Published online Aug 28, 2012. doi: 10.4329/wjr.v4.i8.372
Revised: May 30, 2012
Accepted: April 7, 2012
Published online: August 28, 2012
AIM: To propose a diagnostic algorithm for preoperatively predicting the need for surgical intervention.
METHODS: The study included 56 patients (27 men and 29 women) with a final diagnosis of cystic pancreatic lesions. The following materials were used: ultrasonic equipment with 3.5 and 7 MHz linear, convex and biopsical transducers. Multidetector computed tomography (MDCT) investigations were performed using a 16-slice scanner. Images were obtained following the oral administration of 200 mL water and 100 mL intravenous iopamidol (300 mg/mL) administered by pump injector at a rate of 3 mL/s (40 and 60 s post-injection, respectively) using 0.5 mm detectors, reconstructed at 1 mm (pancreatic phase) or 2 mm (portal venous phase) increments. The table feed was 10 mm per rotation. Images were acquired in the pancreatic and portal venous phases of contrast enhancement. The “Chiba” needles 18, 20, 22, 23 G and an automatic aspiration system were used in conjunction with the following methods of guiding the interventional procedures: (1)“free-hand” biopsy and puncture method under ultrasound (US) or computed tomography (CT) control; (2) guiding method using biopsical transducer.
RESULTS: All 56 patients in this study underwent at least two cuts imaging survey methods, such as US, CT or magnetic resonance imaging (MRI). The most common preoperative diagnostic examination was US scan - 56 patients (100%). MDCT studies were conducted in 49 (87.50%) and MRI in 13 (23.21%). More than half of patients surveyed (37) underwent some type of interventional procedure: 25-fine-needle aspiration and 29-fine needle aspiration biopsy (FNAB), as part of the examination. Thirty-four patients of all 56 patients underwent surgery because of histological evidence of malignancy after the FNAB for cystic lesions of the pancreas. Distal pancreatectomy with splenectomy was the most common operative approach in 13 patients, followed by Whipple resection in 11 and distal pancreatectomy without splenectomy in 7. Three patients were treated with total pancreatectomy due to the presence of a multifocal mucinous neoplasm. Comparing the diagnostic results of US examination with those of MDCT examination and histological verification true positive results were found in 31 patients, true negative in 11 patients, false positive in 5 and false negative in 9 patients. Accordingly we estimated the power of the diagnostic imaging methods for cystic lesions of the pancreas. A specificity of 68.75%, sensitivity of 79.48%, accuracy of 75.00%, positive predictive value of 86.11% and negative predictive value of 55% were obtained. The power increased after applying invasive procedures with immunohistochemical analysis of CEA and P-53 (Fig. 4). In 15 patients with cytological feature of malignant tumour cells, the tumour markers were positive. In our opinion the higher the percentage of reacting cells the higher the percent of malignancy. In patients with clear symptoms and/or clear imaging features of malignant or premalignant cystic neoplasm, the need for surgery was confirmed by histological verification in 34 (60.71%) of cases.
CONCLUSION: By using the proposed algorithm, cystic mucinous tumors of the pancreas were detected and proper operative interventions would have been rendered with fewer diagnostic examinations.
- Citation: Hilendarov AD, Deenichin GP, Velkova KG. Imaging investigation of pancreatic cystic lesions and proposal for therapeutic guidelines. World J Radiol 2012; 4(8): 372-378
- URL: https://www.wjgnet.com/1949-8470/full/v4/i8/372.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i8.372
Although the clinical, radiological and pathologic features of cystic pancreatic lesions are well known, preoperative diagnosis is difficult. Before the widespread use of modern ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) methods, cystic neoplasms of the pancreas were considered very rare, and were thought to constitute 10%-15% of all cystic pancreatic lesions and 1%-5% of pancreatic neoplasms[1]. With the increasing use of advanced imaging technology significantly increased accidental openings of asymptomatic cystic lesions have occurred. In some medical centers more than one third of patients with cystic neoplasms of the pancreas are asymptomatic and neoplasms are discovered incidentally on imaging studies[2].
The problem that surgeons face is how to differentiate benign lesions such as serous cystadenomas and pseudocysts from malignant lesions and premalignant mucinous neoplasms.
With increasing incidental detection of these cystic neoplasms, especially in light of the understanding that most of these lesions are benign in nature, resection in all cases is unnecessary[2,3]. No optimal test distinguishing benign from malignant cystic tumors has been provided so far. CT is also accurate in identifying vascular involvement, which complements multidetector computed tomography (MDCT) in predicting resectability[4]. In cases of diagnostic uncertainty, US or CT-guided fine-needle aspiration (FNA) were used to obtain tissue samples from solid lesions and fluid aspirates from cystic lesions, allowing histological, cytological, and biochemical analysis to determine the nature of the lesion.
The aim of this study is to propose a diagnostic algorithm for differentiating cystic pancreatic lesions and define criteria for surgical intervention.
At our institution the proposed algorithm was the method of choice for all of the patients with cystic pancreatic lesions.
Data of imaging research methods and the disease history of patients with cystic lesions of the pancreas diagnosed and treated in the Surgical Clinics of the University Hospital “St. George” for the period 2007-2010 year were collected in a retrospective study. Patients with a clear history of acute pancreatitis and subsequent development of pseudocyst also were confirmed histologically.
The study included 56 patients (27 men and 29 women) with cystic lesions of the pancreas. The information collected included medical records, demographic data, disease symptoms and diagnostic tests. Operational protocols were reviewed with respect to the type of resection and the need for extended resection of neighboring structures. Histological reports were reviewed to confirm the diagnosis and detect abnormal variants. The latter included tumor location and size, histological type and degree of invasion. Lesions were categorized into five histologic subtypes: mucinous tumor, serous cystadenomas, pseudocysts, solid and papillary cystic tumor and mesothelial cysts. Mucinous tumors were subdivided into mucinous cystadenomas (benign and border), intraductal papillary mucinous tumor and mucinous cystadenocarcinoma.
In addition to routine laboratory specific behavior the examination of pancreatic cystic lesions was followed using imaging studies. Diagnostic examinations are divided into invasive and non-invasive. Non-invasive examinations included abdominal US, CT scan and MRI study. Invasive procedures were fine-needle aspiration biopsy (FNAB) and FNA under imaging (US and CT) control. In some cases immunocytochemical examination of CEA and P-53 as malignant markers was performed[3,5]. In all patients at least two diagnostic imaging methods were applied to characterize the cystic pancreatic lesions.
All patients with cystic pancreatic lesions (regardless of treatment) were followed by diagnostic imaging methods for a period of 3 years to search for suspected malignant features. In case of malignancy additional invasive (FNAB) diagnostic procedures were applied for histological verification.
The following materials and methods were used: (1) ultrasonic equipment with 3.5 and 7 MHz linear, convex and biopsical transducers for guidance of the interventional procedures; (2) MDCT investigations and guidance were done with a 16-slice scanner. Images were obtained after oral administration of 200 mL water and 100 mL intravenous iopamidol (300 mg/mL) administered by pump injector at a rate of 3 mL/s. Images were acquired in the pancreatic and portal venous phases of contrast enhancement; (3) the “Chiba” needles 18, 20, 22, 23 G catheters pig-tail 7, 8 F were used; and (4) cytopathologic preparations were either air-dried for Diff-Quik staining or fixed in alcohol for Papanicolaou staining. Serial sections of the prepared from aspirates blocks were stained with hematoxylin-eosin. Immunostaining with antibodies against the following antigens was performed: cytokeratin (AE1, AE3), vimentin, CEA and P-53.
Forty-two (75%) patients were symptomatic at the time of presentation. The most common symptom was abdominal pain observed in 39 patients, followed by weight loss in 14 and nausea with vomiting in 3 patients. Twelve patients presented without symptoms. In those patients, cysts were found during preliminary examination for other complaints (Table 1).
| Magnitude | n (%) |
| Sex | |
| Men | 27 (48.21) |
| Women | 29 (51.78) |
| Symptoms | |
| Pain | 39 (69.64) |
| Loss of weight (kg) | 14 (25.00) |
| Asymptomatic | 11 (19.64) |
| Vomiting | 3 (5.35) |
| Reflux | 6 (10.71) |
| Anorexia | 3 (5.35) |
| Jaundice | 1 (1.78) |
| Operations | |
| Whipple | 11 (19.64) |
| Distal pancreatectomy with splenectomy | 13 (23.21) |
| Distal pancreatectomy without splenectomy | 7 (12.50) |
| Total pancreatectomy | 3 (5.35) |
| All | 34 (60.71) |
Thirty-four (60.71%) of all 56 patients underwent surgery because of histological features of malignancy after the FNAB. Distal pancreatectomy with splenectomy was the most common operative approach in 13 patients, followed by Whipple resection in 11 and distal pancreatectomy without splenectomy in 7 of the patients with pancreatic cystic lesions. Three patients were treated with total pancreatectomy due to the presence of multifocal mucinous neoplasm.
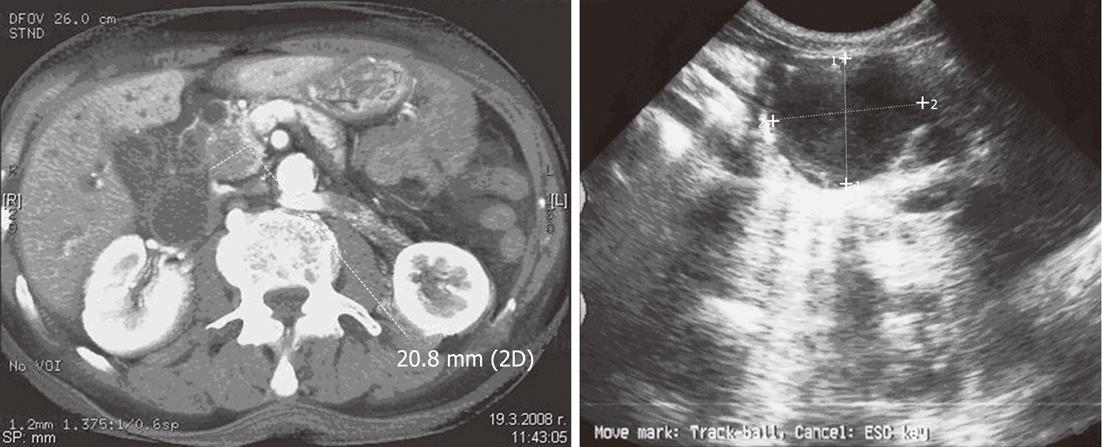
In the process of US and CT diagnostic examination for cystic lesions of the pancreas and their differential diagnostic clarification characteristic images were seen in various neoplasms: in 9 (83.33%) of the cases with serous cystic neoplasm (SCN) the cystic entity presented as multiple cavities (up to 2 cm in diameter) alluding to the so-called “honeycomb” with polycyclic borders (Figure 1). Five (41.66%) of the patients with SCN presented with a central scar of fibrous tissue, forming a typical “star” image.
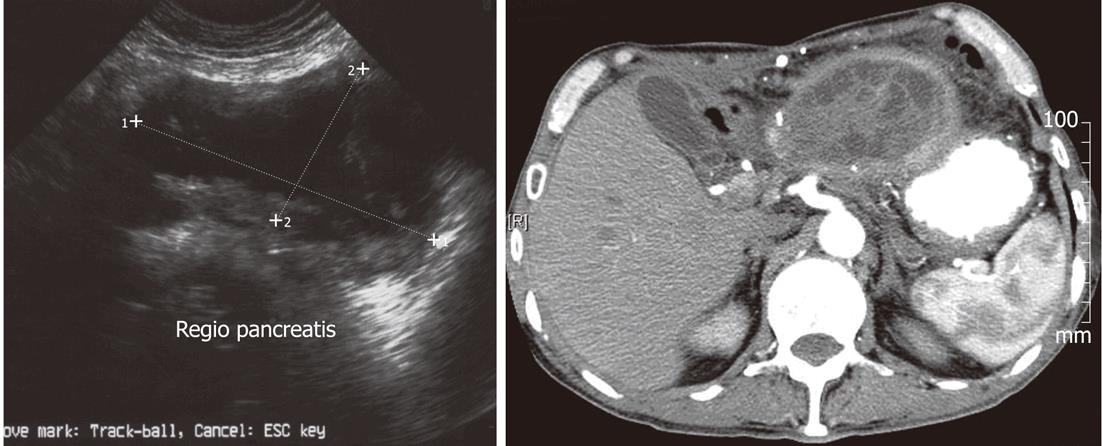
In 18 (69.23%) of 26 cases with mucinous cystic neoplasm (MCN) presented on US and CT images as a large cyst, rarely multicavitary. These neoplasms developed mainly in the body-tail of the pancreas and although it has no communication with the pancreatic duct can cause its obstruction. Usually the lesions presented with an image of a cyst with thick, irregular walls with papillary growths or septation (Figure 2).
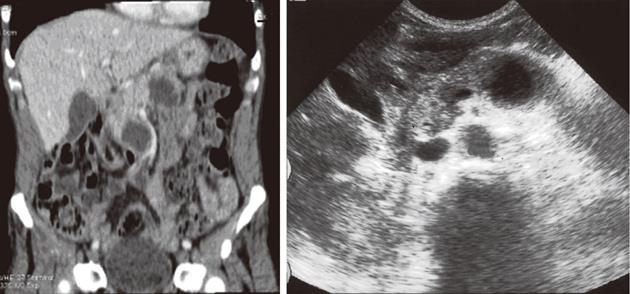
In 5 (55.55%) patients with intraductal mucinous cystic neoplasm (IPMN) cystic lesions located in the processus uncinatus presented with images of echogenic/hypodense lesions with papillary defects and cystic dilation of the main pancreatic duct (Figure 3).
The distribution of the lesions by their location was as follows: in the head/processus uncinatus - 15; in the body/neck - 11; and in the tail - 30. The average size of lesions was 4.3 cm with a range of 0.5-22 cm. Histological analysis showed that the most common neoplasm in this patient group was mucinous tumor - 34, followed by serous cystadenoma - 12 ( Table 2).
| Histology | n (%) |
| Serous cystadenoma | 12 (21.42) |
| Mucinous cystadenoma-benign | 15 (26.78) |
| Mucinous cystadenoma -borderline | 5 (8.92) |
| Mucinous cystadenocarcinoma | 6 (10.71) |
| Intraductal papillary mucinous neoplasm | 9 (16.07) |
| Adenocarcinoma | 1 (1.78) |
| Solid and papillary cystic ТU | 1 (1.78) |
| Lymphoepithelial cyst | 3 (5.35) |
| Pseudocyst | 4 (7.14) |
| All | 56 (100) |
Of mucinous tumors, mucinous cystadenomas with benign features were the most frequent (15 cases); in 11 patients the lesions were with both borderline and malignant signs and 9 lesions were intraductal papillary mucinous neoplasms. In 4 patients without a clear prior history of acute pancreatitis or pseudocyst a final diagnosis of pseudocyst was made.
Comparing the diagnostic results of US examination with those of MDCT examination and histological verification true positive results were found in 31 patients, true negative in 11 patients, false positive in 5 and false negative in 9 patients. Accordingly we estimated the power of the diagnostic imaging methods for cystic lesions of the pancreas. A specificity of 68.75%, sensitivity of 79.48%, accuracy of 75.00%, positive predictive value of 86.11% and negative predictive value of 55% were obtained. The power increased after applying invasive procedures with immunohistochemical analysis of CEA and P-53 (Figure 4). Thirty-five true positive results, true negative in 13 patients, false positive in 3 and false negative in 5 patients were found. A specificity of 81.25%, sensitivity of 87.50%, accuracy of 85.71%, positive predictive value of 92.10% and negative predictive value of 72.22% was obtained. In 15 patients with cytological features of malignant tumour cells the tumour markers were positive. In our opinion the higher the percentage of reacting cells the higher the percent of malignancy. In patients with clear symptoms and/or clear imaging features of malignant or premalignant cystic neoplasm, the need for surgery was confirmed by histological verification in 34 (60.71%) of cases.
Adding the results of invasive procedures this percentage increased to 46 (82.14%) because the high expression of P-53 antigen and CEA combined with degenerative cells and intra/extracellular mucin provided almost certain diagnosis of malignant cystic lesions.
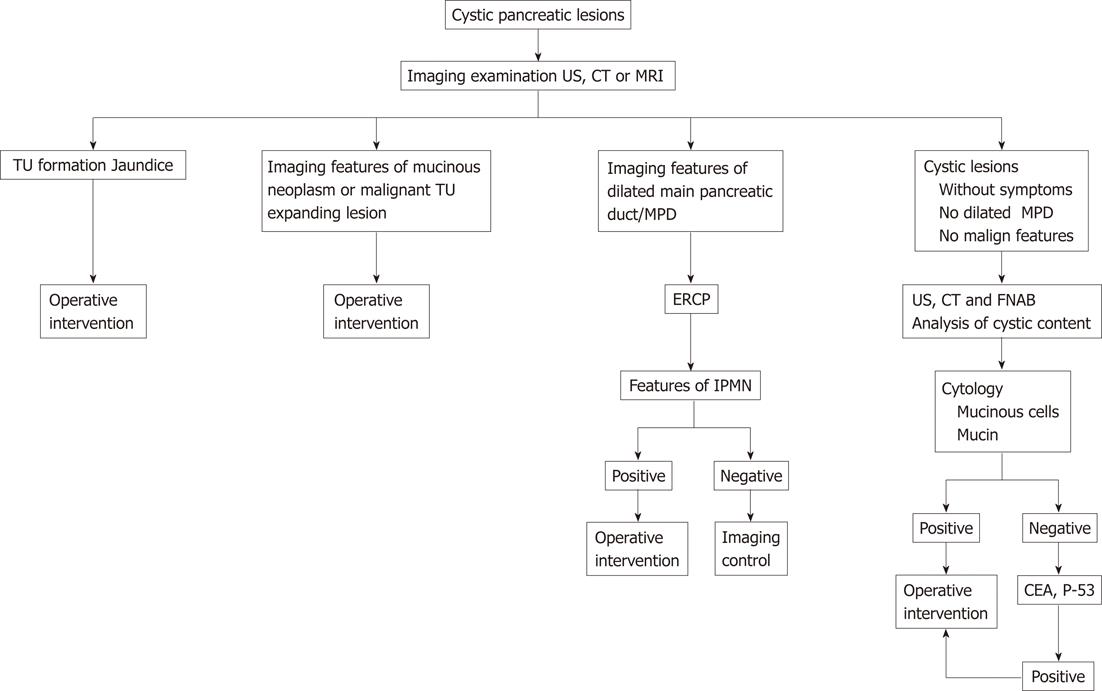
Based on the presence or absence of clinical symptoms, clear and reliable imaging of MCNs, and high specificity of the criteria of invasive manipulations, we proposed a diagnostic-therapeutic algorithm for cystic lesions of the pancreas (Figure 5).
The algorithm proposed and used routinely in our institution allowed us to detect mucinous tumors and perform proper operative interventions at low risk of missing malignant lesions.
The use of multiple imaging methods significantly increases the reports of accidental detection of cystic lesions of the pancreas. In some centres, the increase in operational interventions is almost 50% in cystic lesions of the pancreas[6]. With a growing body of information about the biological behavior of the pancreatic cystic lesions the necessity of performing resections in all cases with cystic lesions is changing[7-9]. These lesions should be resected only in the presence of significant symptomatology related to their increase[8-10].
In our study, the average number of tests performed for diagnostic clarification of cystic lesions of the pancreas was two. After the first clinical application of CT in 1973, a growing development in technology and related innovations has been observed[11]. There is a large range of variations in CT sensitivity in cystic pancreatic neoplasm (20%-70%)[12-14]. Some authors recommend CEUS, as a better method for identification of vascularization of pancreatic tumors when the pancreatic gland is optimally visualized[15]. CT is recommended as the primary method in the study of cystic pancreatic neoplasms[16,17]. Typical serous cystadenoma is characterized by numerous small cysts usually more than 6 mm up to 2-3 cm in diameter. The central “star” feature of fibrotic changes and calcifications is a pathognomonic scar[10,12,17]. Our algorithm proposed imaging features highly suspicious of mucinous or malignant tumor, including CT and MRI signs of thick walls with septations with or without calcifications and adjacent mass. Performing FNAB and obtaining mucin containing cells eliminates the need for further examination because of the necessity of surgical intervention. One exception may be the suspicion of IPMN on MDCT and MRI examination. Dilation of the main pancreatic duct over 10 mm could then be secondary to chronic pancreatitis, obstruction of lumen and tumor - IPMN[17,18]. ERCP preoperatively allows the distribution and localization of pancreatic duct involvement. Very important information on the needs and volume of operative intervention can be obtained using these techniques. US morphological diagnosis with FNAB and analysis of cystic fluid have the same restrictions as other scanning methods. Many studies show that this is less sensitive than CT[19-21]. To increase the accuracy of diagnostic imaging methods we offer a combination of FNAB and FNA[22]. Biopsy findings of mucinous columnar cells, atypical cytology or malignant cells clearly require operation[23]. Many authors recommend examination of the fluid with cytological preparations and examination for P-53 and CEA antigens[22,24-26].
Cystic tumors of the pancreas represent a diagnostic challenge for both radiologists and surgeons because of their overlapping clinical, radiological and pathologic features. In patients with cystic lesions of the pancreas presenting with clinical symptoms, imaging signs or malignant mucinous cells from FNA additional tests and examinations are not necessary. Cytological preparations of FNA and FNAB are sufficient to differentiate mucinous neoplasms of the pancreas. The algorithm presented, based on symptomatology, imaging features and examination of cytology FNA and FNAB, enables satisfactory basis for an adequate therapeutic behavior in cystic lesions of the pancreas.
Based on the results obtained and our understanding of the limited possibilities of accurate diagnosis of cystic pancreatic neoplasms, we believe that all cystic lesions should be suspected and biopsied in search of early signs of malignant changes.
Cystic tumours comprise 10%-15% of all cystic masses and 1%-5% of all pancreatic malignancies. The clinical, radiologic and pathologic features of cystic pancreatic lesions are well known, but still preoperative diagnosis is difficult. With advancements in diagnostic imaging, cystic lesions of the pancreas are being detected with increasing frequency and this requires looking for signs of malignancy.
With increasing incidental detection of these cystic neoplasms, especially in light of the understanding that most of these lesions are benign in nature, resection in all cases is unnecessary. No optimal test distinguishing benign from malignant cystic tumors has been provided so far. Computed tomography (CT) is also accurate in identifying vascular involvement, which complements multidetector computed tomography (MDCT) in predicting resectability.
Contrast-enhanced, single-section, helical CT is almost 100% accurate in determining unresectability, but is less accurate in predicting resectability. However, the latest MDCT technology provides three-dimensional multiplanar reconstruction techniques enabling accurate determination of tumour involvement of the pancreatic duct, and peripancreatic vasculature. This has improved the preoperative determination of surgical respectability.
The study proposes a diagnostic algorithm for differentiating cystic neoplasms and to define criteria for preoperatively predicting the need for surgical intervention. The power increased after applying invasive procedures with immunohistochemical analysis of CEA and P-53.
Cystic tumors of the pancreas represent a diagnostic challenge for both radiologists and surgeons because of their overlapping clinical, radiological and pathologic features. The algorithm presented, based on symptomatology, imaging features and examination of cytology fine-needle aspiration and fine needle aspiration biopsy enables satisfactory basis for an adequate therapeutic behavior in cystic lesions of the pancreas.
This is a very interesting paper addressing a common clinical condition. The authors reported an algorithm to guide the diagnostic and treatment approach for cystic pancreatic lesion, which was not reported before. However, the manuscript needs revision
Peer reviewer: Wenbao Wang, MD, Department of Orthopaedic, Columbia University Medical Center, 106 Fort Washington Avenue, Apt 3H, New York, NY 10032, United States
S- Editor Cheng JX L- Editor O’Neill M E- Editor Xiong L
| 1. | Becker WF, Welsh RA, Pratt HS. Cystadenoma and cystadenocarcinoma of the pancreas. Ann Surg. 1965;161:845-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427-437; discussion 427-437. [PubMed] |
| 3. | Warshaw AL, Compton CC, Lewandrowski K, Cardenosa G, Mueller PR. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg. 1990;212:432-43; discussion 444-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 386] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Rafique A, Freeman S, Carroll N. A clinical algorithm for the assessment of pancreatic lesions: utilization of 16- and 64-section multidetector CT and endoscopic ultrasound. Clin Radiol. 2007;62:1142-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Brugge WR. Management and outcomes of pancreatic cystic lesions. Digestive and Liver Disease. 2008;40:854-859. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Balcom JH, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 584] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 7. | Sarr MG, Murr M, Smyrk TC, Yeo CJ, Fernandez-del-Castillo C, Hawes RH, Freeny PC. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg. 2003;7:417-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 490] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 9. | Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, Wilson SD, Pitt HA. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651-657; discussion 651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 387] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 10. | Allen PJ, Jaques DP, D'Angelica M, Bowne WB, Conlon KC, Brennan MF. Cystic lesions of the pancreas: selection criteria for operative and nonoperative management in 209 patients. J Gastrointest Surg. 2003;7:970-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Stockburger WT. CT imaging, then and now: a 30-year review of the economics of computed tomography. Radiol Manage. 2004;26:20-22, 24-27; quiz 28-30. [PubMed] |
| 12. | Procacci C, Biasiutti C, Carbognin G, Accordini S, Bicego E, Guarise A, Spoto E, Andreis IA, De Marco R, Megibow AJ. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999;23:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Scott J, Martin I, Redhead D, Hammond P, Garden OJ. Mucinous cystic neoplasms of the pancreas: imaging features and diagnostic difficulties. Clin Radiol. 2000;55:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, Fishman EK. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000;175:99-103. [PubMed] |
| 15. | D'Onofrio M, Malagò R, Zamboni G, Vasori S, Falconi M, Capelli P, Mansueto G. Contrast-enhanced ultrasonography better identifies pancreatic tumor vascularization than helical CT. Pancreatology. 2005;5:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Minami M, Itai Y, Ohtomo K, Yoshida H, Yoshikawa K, Iio M. Cystic neoplasms of the pancreas: comparison of MR imaging with CT. Radiology. 1989;171:53-56. [PubMed] |
| 17. | Hashimoto L, Walsh RM, Vogt D, Henderson JM, Mayes J, Hermann R. Presentation and management of cystic neoplasms of the pancreas. J Gastrointest Surg. 1998;2:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Adsay NV, Conlon KC, Zee SY, Brennan MF, Klimstra DS. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Madura JA, Wiebke EA, Howard TJ, Cummings OW, Hull MT, Sherman S, Lehman GA. Mucin-hypersecreting intraductal neoplasms of the pancreas: a precursor to cystic pancreatic malignancies. Surgery. 1997;122:786-792; discussion 792-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Sedlack R, Affi A, Vazquez-Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc. 2002;56:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Brandwein SL, Farrell JJ, Centeno BA, Brugge WR. Detection and tumor staging of malignancy in cystic, intraductal, and solid tumors of the pancreas by EUS. Gastrointest Endosc. 2001;53:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 736] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 23. | Jones EC, Suen KC, Grant DR, Chan NH. Fine-needle aspiration cytology of neoplastic cysts of the pancreas. Diagn Cytopathol. 1987;3:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Lewandrowski KB, Southern JF, Pins MR, Compton CC, Warshaw AL. Cyst fluid analysis in the differential diagnosis of pancreatic cysts. A comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg. 1993;217:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 189] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Sand JA, Hyoty MK, Mattila J, Dagorn JC, Nordback IH. Clinical assessment compared with cyst fluid analysis in the differential diagnosis of cystic lesions in the pancreas. Surgery. 1996;119:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Hammel P, Levy P, Voitot H, Levy M, Vilgrain V, Zins M, Flejou JF, Molas G, Ruszniewski P, Bernades P. Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas. Gastroenterology. 1995;108:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 166] [Article Influence: 5.5] [Reference Citation Analysis (0)] |