Published online Sep 28, 2012. doi: 10.4329/wjr.v4.i9.391
Revised: September 3, 2012
Accepted: September 10, 2012
Published online: September 28, 2012
Percutaneous angioplasty and stenting for the treatment of extracranial vertebral artery (VA) stenosis seems a safe, effective and useful technique for resolving symptoms and improving blood flow to the posterior circulation, with a low complication rate and good long-term results. In patients with severe tortuosity of the vessel, stent placement is a real challenge. The new coronary balloon-expandable stents may be preferred. A large variability of restenosis rates has been reported. Drug-eluting stents may be the solution. After a comprehensive review of the literature, it can be concluded that percutaneous angioplasty and stenting of extracranial VA stenosis is technically feasible, but there is insufficient evidence from randomized trials to demonstrate that endovascular management is superior to best medical management.
- Citation: Kocak B, Korkmazer B, Islak C, Kocer N, Kizilkilic O. Endovascular treatment of extracranial vertebral artery stenosis. World J Radiol 2012; 4(9): 391-400
- URL: https://www.wjgnet.com/1949-8470/full/v4/i9/391.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i9.391
About 25% of ischemic strokes occur in the vertebrobasilar territory[1,2]. Around one fifth of posterior circulation strokes occur in the setting of extracranial vertebral artery (VA) stenosis[3-6]. VA stenosis may occur either extra- or intra-cranially, but it is often localized at the origin of the vessel as it arises from the subclavian artery[7]. In a large series which included 4748 patients with ischemic stroke, some degree of proximal extracranial VA stenosis was seen in 18% of cases on the right and 22.5% on the left[8]. This is the second most common location of stenosis after internal carotid artery stenosis at the carotid bifurcation[9].
There are three treatment options for extracranial VA stenosis: medical, surgical and endovascular. Recently, management of the VA stenosis has shifted to percutaneous techniques with the evolution of endovascular device technology. The first VA intraluminal angioplasty was reported by Sundt et al[10] in 1980. Since then, multiple case reports and clinical series have described the use of balloon angioplasty and stenting to treat vertebrobasilar atherosclerotic disease[11-20]. This review focuses on the endovascular treatment of extracranial VA stenosis.
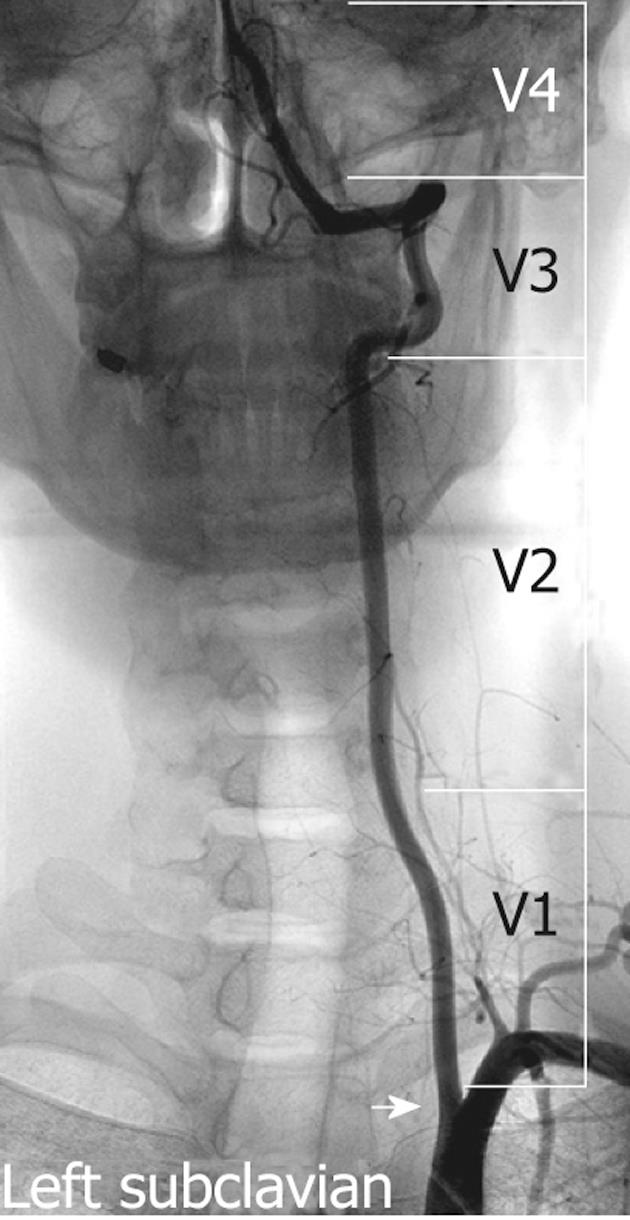
The VA is typically divided into four segments: V1, V2, V3 and V4 (Figure 1). The first 3 of these are extracranial and the last segment is intracranial. The first segment (V1) begins from the origin of the VA and extends between the longus colli and scalenus anterior muscles to where it enters the transverse foramina at the fifth or sixth cervical vertebra. The second segment (V2) begins from the level of the fifth or sixth cervical vertebra to the second cervical vertebra travelling through the transverse foramina at each vertebral level, with an alternating intra and inter-osseous course. On account of having such a unique anatomic environment, the V2 segment may be exposed to the extrinsic compression from spondylotic exostosis of the spine[21]. This segment can be extremely tortuous, which can make the placement of a stent in the mid or distal extracranial VA difficult[22]. The third segment (V3) extends between the C2 transverse process and base of the skull where it enters the foramen magnum. The last segment (V4) extends from the point at which the arteries enter the dura to the termination of both VAs at the vertebrobasilar junction.
The branches of the VA can be classified as cervical and cranial. The cervical VA produces spinal and muscular branches. The muscular branches typically originate from the second (V2) and third (V3) segments of the VA. These branches typically supply the dorsal cervical musculature and are best visualized in the presence of the common carotid artery or VA occlusion. Small branches from the second segment (V2) may anastomose with spinal arteries. Branches of the V3 segment typically anastomose with a branch of the occipital artery. The cranial branches of the VA are meningeal, posterior spinal, anterior spinal, posterior inferior cerebellar artery (PICA) and medullary arteries. The PICA is the largest branch of the VA, coursing backward to the inferior surface of the cerebellum. It is divided into medial and lateral branches and may anastomose with the anterior cerebellar artery and superior cerebellar artery of the basilar artery.
Recognizing the location of these branches is important to avoid perforation during catheter or wire manipulation.
Anatomic variants of VA are much more common than those of the carotid artery and most often involve the origin of VA from the aortic arch in V1 segment or the distal branches in V3 and V4 segments[21]. The VA generally arises from the superior-posterior aspect of the first part of the subclavian artery. However, in approximately 5%-6% of cases, the left VA arises directly from the aortic arch between the left common carotid artery and the left subclavian artery[23]. On rare occasions, the right VA may arise distal to the left subclavian artery or from the right common carotid artery (0.18%)[24,25]. In 50% of individuals, the diameter of the left VA is larger than the right VA diameter[21]. In 25% of individuals, the VA diameters are equal to each other. On the other hand, in approximately 10% of individuals one VA is prominently smaller in diameter than the other[21]. In these kinds of cases, the smaller VA may terminate in the PICA or have a hypoplastic segment between the PICA and basilar artery that contributes little to basilar artery blood flow[21].
The PICA generally originates from the intradural segment of the VA, but may alternatively originate from the extracranial segment[22]. The PICA may be absent unilaterally or bilaterally, in approximately 20% and 2% of individuals, respectively. In 1% of cases, the VA terminates in the PICA[26].
In the literature, the incidence of normal entrance to the transverse foramina ranges from 90% to 93%[22]. Additionally, it may enter into the transverse foramen at other levels than C6[27]. The prevalence of VA entry at C4 level ranges from 0.5% to 1.3% and at C5 level ranges from 5% to 6.6%, which often makes the V1 segment longer[27,28]. However, it may enter at the C7 level (0.8%-5.4%), which makes the V1 segment shorter.
Tortuosity of the VA from the origin to the transverse foramen is another variation. Matula et al[29] reported 47.2% tortuosity of the V1 segment. It is important in endovascular procedures, because severe tortuosity of the proximal V1 segment combined with a stenosis can preclude safe stent placement. Moreover, it was also reported as an independent and significant predictor of in-stent restenosis owing to unnatural straightening of the tortuous segment[30].
These anatomic variations must be considered in clinical assessment and treatment.
Atherosclerosis is the most common cause of extracranial VA stenosis. However, an atherosclerotic plaque situated at the VA origin is considered to be less prone to ulceration and smoother than that seen at the carotid system[31]. The less common causes of extracranial VA stenosis are arterial dissection, extrinsic compression due to trauma, osteophytes, fibrous bands and vasculitis (most commonly in giant cell arteritis)[9].
The most common mechanism of stroke in patients with VA stenosis is intra-arterial embolism, rather than hemodynamic failure[6]. Hemodynamic stroke, however, is less commonly caused by VA stenosis, because both VAs feed into one basilar artery[7]. Also, in contrast to the internal carotid artery, the VA gives off numerous branches at the neck region, therefore facilitating a considerable collateral blood supply, which often reconstitutes the distal artery after occlusion at the origin[7].
Individuals with occlusive disease of proximal segments of the VA are at relatively high risk for posterior or vertebrobasilar circulation ischemia[6]. Indeed, a systematic review suggested that patients with symptomatic VA stenosis may have a greater recurrent stroke risk in the first 7 d after symptoms onset than patients with recently symptomatic carotid stenosis[32]. Nevertheless, the best medical therapy for these patients is unclear, and the precise role of invasive treatment remains uncertain[33].
The American Heart Association/American Stroke Association guideline recommends an evidence-based diagnostic approach for diagnosing VA disease[21]. The recommendations are presented in Table 1. In this guideline, 11 studies about comparing noninvasive methods and digital subtraction angiography (DSA) for the detection of VA stenosis have been systematically reviewed. According to these studies, computed tomography angiography (CTA) and contrast-enhanced magnetic resonance angiography (MRA) were associated with higher sensitivity (94%) and specificity (95%) than Doppler ultrasonography (US) (sensitivity 70%)[21]. Also, of these noninvasive imaging methods, CTA had slightly superior accuracy[21]. Doppler US is relatively less suitable and technically difficult for detection of VA stenosis in related anatomic regions. DSA as a noninvasive method is typically required before revascularization for patients with symptomatic posterior cerebral ischemia, because of the fact that neither MRA nor CTA reliably delineates the origins of the VAs[34]. Thus the gold standard for diagnosing VA stenosis remains DSA, although it has a small morbidity and associated mortality. The complications of DSA associated with morbidity and mortality can be divided into two major groups: clinical and technical. The former includes groin hematoma, contrast medium reaction, transient neurological event and permanent neurological deficit; the latter includes carotid and VA dissection and femoral or iliac artery dissection[35]. Although the overall neurological complication rate related to cerebral angiography is 1.5%[36], recent reports using diffusion-weighted imaging suggest that there is a much higher rate of subclinical neurological events[37-40].
| Size of treatment effect | Indications | Recommended imaging study | Level of evidence (A,B,C1) |
| Class 1 (benefit>>>risk) | |||
| 1 | -With symptoms referable to posterior circulation and subclavian steal syndrome | Noninvasive imaging | C |
| 2 | -With asymptomatic bilateral carotid occlusions or-Unilateral carotid artery occlusion and incomplete circle of Willis | Noninvasive imaging | C |
| 3 | -With symptoms of posterior cerebral ischemia or -With symptoms of cerebellar ischemia | Noninvasive imaging (MRA or CTA rather than Doppler US) | C |
| Class 2a (benefit>>risk) | |||
| 1 | -With symptoms of posterior cerebral ischemia or -With symptoms of cerebellar ischemia | Serial noninvasive imaging | C |
| 2 | -Revascularization candidates with symptoms of posterior cerebral ischemia or -Revascularization candidates with symptoms of cerebellar ischemia | -Noninvasive imaging -DSA (if noninvasive imaging fails) | C |
| 3 | -Patients undergone vertebral artery revascularization | -Serial noninvasive imaging | C |
Patients with posterior circulation ischemic symptoms that are present despite optimal medical therapy and VA origin stenosis greater than 50% demonstrated at DSA are considered for endovascular therapy.
The treatment of asymptomatic patients with significant stenosis of VA origin is a subject of controversy. Although most asymptomatic patients do not require endovascular treatment, some investigators believe that high-grade stenosis (greater than 70%) affecting the origin of a dominant or single VA should be treated because of increased risk of stroke[22]. Other investigators believe asymptomatic patients should be treated when the necessity of collateral support is of major importance, as in cases of carotid occlusion[41].
Although optimum management of patients with VA stenosis is not well established in the literature, treatment options fundamentally include medical, surgical and endovascular therapies. For all patients with VA stenosis, optimal medical therapy should include risk factor modifications, antiplatelet and statin therapies[21]. In patients with ongoing symptoms despite optimal medical treatment, endovascular and surgical options should be considered.
Aspirin (81-325 mg daily), clopidogrel (75 mg daily) or the combination of aspirin and extended-release dipyridamole (25 and 200 mg twice daily, respectively) are acceptable options. Combination antiplatelet regimens (e.g., aspirin and clopidogrel) are emerging as the mainstay of medical therapy for patients with vertebrobasilar insufficiency. A combination of aspirin and dipyridamole was shown to significantly reduce the rate of stroke in patients with vertebrobasilar insufficiency compared to placebo[42]. Selection of an antiplatelet regimen should be individualized on the basis of patient risk factor profiles, cost, tolerance, resistance and other clinical characteristics[43-48].
Surgery to this region of the VA is technically difficult due to poor access to the vessel origin, hence surgery is not considered in most centers. It may be the only viable treatment option in those patients who fail medical therapy but have lesions or anatomy that are unfavorable for angioplasty and/or stent therapy. In the study by Buerger et al[49] that includes 369 consecutive extracranial VA reconstructions, stroke and death rates of the procedure were found to be low (5.1% in the 215 patients treated before 1991 and 1.9% in the 154 patients treated since 1991). The combined morbidity and mortality rates of surgical therapy for VA stenosis range from 10% to 20% and have dampened enthusiasm for this option[50-55]. Horner’s syndrome and lymphocele are considerable postoperative complications of the surgery.
Conscious sedation is not used as a rule, because it may mimic some of the posterior circulation stroke symptoms. Therefore patients are kept awake during the procedure so that it allows prompt assessment of the neurologic symptoms. The pretreatment antiplatelet regimen is extremely important to decrease the risk of stent thrombosis after endothelial injury or plaque rupture following angioplasty and stenting[22]. Aspirin (325 mg daily) should be started at least 3 d before the procedure and continued indefinitely[55]. Clopidogrel (75 mg/d) should be administered 5-7 d before the procedure. On the other hand, a suitable alternative is to give a 600 mg loading dose at least 2 h before the intervention. In addition, novel thienopyridines may provide a therapeutic advantage over clopidogrel. For instance, prasugrel has twice the platelet inhibitory effect of clopidogrel with much less inter-individual variability in platelet response to ADP[56,57]. Unfractionated heparin (50-100 U/kg) is given intravenously after the sheath is inserted and before the angiography to maintain the activated clotting time between 250 and 300 s. Alternatively, low molecular weight heparin (enoxaparin 1 mg/kg, subcutaneously) may be used, but no published data are available. Hirudin derivatives may also be used in place of heparin (a bolus of 0.75 mg/kg followed by an infusion of 1.75 mg/kg per hour for the duration of the procedure), although this has also not been evaluated for vertebral interventions.
With regard to the antiplatelet resistance, response to antiplatelet medications may vary among individuals. It can be assessed with several techniques including light transmittance aggregometry, flow cytometry and point-of-care assays[58-60]. In patients with antiplatelet resistance, higher doses of oral antiplatelet therapies (especially for clopidogrel) or more potent antiplatelet therapies (e.g., GP IIb/IIIa inhibitors or novel thienopyridines) may be required to optimize outcomes[59-61].
Most of the interventions are performed via the femoral artery approach. A 6-8 Fr sheath and a 5 Fr diagnostic catheter are usually sufficient to perform a DSA. If bilateral occlusive iliac disease is present, access may be obtained via the ipsilateral brachial artery or radial artery (e.g., right vertebral stenosis, right brachial artery access)[62,63].The transradial approach has been proposed recently[62,63]. Advantages of this approach include easy hemostasis and comfort to the patient so that the patient is able to sit and walk immediately after the procedure[63]. To perform this approach, the patient must have adequate ulnar arterial supply to the hand to prevent ischemia of the hand due to occlusion of the radial artery. Ulnar arterial supply can be assessed before the procedure with the Allen test or Doppler US.
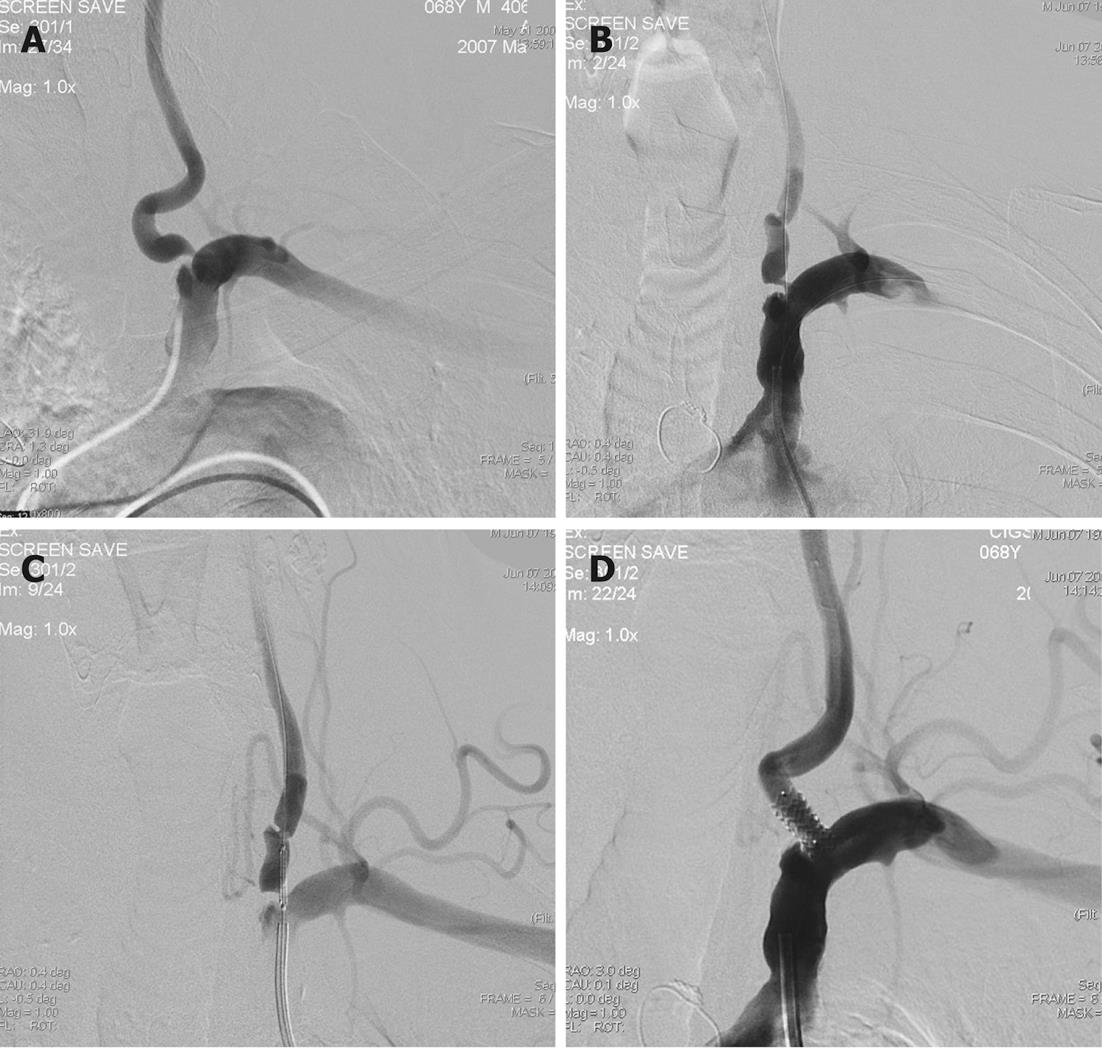
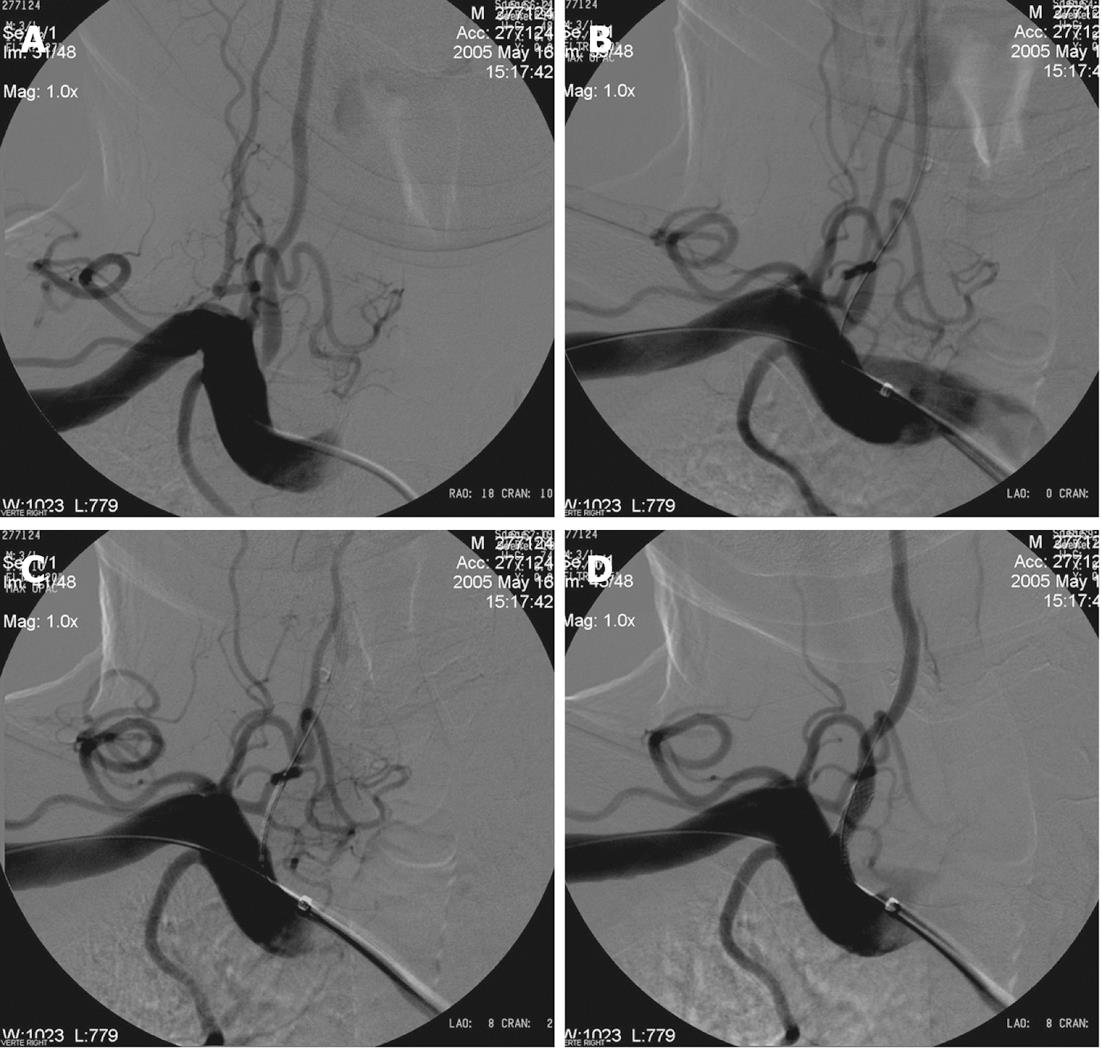
Either a guide or sheath approach is suitable for treatment of V1-V3 segment stenosis. A sheath approach requires a 6 Fr system. A guide approach requires typically an 8 Fr system although a 6-7 Fr system may be suitable if a coronary balloon-expandable stent is used. Using a standard hydrophilic guide wire and a 6F guide catheter, the target subclavian artery is catheterized and the guide catheter is advanced to just proximal to the origin of the VA. The 6F guide catheter usually provides adequate stability (Figure 2). For a tortuous subclavian artery, a 0.014-inch buddy wire[64] or a large caliber coronary guiding catheter[65] may be left in place in the subclavian artery for support (Figure 3). Biplane road map images are then obtained and the stenosis is crossed with a curved-tip 0.014-inch or 0.018-inch guide wire. The curved tip helps to negotiate the stenosis and prevent subintimal dissection at the site of stenosis or distal segments within the VA. The wire tip is positioned in the distal cervical VA within the fluoroscopic field-of-view, providing additional stability to the system. Operators may decide to use an embolus protection device instead. The degree of stenosis is determined in relation to the diameter of the normal segment of vessel immediately distal to the stenosis. Angioplasty with a small balloon may be necessary for very tight stenosis to allow good positioning of the definitive balloon stent system. Using of coronary balloon-expandable stents to treat stenosis of VA origin is much more common than the others because of accuracy in placement. They have a good combination of adequate radial force, low crossing profile and limited foreshortening. Recently, drug-eluting stents (DES) (sirolimus or paclitaxel coating) have been produced which are useful especially if the patient is diabetic. It is noteworthy that there is very limited data with only a few patients on the use of DES in the VA. The expectation from DES is a decrease in restenosis through inhibition of smooth muscle and endothelial proliferation. Although experience described in the coronary literature largely supports such a practice, DES in cardiac procedures have recently been found to be associated with clot formation in some cases, resulting in thrombosis at the stent site[66,67]. On the other hand, there are self-expanding stents, but they suffer from size limitations of currently available stent diameter and occasional misplacement of the stent requiring placement of an additional stent[68]. The use of monorail or over-the-wire systems depends on the experience and comfort level of the operator. Therefore, in patients with severe tortuosity of the vessel in whom support may be an issue, a coronary stent may be preferred. The stent length should be enough to extend proximally 1 mm to 2 mm into the lumen of the ipsilateral subclavian artery and at least 3 mm into the normal distal VA, covering the entire lesion. For stenosis involving the V2 segment, because of the fixed bony location, the coronary balloon-expandable stents can be chosen. For stenosis involving the V3 segment, a nitinol self-expanding stent is suitable because of vessel tortuosity. After positioning of the stent, an angiogram is performed in the working projection (used to deploy the stent) to document the technical result of the procedure. The final angiogram is compared with the initial pre-procedure angiogram.
The success of the intervention depends on anatomy and collateral vasculature of the individual patient. It is sometimes acceptable to have a residual 50% stenosis if the risks of re-intervention outweigh observation and it should be noted that these patients often do well even with a 50% stenosis.
Clopidogrel (75 mg daily) should be continued for at least 1 mo with bare metal stents. If DESs are used it should be continued for 6 mo to 1 year based on coronary artery intervention data[69,70]. Aspirin (80-325 mg daily) should be administered indefinitely.
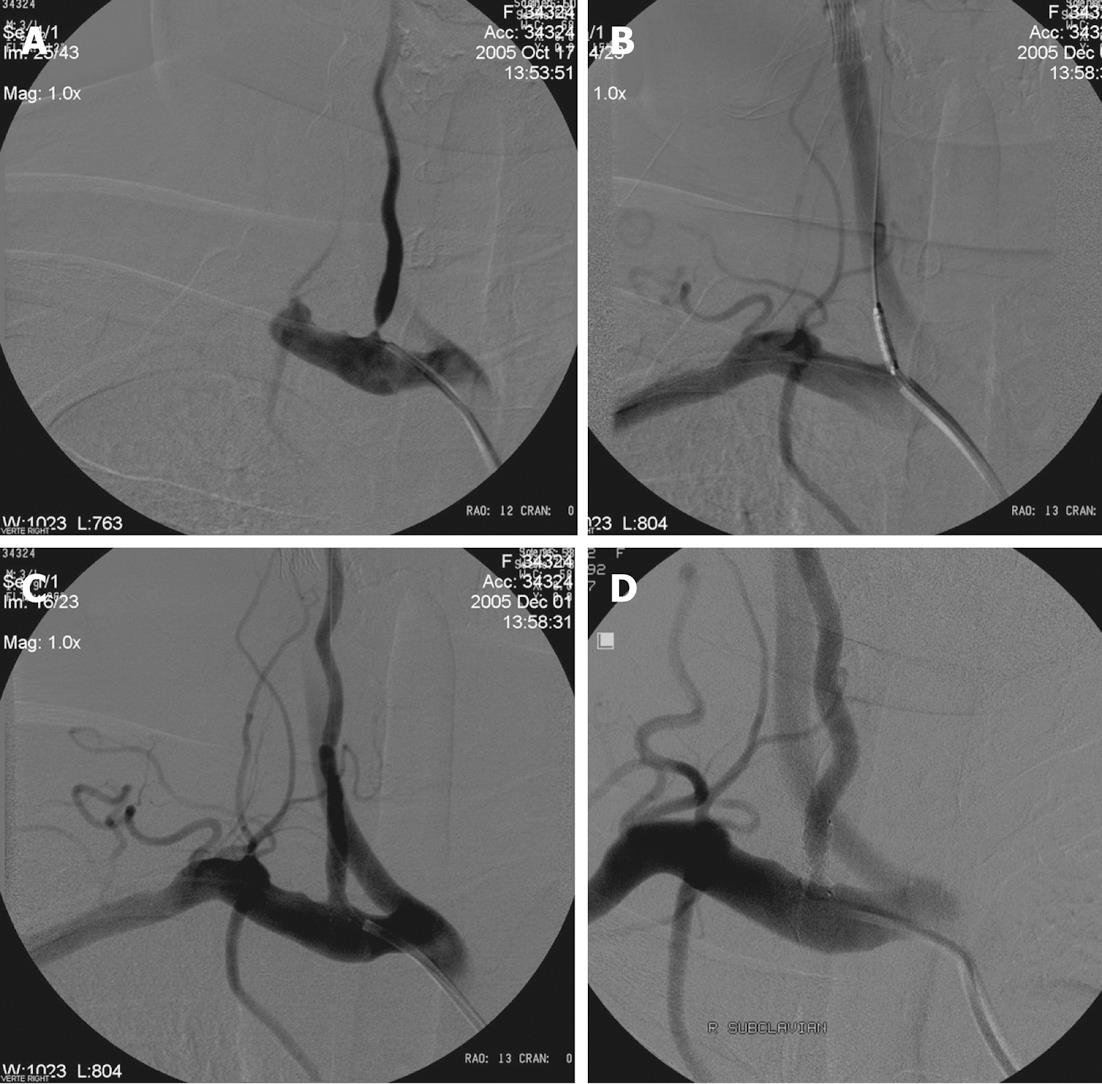
DSA is the gold standard for the follow-up of VA stenting procedures, although it has a small morbidity and associated mortality[71-73]. Despite the fact that no standardized follow-up is recognized, timing for DSA after intervention ranges from 3-12 mo (Figure 4).
Doppler US should be performed for VA stents within the ostial (V1) or proximal region (V2) every 6 mo for the first year and yearly thereafter as a non-invasive procedure. Additionally CTA and MRA can be used; however, neither of these reliably delineates the VA orifice and visualizes the stent well.
Based on published VA data, the best treatment option of extracranial VA stent is still uncertain. Endovascular treatment of extracranial VA stent remains a major challenge with unsatisfactory long term results and a lack of randomized controlled trials. A review of previous studies on stent treatment of extracranial VA is given in Table 2. In all studies, low technical and clinical complication rates indicate that stenting of extracranial VA is feasible. On the other hand, the rate of significant restenosis (greater than 50%) after either bare metal or drug-eluting stent placement is extremely variable in the literature. After bare stent placement, the rate can reach 48% and when DES are used it can reach 63%[79,86]. These large variations in the restenosis rates may have been affected by case series, duration, post-procedural medical therapy and/or diameter of the stent. In the study of Zhou et al[30], restenosis rates were found to be associated with tortuosity of extracranial VA and diameter of stent.
| Studies | Stent type | Number of patients | Number of treated stenoses | Rate of complication (%) | Follow-up | ||
| Technical | Clinical | Period (mo) | Rate of significant restenosis2 (%) | ||||
| Lin et al[74] | Bare stent | 58 | 67 | 0 | 7 | 11 | 25 |
| Albuquerque et al[11] | Bare stent | 33 | 33 | 3 | 0 | 16 | 43 |
| Chastain et al[12] | Bare stent | 50 | 55 | 2 | 0 | 6 | 10 |
| Weber et al[75] | Bare stent | 38 | 38 | 2 | 1 | 11 | 38 |
| Cloud et al[76] | Bare stent | 14 | 14 | 0 | 0 | 20 | 9 |
| SSYLVIA1 study[77] | Bare stent | 18 | 18 | - | - | 6 | 43 |
| Akins et al[78] | Bare stent | 7 | 7 | 0 | 0 | 36 | 43 |
| Taylor et al[79] | Bare stent | 44 | 48 | 0 | 0 | 7 | 48 |
| Hatano et al[80] | Bare stent | 117 | 117 | 0 | 0 | 6 | 10 |
| Lin et al[81] | Bare stent | 80 | 90 | 0 | 0 | 12 | 28 |
| Jenkins et al[16] | Bare stent | 32 | 38 | 0 | 0 | 10 | 3 |
| Karamehev et al[82] | Bare stent, DES | 10 | 12 | 0 | 0 | 34 | 10 |
| Lin et al[27] | DES | 11 | 11 | 0 | 0 | 8 | 0 |
| Zhou et al[30] | DES | 86 | 92 | 0 | 0 | 12 | 17 |
| Gupta et al[83] | DES | 27 | 27 | 0 | 2 | 6 | 7 |
| Akins et al[78] | DES | 5 | 5 | 0 | 0 | 17 | 0 |
| Edgell et al[84] | DES | 5 | 5 | 0 | 0 | 15 | 0 |
| Vajda et al[85] | DES | 48 | 52 | 0 | 0 | 7 | 12 |
| Lugmayr et al[86] | DES | 7 | 8 | 0 | 0 | 6 | 63 |
In a review of 300 interventions for proximal VA stenosis, the risk of death was 0.3%, the risk of periprocedural neurological complications was 5.5%, and the risk of posterior system stroke was 0.7% at a mean follow-up of 14.2 mo. Restenosis occurred in 26% of cases (range: 0%-43%) after a mean of 12 mo (range: 3-25 mo), although restenosis was not consistently correlated with recurrent symptoms[14]. In a Cochrane review of 313 cases of VA intervention, 173 cases which underwent a VA stenting procedure were identified from 20 studies[87]. Analysis of these studies revealed a 30-d major stroke and death rate of 3.2% and a 30-d transient ischemic attack and non-disabling stroke rate of 3.2%[87]. These analyses suggest that VA stenting is safe and effective.
Recently, there have been some studies about use of embolism protection devices to preclude embolization during angioplasty and stenting of VA origin. Qureshi et al[88] treated 12 patients using distal protection devices; they had technical success in 11 patients, and no stroke or death was observed at 1-mo follow-up. This study demonstrated that angioplasty and stenting of the VA orifice using an embolism protection device is feasible and safe. Wehman et al[22] recommended the use of an embolism protection device for larger VAs (diameter greater than 3.5 mm), in patients that have a favorable angle of the VA orifice and treatment of ulcerated lesions. These studies demonstrated that angioplasty and stenting of the VA orifice using embolism protection devices is feasible and safe but widespread use of embolism protection devices needs further study on their efficacy.
In fact, in a literature review, only one completed randomized trial, Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS), had been published, comparing percutaneous intervention and medical treatment for VA disease[89]. In the CAVATAS study (n = 16), 8 patients were randomized to medical therapy, and 8 patients underwent successful endovascular stenting, with no strokes or death occurring within 30 d in either group. At a mean follow-up of 4.7 years, there were no vertebral strokes in either group, thus there was no difference in outcomes among patients treated by stenting and medical therapy. Although data from small case series have demonstrated safety and acceptable long-term patency, the long-term clinical efficacy, especially the reduction of posterior circulation strokes, is still unknown and would require randomized comparison with best medical therapy.
In conclusion, although angioplasty and stenting of the vertebral vessels are technically feasible, there is insufficient evidence from randomized trials to demonstrate that endovascular management is superior to best medical management.
Peer reviewer: Kennith F Layton, MD, FAHA, Director of Interventional Neuroradiology, Department of Radiology, Baylor University Medical Center, 3500 Gaston Avenue, Dallas, TX 75246, United States
S- Editor Cheng JX L- Editor O’Neill M E- Editor Xiong L
| 1. | Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 2328] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 2. | Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 732] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 3. | Caplan LR, Amarenco P, Rosengart A, Lafranchise EF, Teal PA, Belkin M, DeWitt LD, Pessin MS. Embolism from vertebral artery origin occlusive disease. Neurology. 1992;42:1505-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 113] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Koroshetz WJ, Ropper AH. Artery-to-artery embolism causing stroke in the posterior circulation. Neurology. 1987;37:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Pessin MS, Daneault N, Kwan ES, Eisengart MA, Caplan LR. Local embolism from vertebral artery occlusion. Stroke. 1988;19:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Wityk RJ, Chang HM, Rosengart A, Han WC, DeWitt LD, Pessin MS, Caplan LR. Proximal extracranial vertebral artery disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 1998;55:470-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 161] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Caplan L. Posterior circulation ischemia: then, now, and tomorrow. The Thomas Willis Lecture-2000. Stroke. 2000;31:2011-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Hass WK, Fields WS, North RR, Kircheff II, Chase NE, Bauer RB. Joint study of extracranial arterial occlusion. II. Arteriography, techniques, sites, and complications. JAMA. 1968;203:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 283] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Cloud GC, Markus HS. Diagnosis and management of vertebral artery stenosis. QJM. 2003;96:27-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Sundt TM, Smith HC, Campbell JK, Vlietstra RE, Cucchiara RF, Stanson AW. Transluminal angioplasty for basilar artery stenosis. Mayo Clin Proc. 1980;55:673-680. [PubMed] |
| 11. | Albuquerque FC, Fiorella D, Han P, Spetzler RF, McDougall CG. A reappraisal of angioplasty and stenting for the treatment of vertebral origin stenosis. Neurosurgery. 2003;53:607-614; discussion 614-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Chastain HD, Campbell MS, Iyer S, Roubin GS, Vitek J, Mathur A, Al-Mubarak NA, Terry JB, Yates V, Kretzer K. Extracranial vertebral artery stent placement: in-hospital and follow-up results. J Neurosurg. 1999;91:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 13. | Dabus G, Moran CJ, Derdeyn CP, Cross DT. Endovascular treatment of vertebral artery-origin and innominate/subclavian disease: indications and technique. Neuroimaging Clin N Am. 2007;17:381-392, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Eberhardt O, Naegele T, Raygrotzki S, Weller M, Ernemann U. Stenting of vertebrobasilar arteries in symptomatic atherosclerotic disease and acute occlusion: case series and review of the literature. J Vasc Surg. 2006;43:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Henry M, Polydorou A, Henry I, Ad Polydorou I, Hugel IM, Anagnostopoulou S. Angioplasty and stenting of extracranial vertebral artery stenosis. Int Angiol. 2005;24:311-324. [PubMed] |
| 16. | Jenkins JS, White CJ, Ramee SR, Collins TJ, Chilakamarri VK, McKinley KL, Jain SP. Vertebral artery stenting. Catheter Cardiovasc Interv. 2001;54:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Malek AM, Higashida RT, Phatouros CC, Lempert TE, Meyers PM, Gress DR, Dowd CF, Halbach VV. Treatment of posterior circulation ischemia with extracranial percutaneous balloon angioplasty and stent placement. Stroke. 1999;30:2073-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Motarjeme A. Percutaneous transluminal angioplasty of supra-aortic vessels. J Endovasc Surg. 1996;3:171-181. [PubMed] |
| 19. | Mukherjee D, Roffi M, Kapadia SR, Bhatt DL, Bajzer C, Ziada KM, Kalahasti V, Hughes K, Yadav JS. Percutaneous intervention for symptomatic vertebral artery stenosis using coronary stents. J Invasive Cardiol. 2001;13:363-366. [PubMed] |
| 20. | Jenkins JS, Patel SN, White CJ, Collins TJ, Reilly JP, McMullan PW, Grise MA, Grant AG, Ramee SR. Endovascular stenting for vertebral artery stenosis. J Am Coll Cardiol. 2010;55:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Stroke. 2011;42:e464-e540. [PubMed] |
| 22. | Wehman JC, Hanel RA, Guidot CA, Guterman LR, Hopkins LN. Atherosclerotic occlusive extracranial vertebral artery disease: indications for intervention, endovascular techniques, short-term and long-term results. J Interv Cardiol. 2004;17:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Lemke AJ, Benndorf G, Liebig T, Felix R. Anomalous origin of the right vertebral artery: review of the literature and case report of right vertebral artery origin distal to the left subclavian artery. AJNR Am J Neuroradiol. 1999;20:1318-1321. [PubMed] |
| 24. | Gluncic V, Ivkic G, Marin D, Percac S. Anomalous origin of both vertebral arteries. Clin Anat. 1999;12:281-284. [PubMed] |
| 25. | Palmer FJ. Origin of the right vertebral artery from the right common carotid artery: angiographic demonstration of three cases. Br J Radiol. 1977;50:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Wollschlaeger G, Wollschlaeger PB, Lucas FV, Lopez VF. Experience and result with postmortem cerebral angiography performed as routine procedure of the autopsy. Am J Roentgenol Radium Ther Nucl Med. 1967;101:68-87. [PubMed] |
| 27. | Lin YH, Hung CS, Tseng WY, Lee RK, Wang YC, Lin MS, Yeh MH, Chao CL, Ho YL, Jeng JS. Safety and feasibility of drug-eluting stent implantation at vertebral artery origin: the first case series in Asians. J Formos Med Assoc. 2008;107:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Voetsch B, DeWitt LD, Pessin MS, Caplan LR. Basilar artery occlusive disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 2004;61:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Matula C, Trattnig S, Tschabitscher M, Day JD, Koos WT. The course of the prevertebral segment of the vertebral artery: anatomy and clinical significance. Surg Neurol. 1997;48:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Zhou Z, Yin Q, Xu G, Yue X, Zhang R, Zhu W, Fan X, Ma M, Liu X. Influence of vessel size and tortuosity on in-stent restenosis after stent implantation in the vertebral artery ostium. Cardiovasc Intervent Radiol. 2011;34:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Caplan L. Stroke: A Clinical Approach. 3rd ed. Stoneham, MA: Butterworth-Heinemann 2000; . |
| 32. | Flossmann E, Rothwell PM. Prognosis of vertebrobasilar transient ischaemic attack and minor stroke. Brain. 2003;126:1940-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1088] [Cited by in RCA: 972] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 34. | Long A, Lepoutre A, Corbillon E, Branchereau A. Critical review of non- or minimally invasive methods (duplex ultrasonography, MR- and CT-angiography) for evaluating stenosis of the proximal internal carotid artery. Eur J Vasc Endovasc Surg. 2002;24:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Dawkins AA, Evans AL, Wattam J, Romanowski CA, Connolly DJ, Hodgson TJ, Coley SC. Complications of cerebral angiography: a prospective analysis of 2,924 consecutive procedures. Neuroradiology. 2007;49:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227:522-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 585] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 37. | Chuah KC, Stuckey SL, Berman IG. Silent embolism in diagnostic cerebral angiography: detection with diffusion-weighted imaging. Australas Radiol. 2004;48:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet. 1999;354:1594-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 333] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 39. | Kato K, Tomura N, Takahashi S, Sakuma I, Watarai J. Ischemic lesions related to cerebral angiography: Evaluation by diffusion weighted MR imaging. Neuroradiology. 2003;45:39-43. [PubMed] |
| 40. | Bendszus M, Koltzenburg M, Bartsch AJ, Goldbrunner R, Günthner-Lengsfeld T, Weilbach FX, Roosen K, Toyka KV, Solymosi L. Heparin and air filters reduce embolic events caused by intra-arterial cerebral angiography: a prospective, randomized trial. Circulation. 2004;110:2210-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Henry M, Henry I, Klonaris C. Percutaneous transluminal angioplasty and stenting of extracranial VA stenosis. In Henry M, Ohki T, Polydorou A, et al, editors. Angioplasty and stenting of the carotid and supra-aortic trunks. 1st ed. London: Taylor and Francis Medicine 2003; 673-682. |
| 42. | Sivenius J, Riekkinen PJ, Smets P, Laakso M, Lowenthal A. The European Stroke Prevention Study (ESPS): results by arterial distribution. Ann Neurol. 1991;29:596-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Adams RJ, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39:1647-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 323] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 44. | Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4959] [Cited by in RCA: 4590] [Article Influence: 199.6] [Reference Citation Analysis (0)] |
| 45. | CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4584] [Cited by in RCA: 4179] [Article Influence: 144.1] [Reference Citation Analysis (0)] |
| 46. | Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1618] [Cited by in RCA: 1494] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 47. | Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 758] [Cited by in RCA: 667] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 48. | Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1082] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 49. | Berguer R, Flynn LM, Kline RA, Caplan L. Surgical reconstruction of the extracranial vertebral artery: management and outcome. J Vasc Surg. 2000;31:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Imparato AM. Vertebral arterial reconstruction: a nineteen-year experience. J Vasc Surg. 1985;2:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Berguer R. Long-term results of vertebral artery reconstruction. Long-term results in vascular surgery. Norwalk, CT: Appleton and Lange 1993; 69. |
| 52. | Hass WK, Easton JD, Adams HP, Pryse-Phillips W, Molony BA, Anderson S, Kamm B. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med. 1989;321:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 721] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 53. | Thevenet A, Ruotolo C. Surgical repair of vertebral artery stenoses. J Cardiovasc Surg (Torino). 1984;25:101-110. [PubMed] |
| 54. | Koskas F, Kieffer E, Rancurel G, Bahnini A, Ruotolo C, Illuminati G. Direct transposition of the distal cervical vertebral artery into the internal carotid artery. Ann Vasc Surg. 1995;9:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Bhatt DL, Kapadia SR, Bajzer CT, Chew DP, Ziada KM, Mukherjee D, Roffi M, Topol EJ, Yadav JS. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol. 2001;13:767-771. [PubMed] |
| 56. | Wiviott SD, Antman EM, Winters KJ, Weerakkody G, Murphy SA, Behounek BD, Carney RJ, Lazzam C, McKay RG, McCabe CH. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 trial. Circulation. 2005;111:3366-3373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 276] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 57. | Frelinger AL, Jakubowski JA, Li Y, Barnard MR, Fox ML, Linden MD, Sugidachi A, Winters KJ, Furman MI, Michelson AD. The active metabolite of prasugrel inhibits ADP-stimulated thrombo-inflammatory markers of platelet activation: Influence of other blood cells, calcium, and aspirin. Thromb Haemost. 2007;98:192-200. [PubMed] |
| 58. | Azam SM, Jozic J. Variable platelet responsiveness to aspirin and clopidogrel: role of platelet function and genetic polymorphism testing. Transl Res. 2009;154:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 751] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 60. | Michelson AD, Frelinger AL, Furman MI. Current options in platelet function testing. Am J Cardiol. 2006;98:4N-10N. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 61. | Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, Zenni MM, Guzman LA, Bass TA, Costa MA. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation. 2007;115:708-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 350] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 62. | Layton KF, Kallmes DF, Cloft HJ. The radial artery access site for interventional neuroradiology procedures. AJNR Am J Neuroradiol. 2006;27:1151-1154. [PubMed] |
| 63. | Fessler RD, Wakhloo AK, Lanzino G, Guterman LR, Hopkins LN. Transradial approach for vertebral artery stenting: technical case report. Neurosurgery. 2000;46:1524-1527; discussion 1524-1527. [PubMed] |
| 64. | Kizilkilic O. Vertebral artery origin stenting with buddy wire technique in tortuous subclavian artery. Eur J Radiol. 2007;61:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Putman CM, Chaloupka JC. Use of large-caliber coronary guiding catheters for neurointerventional applications. AJNR Am J Neuroradiol. 1996;17:697-704. [PubMed] |
| 66. | Abizaid A, Costa MA, Blanchard D, Albertal M, Eltchaninoff H, Guagliumi G, Geert-Jan L, Abizaid AS, Sousa AG, Wuelfert E. Sirolimus-eluting stents inhibit neointimal hyperplasia in diabetic patients. Insights from the RAVEL Trial. Eur Heart J. 2004;25:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Regar E, Serruys PW, Bode C, Holubarsch C, Guermonprez JL, Wijns W, Bartorelli A, Constantini C, Degertekin M, Tanabe K. Angiographic findings of the multicenter Randomized Study With the Sirolimus-Eluting Bx Velocity Balloon-Expandable Stent (RAVEL): sirolimus-eluting stents inhibit restenosis irrespective of the vessel size. Circulation. 2002;106:1949-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 68. | Chung SY, Lee DH, Choi JW, Choi BS, In HS, Kim SM, Choi CG, Kim SJ, Suh DC. Use of self-expanding stents for the treatment of vertebral artery ostial stenosis: a single center experience. Korean J Radiol. 2010;11:156-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Gruberg L, Beyar R. Optimized combination of antiplatelet treatment and anticoagulation for percutaneous coronary intervention: the final word is not out yet! J Invasive Cardiol. 2002;14:251-253. [PubMed] |
| 70. | Steinhubl SR, Berger PB, Mann JT, Fry ET, DeLago A, Wilmer C, Topol EJ. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2349] [Cited by in RCA: 2221] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 71. | Berteloot D, Leclerc X, Leys D, Krivosic R, Pruvo JP. [Cerebral angiography: a study of complications in 450 consecutive procedures]. J Radiol. 1999;80:843-848. [PubMed] |
| 72. | Hankey GJ, Warlow CP, Molyneux AJ. Complications of cerebral angiography for patients with mild carotid territory ischaemia being considered for carotid endarterectomy. J Neurol Neurosurg Psychiatry. 1990;53:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke. 1990;21:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 281] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 74. | Lin YH, Juang JM, Jeng JS, Yip PK, Kao HL. Symptomatic ostial vertebral artery stenosis treated with tubular coronary stents: clinical results and restenosis analysis. J Endovasc Ther. 2004;11:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Weber W, Mayer TE, Henkes H, Kis B, Hamann GF, Holtmannspoetter M, Brueckmann H, Kuehne D. Efficacy of stent angioplasty for symptomatic stenoses of the proximal vertebral artery. Eur J Radiol. 2005;56:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Cloud GC, Crawley F, Clifton A, McCabe DJ, Brown MM, Markus HS. Vertebral artery origin angioplasty and primary stenting: safety and restenosis rates in a prospective series. J Neurol Neurosurg Psychiatry. 2003;74:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | SSYLVIA Study Investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004;35:1388-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 402] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 78. | Akins PT, Kerber CW, Pakbaz RS. Stenting of vertebral artery origin atherosclerosis in high-risk patients: bare or coated? A single-center consecutive case series. J Invasive Cardiol. 2008;20:14-20. [PubMed] |
| 79. | Taylor RA, Siddiq F, Suri MF, Martin CO, Hayakawa M, Chaloupka JC. Risk factors for in-stent restenosis after vertebral ostium stenting. J Endovasc Ther. 2008;15:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Hatano T, Tsukahara T, Miyakoshi A, Arai D, Yamaguchi S, Murakami M. Stent placement for atherosclerotic stenosis of the vertebral artery ostium: angiographic and clinical outcomes in 117 consecutive patients. Neurosurgery. 2011;68:108-16; discussion 116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Lin YH, Liu YC, Tseng WY, Juang JM, Hung CS, Lin JW, Jeng JS, Yip PK, Kao HL. The impact of lesion length on angiographic restenosis after vertebral artery origin stenting. Eur J Vasc Endovasc Surg. 2006;32:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Karameshev A, Schroth G, Mordasini P, Gralla J, Brekenfeld C, Arnold M, Mono ML, Mattle HP, Do DD, Nedeltchev K. Long-term outcome of symptomatic severe ostial vertebral artery stenosis (OVAS). Neuroradiology. 2010;52:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Gupta R, Al-Ali F, Thomas AJ, Horowitz MB, Barrow T, Vora NA, Uchino K, Hammer MD, Wechsler LR, Jovin TG. Safety, feasibility, and short-term follow-up of drug-eluting stent placement in the intracranial and extracranial circulation. Stroke. 2006;37:2562-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 84. | Edgell RC, Yavagal DR, Drazin D, Olivera R, Boulos AS. Treatment of vertebral artery origin stenosis with anti-proliferative drug-eluting stents. J Neuroimaging. 2010;20:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Vajda Z, Miloslavski E, Güthe T, Fischer S, Albes G, Heuschmid A, Henkes H. Treatment of stenoses of vertebral artery origin using short drug-eluting coronary stents: improved follow-up results. AJNR Am J Neuroradiol. 2009;30:1653-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 86. | Lugmayr H, Kastner M, Fröhler W, Meindl S, Zisch R. [Sirolimus-eluting stents for the treatment of symptomatic extracranial vertebral artery stenoses: early experience and 6-month follow-up]. Rofo. 2004;176:1431-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Coward LJ, Featherstone RL, Brown MM. Percutaneous transluminal angioplasty and stenting for vertebral artery stenosis. Cochrane Database Syst Rev. 2005;CD000516. [PubMed] |
| 88. | Qureshi AI, Kirmani JF, Harris-Lane P, Divani AA, Ahmed S, Ebrihimi A, Al Kawi A, Janjua N. Vertebral artery origin stent placement with distal protection: technical and clinical results. AJNR Am J Neuroradiol. 2006;27:1140-1145. [PubMed] |
| 89. | Coward LJ, McCabe DJ, Ederle J, Featherstone RL, Clifton A, Brown MM. Long-term outcome after angioplasty and stenting for symptomatic vertebral artery stenosis compared with medical treatment in the Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Stroke. 2007;38:1526-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |