tassinari D, Trevisani L, Abbasciano V, Sartori S. Transthoracic ultrasonography-guided core needle biopsy of pleural-based lung lesions: prospective randomized comparison between a Tru-cut-type needle and a modified Menghini-type needle. Ultraschall Med. 2009;30:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D’Onofrio M. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 498] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 7. | Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology. 2010;257:24-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 8. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11-29. [PubMed] |
| 9. | Görg C, Bert T, Kring R, Dempfle A. Transcutaneous contrast enhanced sonography of the chest for evaluation of pleural based pulmonary lesions: experience in 137 patients. Ultraschall Med. 2006;27:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Görg C, Kring R, Bert T. Transcutaneous contrast-enhanced sonography of peripheral lung lesions. AJR Am J Roentgenol. 2006;187:W420-W429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Görg C. Transcutaneous contrast-enhanced sonography of pleural-based pulmonary lesions. Eur J Radiol. 2007;64:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Görg C, Bert T, Görg K. Contrast-enhanced sonography for differential diagnosis of pleurisy and focal pleural lesions of unknown cause. Chest. 2005;128:3894-3899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Görg C, Bert T, Kring R. Contrast-enhanced sonography of the lung for differential diagnosis of atelectasis. J Ultrasound Med. 2006;25:35-39. [PubMed] |
| 14. | Sperandeo M, Sperandeo G, Varriale A, Filabozzi P, Decuzzi M, Dimitri L, Vendemiale G. Contrast-enhanced ultrasound (CEUS) for the study of peripheral lung lesions: a preliminary study. Ultrasound Med Biol. 2006;32:1467-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston: Little, Brown & Co 1994; 253–256. |
| 16. | Wernecke K. Ultrasound study of the pleura. Eur Radiol. 2000;10:1515-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Beckh S, Bölcskei PL, Lessnau KD. Real-time chest ultrasonography: a comprehensive review for the pulmonologist. Chest. 2002;122:1759-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Stević R, Jaković R, Masulović D, Nagorni-Obradović L, Mujović N, Jovanović D. [Ultrasonography in diagnosis of thoracic diseases]. Med Pregl. 2010;63:86-90. [PubMed] |
| 19. | Sconfienza LM, Mauri G, Grossi F, Truini M, Serafini G, Sardanelli F, Murolo C. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology. 2013;266:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33-59. [PubMed] |
| 21. | Sartori S, Nielsen I, Trevisani L, Tombesi P, Ceccotti P, Abbasciano V. Contrast-enhanced sonography as guidance for transthoracic biopsy of a peripheral lung lesion with large necrotic areas. J Ultrasound Med. 2004;23:133-136. [PubMed] |
| 22. | Cao BS, Wu JH, Li XL, Deng J, Liao GQ. Sonographically guided transthoracic biopsy of peripheral lung and mediastinal lesions: role of contrast-enhanced sonography. J Ultrasound Med. 2011;30:1479-1490. [PubMed] |
| 23. | Caremani M, Benci A, Lapini L, Tacconi D, Caremani A, Ciccotosto C, Magnolfi AL. Contrast enhanced ultrasonography (CEUS) in peripheral lung lesions: A study of 60 cases. J Ultrasound. 2008;11:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Zheng YL, Yin XY, Xie XY, Xu HX, Xu ZF, Liu GJ, Liang JY, Lu MD. Value of contrast-enhanced ultrasonography in assessing the vascularity of liver metastases: comparison with contrast-enhanced computed tomography. J Ultrasound Med. 2010;29:1403-1410. [PubMed] |
| 25. | Wen Q, Liu XM, Luo ZY, Chen JJ, Hong YR. [Enhancement pattern of peripheral lung carcinoma: comparison between contrast-enhanced ultrasonography and contrast-enhanced computed tomography]. Zhonghua Yixue Zazhi. 2008;88:2779-2782. [PubMed] |
| 26. | Linde HN, Holland A, Greene BH, Görg C. Contrast-enhancend sonography (CEUS) in pneumonia: typical patterns and clinical value-a retrospective study on n = 50 patients. Ultraschall Med. 2012;33:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Zhang H, He Y, Du L, Wu Y. Shorter hepatic transit time can suggest coming metastases: through-monitoring by contrast-enhanced ultrasonography? J Ultrasound Med. 2010;29:719-726. [PubMed] |
| 28. | Pezzella F, Pastorino U, Tagliabue E, Andreola S, Sozzi G, Gasparini G, Menard S, Gatter KC, Harris AL, Fox S. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol. 1997;151:1417-1423. [PubMed] |