INTRODUCTION
The association of ovarian hyperstimulation with pituitary enlargement, although established in children and prepubertal girls with primary hypothyroidism, is rare. The diagnosis is usually suspected clinically in young patients in response to some alarming signs such as growth arrest and/or precocious puberty. In adults, the diagnosis is more challenging except in pregnant women who are known to have higher risks for thyroid disorders and spontaneous ovarian hyperstimulation syndrome (OHSS). To the best of our knowledge, only five cases of ovarian hyperstimulation associated with a pituitary mass in non-pregnant adult women with primary hypothyroidism have been previously described in the literature, and our case is the first to be reported in North America[1-5].
Here, we present a new case of primary hypothyroidism complicated by spontaneous OHSS and pituitary hyperplasia mimicking macroadenoma in a non-pregnant woman. We present imaging evidence of regression of both the ovarian and pituitary masses following thyroid hormone replacement therapy.
CASE REPORT
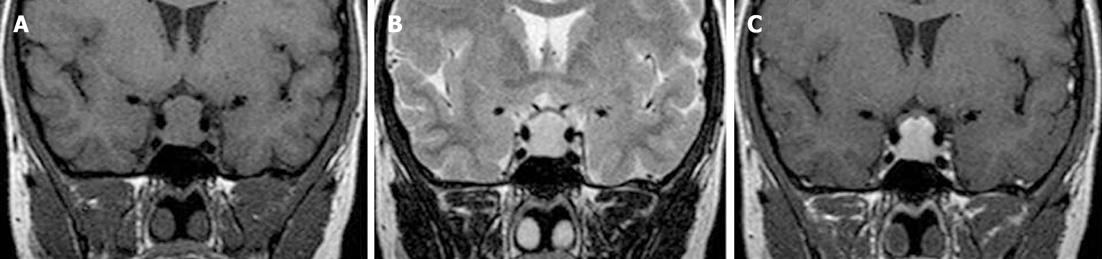
A 19-year-old woman was admitted to our hospital because of lower abdominal pain and distension. The patient had visited another clinic 3 mo before her admission to our hospital due to a 6-mo history of amenorrhea that she said appeared after she discontinued oral contraceptive therapy. At that time, she was diagnosed with a polycystic ovary syndrome (PCOS)-like disease based on clinical, laboratory (mild elevation of serum prolactin levels), and pelvic ultrasonography findings that revealed slightly enlarged ovaries with numerous peripheral follicles. Three weeks prior to the current admission, she was first referred to our department for pituitary magnetic resonance imaging (MRI) as an additional work up for hyperprolactinemia. Those MRI results revealed a 13 mm × 8 mm × 10 mm pituitary mass with suprasellar extension. This lesion was homogeneous and isointense on a T1-weighted image and hypertintense on a T2-weighted image (Figure 1A and B). Homogeneous enhancement of the mass was observed following gadolinium injection (Figure 1C). The mass was reported to be suggestive of macroadenoma by the radiologist. Upon detailed questioning, the patient also reported a long history of slight fatigue and malaise that were relieved by rest but were ignored. The rest of her personal and family medical history was otherwise unremarkable. Physical examination revealed painful facies, tender pelvic distension, and pale dry skin with hair loss but no goiter. Pelvic examination revealed slightly painful and tender masses in the Douglas pouch and the right adnexa.
Figure 1 Initial coronal T1-weighted image (A), T2-weighted image (B), and fat-saturated post-gadolinium T1-weighted image (C) showing a homogenous isointense, hyperintense, and enhancing pituitary mass, respectively, with suprasellar extension and mild compression of the optic chiasma.
Laboratory workup results at admission revealed anemia with a hemoglobin level of 106 g/L (normal range: 117-157 g/L) but normal concentration of hematocrit, platelets, and serum human β-chorionic gonadotropin. However, levels of liver enzymes, serum creatinine, and carbohydrate antigen (CA)-125 were all mildly elevated: alanine transaminase at 124 U/L (normal range: 8-29 U/L); aspartate transaminase at 111 U/L (normal range: 14-37 U/L); creatinine at 90 μm/L (normal range: 37-80 μm/L); and serum CA-125 at 87 kU/L (normal range: 0-35 kU/L).
Hormonal workup showed elevated levels of serum prolactin, at 42 μg/L (normal range: 3-18) and estradiol at 6000 pmol/L; normal levels of follicular stimulating hormone (FSH); and low levels of luteinizing hormone. An additional endocrinological workup revealed markedly elevated thyroid-stimulating hormone (TSH), at levels exceeding 100 mU/L (normal range: 0.47-4.68 mU/L), and low free T4 levels.
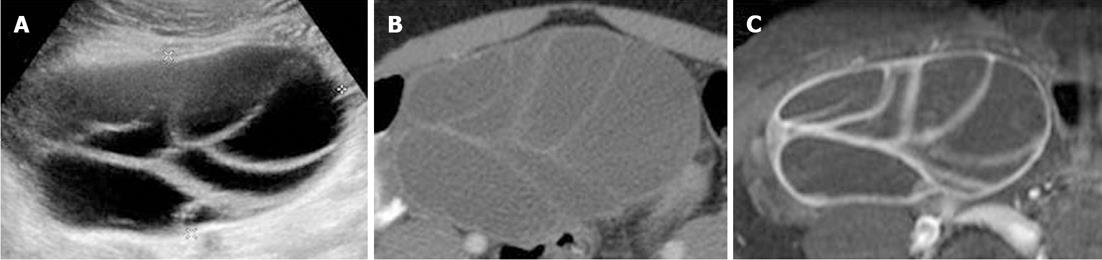
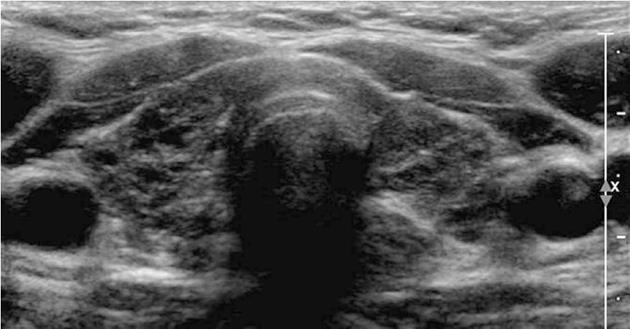
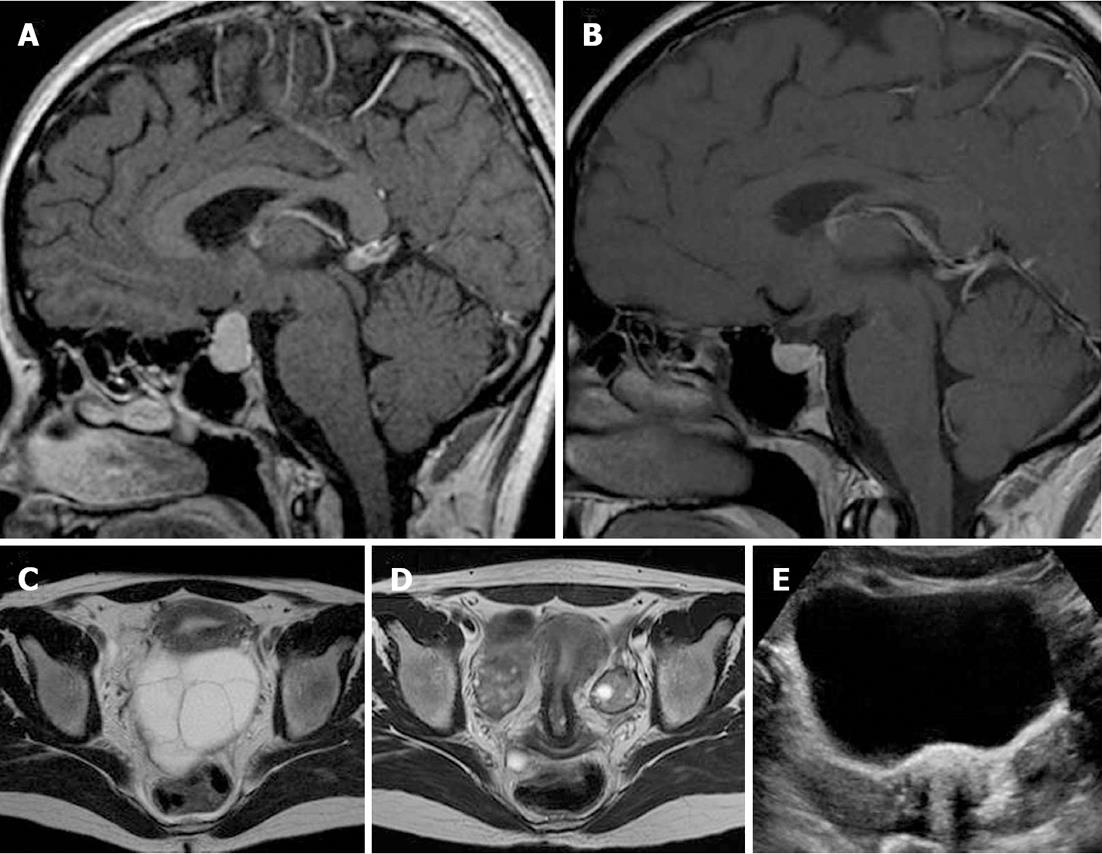
Transabdominal pelvic ultrasonography revealed massive, bilateral, mulitiseptate cystic ovarian masses with a soap-bubble or wheel-spoke appearance in both the ovaries (Figure 2A). Similar findings were observed using abdominal computed tomography (CT) and MRI (Figure 2B and C). The right cystic ovarian mass located in the iliac fossa measured 10 cm × 6 cm × 10 cm. The left ovarian mass occupying the Douglas pouch measured 9 cm × 8 cm × 8 cm. Mild ascites, pericardial effusion and a slight right pleural reaction were also found. No lymphadenopathy or focal liver lesions were noted. Thyroid ultrasonography (Figure 3) revealed alteration in the echotexture of the thyroid gland, consistent with thyroiditis. Based on clinical, laboratory, and imaging findings, we considered a diagnosis of primary hypothyroidism owing to the thyroiditis complicated by spontaneous OHSS and compensatory pituitary hyperplasia. The patient was started on thyroid hormone therapy. She improved clinically and was discharged 6 d later with a prescription of levothyroxine. Follow-up imaging 4 mo later revealed regression of both the pituitary tumor and the ovarian cystic masses (Figure 4).
Figure 2 Initial axial transabdominal pelvic ultrasonography (A), contrast-enhanced CT (B), and fat-saturated post-gadolinium T1-weighted image (C) showing a right ovarian cystic mass with wheel-spoke appearance.
Similar results were observed in the left ovary (data not shown here).
Figure 3 Thyroid ultrasound image showing a marked heterogeneous hypoechoic gland with a small left lobe.
Figure 4 Sellar sagittal MR images with gadolinium administration before (A) and after (B) thyroid hormone replacement therapy and pelvic axial T2-weighted images before (C) and after (D) thyroid hormone replacement therapy revealed regression of the pituitary mass and ovarian cystic enlargement of both ovaries.
E: A corresponding transabdominal ultrasound image after thyroid therapy demonstrating regression of both ovarian cystic masses.
DISCUSSION
OHSS is a rare but well-known iatrogenic complication of ovarian stimulation occurring during fertility therapy[6]. In extremely rare cases, this syndrome can occur without any iatrogenic induction of ovulation. In this case, it is called spontaneous OHSS, which has often been reported in association with pregnancy, FSH-secreting adenoma, or exceptionally TSH-secreting macroadenoma[7]. Only a few cases of hypothyroidism have been described in the literature[1-5,8]. The pathogenesis of OHSS remains unclear. It has been postulated that it might involve the activation of some mediators and vasoactive substances such as histamine, serotonin, prostaglandins, interleukins, estrogen, prolactin, the ovarian renin-angiotensin system, and vascular endothelial growth factor (VEGF). Today, there is growing evidence suggesting a key role of VEGF[9], presumably of a follicular origin, in the pathogenesis of OHSS. Under this model, exogenous gonadotropins or other substances with gonadotropin-like activity can activate the mediators, leading to increased vascular permeability, extravascular fluid accumulation, hemoconcentration, deep vein thrombosis, and other complications observed in OHSS. Moreover, the exact mechanism by which OHSS might occur in the context of hypothyroidism also remains unclear. Different mechanisms have been proposed: (1) TSH-mediated stimulation of the FSH receptor with[9] or without FSH receptor mutation[10], taking into account that TSH has weak FSH activity (this is the likely mechanism in our case); and (2) the preferential formation of estriol in patients with hypothyroidism, which results in excessive gonadotropin release (estriol being a weaker suppressor of gonadotropin release than estradiol)[10]. The main radiological findings in OHSS are multiple cystic enlargements of both ovaries (> 5 cm) associated with fluid accumulation in the peritoneum, pleura, or pericardium. However, these findings are not very specific to this particular disorder because some other conditions (including ovarian cystic neoplasm as serous or mucinous tumors) often display similar characteristics. Therefore, to improve the differential diagnosis based on imaging findings, radiologists should search for the classic soap-bubble or wheel-spoke appearance of the ovarian cystic mass, which results from multiple, enlarged follicles arranged peripherally around a central stroma[11]. Such findings are consistent with a benign cystic mass or a borderline ovarian neoplasm and they are often observed in cases of OHSS. In the case presented here, the soap-bubble appearance of the ovarian cystic mass was demonstrated by transabdominal pelvic ultrasonography, CT and MRI. Considering the appearance of the ovaries after the regression of cystic enlargement (Figure 4D and E), the pseudosepta observed between the cysts appears to have resulted from normal stroma that, due to OHSS, had been extremely compressed between enlarged follicles. Patients with a history of PCOS-like ovaries, such as that in the present patient, are known to have a predisposition for developing this feature in an OHSS setting. In our patient, an elevation of serum CA-125 level was observed, and this could be responsible for stimulating the underlying malignancy, an observation reported before in similar conditions[12]. In fact, several benign conditions, including hypothyroidism, have been recognized as nonmalignant causes of elevated serum CA-125 levels. Theoretically, this may be due to increased secretion or decreased excretion of CA-125 levels. In addition, all conditions that irritate serous membranes (including the peritoneum, pleura, or pericardium) may also increase serum CA-125 levels.
Pituitary hyperplasia is an enlargement of the pituitary gland due to a reversible increase in the number and/or hyperplasia in one or more hormone-producing cell types. It can occur as a normal response to physiological stimulation during infancy, pregnancy and lactation, or as a pathological condition. In primary hypothyroidism, longstanding increases in thyrotropin-releasing hormone levels due to a lack of thyroid hormone induces both thyrotroph and lactophore cell hyperplasia, resulting in TSH and prolactin oversecretion[13]. In the patient presented in this study, pituitary MRI was scheduled after the detection of elevated prolactin levels. The mass was isointense on T1-weighted images and hyperintense on T2-weighted images. Gadolinium-enhanced MRI revealed a homogenously enhancing mass .These findings are similar to those described in a few studies undertaken in pediatric patients[14,15]. Without knowledge of the present patient’s hormonal profile, this mass was first reported as a macroadenoma. Later, with evidence of the patient’s primary hypothyroidism, we considered a diagnosis of reactive pituitary hyperplasia, which was definitively confirmed by the regression of the patient’s mass after thyroid hormone replacement therapy. The main differential diagnosis was a TSH-secreting macroadenoma, which is an extremely rare tumor that would not have shrunk in response to thyroid hormone replacement therapy. In fact, despite recent progress in medical imaging, to date, key imaging techniques including MRI, cannot distinguish pituitary macroadenoma from hyperplasia. Further studies will be needed to elucidate the possible distinguishing features of this condition.
We present a new case of adult primary hypothyroidism complicated by spontaneous ovarian hyperstimulation and pituitary hyperplasia in a non-pregnant woman. The patient’s condition regressed after thyroid hormone replacement therapy. This case highlights the need for clinicians and radiologists to familiarize themselves with the clinical and imaging features detected in this rare medical condition. Such improved knowledge will help to avoid delays in diagnosis and prevent unnecessary surgery.