Published online Jul 28, 2012. doi: 10.4329/wjr.v4.i7.318
Revised: April 13, 2012
Accepted: April 20, 2012
Published online: July 28, 2012
AIM: To retrospectively assess the acute and long-term toxicity using aromatase inhibitors (AI) therapy concurrently with hypofractionated radiotherapy (HFRT) in breast cancer patients.
METHODS: From November 1999 to October 2007, 66 patients were treated with breast HFRT and concurrent AI. In 63 patients (95.5%), HFRT delivered a total dose of 32.5 Gy to the whole breast within 5 wk (five fractions, one fraction per week). Other fractionations were chosen in three patients for the patients’ personal convenience. A subsequent boost to the tumor bed was delivered in 35 patients (53.0%). Acute toxicities were scored according to the Common Toxicity Criteria for Adverse Events v3. Late toxicity was defined as any toxicity occurring more than 6 mo after completion of HFRT and was scored according to the Late Effects Normal Tissue Task Force-Subjective, Objective, Management and Analytic scale.
RESULTS: At the end of the HFRT course, 19 patients (28.8%) had no irradiation-related toxicity. Acute grade 1-2 epithelitis was observed in 46 patients (69.7%). One grade 3 toxicity (1.5%) was observed. With a median follow-up of 34 mo (range: 12-94 mo), 31 patients (47%) had no toxicity, and 35 patients (53%) presented with grade 1-2 fibrosis. No grade 3 or greater delayed toxicity was observed.
CONCLUSION: We found that AI was well tolerated when given concurrently with HFRT. All toxicities were mild to moderate, and no treatment disruption was necessary. Further prospective assessment is warranted.
- Citation: Chargari C, Castro-Pena P, Toledano I, Bollet MA, Savignoni A, Cottu P, Laki F, Campana F, Fourquet A, Kirova YM. Concurrent use of aromatase inhibitors and hypofractionated radiation therapy. World J Radiol 2012; 4(7): 318-323
- URL: https://www.wjgnet.com/1949-8470/full/v4/i7/318.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i7.318
Adjuvant endocrine therapy demonstrated clinical benefit in breast cancer patients with tumors that express hormone receptors[1-4]. More particularly, third-generation aromatase inhibitors (AI) demonstrated improved disease-free survival as adjuvant therapy in postmenopausal patients with hormone positive early breast cancer[5-9]. Postoperative endocrine therapy has become standard clinical practice in this population and it is frequently delivered along with adjuvant radiation therapy (RT). However, the preclinical findings that AI might increase radiosensitivity raised concerns about the safety of such association[10].
Retrospective analysis reported that concurrent use of adjuvant normofractionated RT and endocrine therapy using AI did not increase RT-related side effects[11]. More recently, the prospective randomized phase II trial Concomitant Hormono-Radiotherapy (CO-HO-RT) study demonstrated that patients receiving conventionally fractionated RT and letrozole did not experience more frequent or more serious skin toxicity[12]. Although this trial provided evidence for the safety of normofractionated RT and AI, no conclusion could be drawn regarding hypofractionated radiotherapy (HFRT) and concurrent AI.
HFRT is frequently proposed as an alternative to standard fractionation in elderly patients treated with a breast conservative strategy[13-15]. In this population, an abbreviated course of radiation therapy is more convenient than standard fractionation. Recently, a randomized trial reported by Whelan et al[15] demonstrated that HFRT was not inferior to standard radiation treatment in patients who had undergone breast-conserving surgery for good prognosis breast cancer. Authors found no increase in skin and subcutaneous toxic effects in patients who received accelerated HFRT as compared with those who received the standard regimen. However, since elderly patients are also most likely to receive AI, it would also be clinically relevant to determine whether concurrent HFRT and AI might increase toxicity. Our study is the first to assess the safety of AI therapy concurrently with HFRT.
We retrospectively reviewed the clinical records of 66 consecutive breast cancer patients who were treated at the Institut Curie, Paris, France, from November 1999 to October 2007 for breast HFRT concurrently with AI. Patients were eligible for analysis only if they had more than 12 mo follow-up after completion of breast HFRT. Patients were treated according to the current protocol available in our Institute for women older than 65 years, presenting with voluminous or pendulous breasts and who wished a breast conservation procedure. Local committees approved the study design. Only one patient was less than 65 years old but she presented with metastatic disease and was judged a candidate for HFRT. At first presentation, the median age of the group was 80.5 years (range: 56-92 years). For all patients, the diagnosis of breast cancer been histologically confirmed by biopsy/surgery of the primary lesion. Patients and tumors’ characteristics are reported in Table 1. Regarding associated risk factors, the median body mass index was 26 (range: 16-45), seven patients had type 2 diabetes mellitus, and six patients had active tobacco use.
| Characteristics | |
| Number of patients | 66 |
| Median age in years (range) | 80 (56-92) |
| Stage, n (%) | |
| I | 28 (42.4) |
| II | 23 (34.8) |
| III | 10 (18) |
| IV | 5 (5) |
| Histological type, n (%) | |
| Invasive ductal carcinoma | 54 (81.8) |
| Invasive lobular carcinoma | 11 (16.6) |
| Other histology | 1 (1.6) |
| Grade, n (%) | |
| 1 | 15 (22.7) |
| 2 | 38 (57.6) |
| 3 | 11 (16.6) |
| NR | 2 (3.1) |
| Mitotic index, n (%) | |
| Low | 42 (63.6) |
| Intermediate | 11 (16.7) |
| High | 7 (10.6) |
| NR | 6 (9.1) |
| Expression of endocrine receptors, % (median) | |
| ER | 100 (60-100) |
| PgR | 100 (60–100) |
| HER2 status, n (%) | |
| Positive | 11 (16.6) |
| Negative | 55 (83.4) |
Breast conservative surgery (BCS) ± axillary lymph node dissections were performed in 35 patients (53.0%). All of them had received neoadjuvant endocrine therapy, median duration 6 mo (range: 1-12 mo). The remaining patients were not candidates for surgery, due to poor performance status. Following surgery (or following histological confirmation of the diagnosis in patients who had no surgery), AIs were administered daily and was planned for 5 years, either letrozole 2.5 mg daily (n = 16) or anastrozole 1 mg daily (n = 47), or exemestane 25 mg daily (n = 3). Although concurrent endocrine and HFRT was not in our current protocol in 1999-2007, all patients received breast HFRT and concurrent AI because they were referred to our department after having initiated AI therapy, which was not discontinued for HFRT.
HFRT was delivered according to the recommendations of the International Commission on Radiation Units and Measurements report 50 using a high-energy linear accelerator or a Cobalt unit[16]. In 63 patients (95.5%), HFRT delivered a total dose of 32.5 Gy to the whole breast within 5 wk (five fractions, one fraction per week). Other fractionations were chosen in three patients because of patients’ personal convenience. A subsequent boost to the tumor bed was delivered in 35 patients (53.0%) either because of risk factors for local relapse following BCS or in the setting of exclusive HFRT (Table 2). Axillary lymph node or supraclavicular HFRT could be delivered as 5 weekly fractions of 5.5 Gy in the case of clinical or pathological lymph node involvement. Internal mammary chain (IMC) irradiation was not delivered. A standard technique was used with the patient either in lateral decubitus position (n = 63, 95.4 %) or in dorsal decubitus (n = 3). Treatment characteristics are detailed in Table 2.
| Treatments | n (%) |
| Surgery | |
| Surgery | |
| Yes (BCS) | 35 (53.0)1 |
| No | 31 (47.0) |
| Axillary LND | |
| Yes | 17 (25.8) |
| No | 49 (74.2) |
| Sentinel LN | |
| Yes | 20 (30.3) |
| No | 46 (69.7) |
| Aromatase inhibitor | |
| Letrozole | 16 (24.2) |
| Anastrozole | 47 (71.2) |
| Exemestane | 3 (4.6) |
| RT | |
| Position | |
| Lateral decubitus | 63 (95.4) |
| Dorsal decubitus | 3 (4.6) |
| Source | |
| Cobalt 60 | 57 (86.3) |
| RX 4 MV | 8 (12.2) |
| RX 6 MV | 1 (1.5) |
| Volume | |
| Whole breast | 66 (100) |
| Axillary LN | 4 (6.1) |
| Susclavicular LN | 3 (4.5) |
| Boost | 35 (4.5) |
| Protocol for the whole breast | |
| 5 fractions of 6.5 Gy | 63 (95) |
| Other fractionation | 3 (5) |
| Protocol for the boost | |
| 2 fractions of 6.5 Gy | 28 (42.4) |
| 1 fraction of 6.5 Gy | 5 (7.5) |
| Other fractionation | 2 (3) |
| Median duration in days (range) | 29 (25-52) |
Weekly examination was performed during HFRT, then every 6 mo after HFRT completion. Local symptomatic therapies could be delivered, at the discretion of the radiation oncologist. Acute skin toxicities were scored according to the Common Toxicity Criteria for Adverse Events v3. Late skin toxicity was defined as any skin toxicity occurring more than 6 mo after completion HFRT and was scored according to the Late Effects Normal Tissue Task Force-Subjective, Objective, Management and Analytic scale[17]. For both acute and late toxicity, the maximal skin reaction was assessed independent of the location within the irradiated breast. Our retrospective design did not allow a thorough assessment of cardiac or lung toxicity, but most patients were treated in the lateral decubitus position. We previously reported that very low doses are delivered to the underlying lung and heart using this technique[18]. Moreover, no IMC irradiation was delivered for minimizing the doses to the heart.
At the end of the HFRT course, acute toxicity was low in most patients. Nineteen patients (28.8%) experienced no toxicity. Acute grade 1 epithelitis was observed in 38 patients (57.6%). Eight patients (12.1%) developed grade 2 epithelitis. One grade 3 skin toxicity (1.5%) was observed. No grade 4 or greater acute skin toxicity was observed. Median delay between HFRT initiating and first skin reaction was 28 d (range: 13-50 d). Median dose to first skin reaction was 31.25 Gy (range: 13-45.5 Gy). No treatment disruption was necessary and no clinical acute lung or cardiac toxicity was reported.
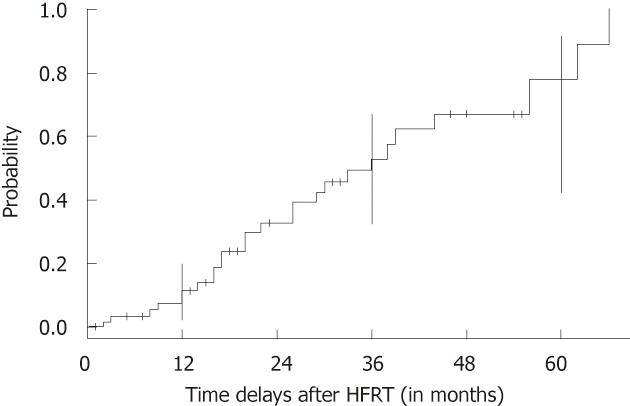
With a median follow-up of 34 mo (range: 12-94 mo), 31 patients (47%) had no delayed skin or subcutaneous toxicity, and 35 patients (53%) presented with grade 1-2 fibrosis. No grade 3 or greater delayed skin toxicity was observed. Figure 1 shows the probability of developing late skin sequelae according to the time delay after breast HFRT. No irradiation-related cardiac or pulmonary delayed toxicity was reported. A multivariate analysis was performed for determining whether fractionation, surgery, or boost delivery could impact on the probability of developing acute or late skin reaction. No significant relation was found between these factors and the cosmetic outcome. Similarly, acute and long-term grade 1 and 2 skin toxicity did not differ among different AIs. Acute and late skin toxicity data are summarized in Table 3.
| Skin toxicity | n (%) |
| Acute toxicity | |
| Grade 0 | 19 (28.8) |
| Grade 1 | 38 (57.6) |
| Grade 2 | 8 (12.1) |
| Grade 3 | 1 (1.5) |
| Grade 4 | 0 (0) |
| Long-term toxicity | |
| Grade 0 | 31 (47.0) |
| Grade 1-2 | 35 (53.0) |
| Grade 3-4 | 0 (0) |
Seventeen patients (25.5%) discontinued endocrine therapy before completion of treatment for 5 years. Reasons for endocrine therapy discontinuation are detailed in Table 4.
| Reasons for discontinuation | n (%) |
| Thromboembolic event | 4 (6.0) |
| Progression | 3 (4.5) |
| Patient death | 5 (7.5) |
| Clinical intolerance | 5 (7.5) |
| Total discontinuations | 17 (25.5) |
There is clinical evidence that 5 years of adjuvant AI anastrozole improves recurrence-free survival in postmenopausal early breast cancer patients. Results from the ATAC trial demonstrated that recurrence rates remained significantly lower on anastrozole compared with tamoxifen [HR 0.75 (0.61-0.94), P = 0.01][19]. As a matter of fact, AI therapy inhibits the aromatase enzyme function and prevents the conversion of androgens to estrogens. AI therapy has logically become standard adjuvant therapy for postmenopausal women with hormone receptor positive cancers. However, most breast cancer patients also receive adjuvant breast or chest well RT. Up till now, there has been little data available on the rationale for concomitant use of AI in adjuvant RT settings.
The optimal sequence for adjuvant endocrine therapy and RT represents a challenge for the clinician[20]. In vitro results by Azria et al[10] demonstrated that letrozole sensitizes breast cancer cells to radiation doses ranging from 0 to 4 Gy. Their results suggested possible additive effects for the combined treatment, supporting concurrent use of AI and RT in postsurgical settings for more clinical efficacy. Although this increased sensitivity might theoretically translate into greater toxicity, most data from literature suggest that AI could be safely given concurrently with normofractionated RT.
We have already reported that hormone therapy and RT could be given concurrently to post-menopausal patients with both good efficacy (57% partial responses, 24% partial response and 21% stable disease) and acceptable tolerance[21]. However, only 10% patients had received AI[21]. Ishitobi et al[11] assessed the optimal sequence of adjuvant AI and RT. They compared concurrent vs sequential treatment for patients with hormone receptor-positive breast cancer treated with BCS. At a median follow-up of 2.9 years, authors found no difference in the breast cancer outcomes and treatment-related complications between the two treatment groups. These retrospective results suggested that both concurrent and sequential use of normofractionated postoperative RT and adjuvant AI therapy were feasible in terms of the breast cancer outcomes and toxicity[11]. Finally, the safety of AI and concurrent adjuvant radiotherapy was prospectively confirmed by the CO-HO-RT study. In this randomized phase II study, Azria et al[12] found no increase in skin toxicity in breast cancer patients receiving letrozole and concurrent normofractionated breast radiotherapy, delivering 2 Gy per daily fraction. Of importance, concurrent AI did not influence the efficacy of irradiation at a median follow-up of 26 mo.
HFRT could be safe and could be used in post menopausal and or in elderly patients with good local control and acceptable toxicity[13,15]. In some cases these patients are already being treated with IA and the interruption of this treatment is, in some cases, a problem. Therefore the question being asked is interesting and the same time important for everyday practice. While HFRT alone might theoretically increase skin toxicity, no data has been previously reported on AI and concurrent HFRT. Since elderly patients are likely to receive concurrent AI, it is also clinically relevant to determine whether concurrent HFRT and AI could increase toxicity. We found that this association was well tolerated. All skin toxicities were mild to moderate and no treatment disruption was necessary. Multiple known factors influence the severity of acute and late reactions, for example the total dose, beam energy, breast volume, observation time, or type of surgery. Our study population was quite heterogeneous in regard to these factors: 53% of the patients received a boost, 53% underwent surgery, which may significantly influence the severity of fibrosis compared to patients without surgery, 86% of patients were treated with cobalt units, and finally, different AIs were used. We failed to evidence any significant relation between all these factors and the risk of skin toxicity, but our patients’ population was probably insufficient for such analysis. Although biased by retrospective analysis and limits inherent to the study design, we found that AI could be safely administered concurrently with HFRT. In the prospective trial by Whelan et al[15], 77.9% of patients had an excellent or good global cosmetic outcome. The results presented here are rather comparable for cosmetic outcome. In their prospective trial, the authors reported that poor cosmetic outcome was reported in only 1.6% of patients. Although any indirect comparison is debatable, we observed no grade 3 or greater late skin toxicity when combining HFRT with AIs. Good tolerance could be obtained using techniques adapted to be less toxic and adapted to patients’ anatomy as previously reported[18]. This report was not designed for assessment of potential cardiac or lung toxicity, but our patients were treated in the lateral decubitus position. This technique provides several advantages over more conventional techniques, including total avoidance of cardiac and/or lung irradiation[18]. However, cardiac toxicity may be associated with exposure to radiation doses lower than 4 Gy, suggesting that the theoretical risk of cardiac toxicity should not be underestimated in the setting of adjuvant HFRT[22]. Obviously, our study is limited by biases inherent to the retrospective nature of the analysis. Prospective confirmation will be mandatory.
We found that AI was well tolerated concurrently with HFRT. All toxicities were mild to moderate, and no treatment disruption was necessary. Although retrospective, our study suggests that AI could be given concurrently with HFRT in postmenopausal breast cancer patients without jeopardizing the cosmetic results. Confirmatory prospective assessments are, however, warranted before translating these results into clinical practice.
A part of this work has been presented at the 2010 annual meeting of the American Society for Therapeutic Radiology and Oncology, San Diego, United States, the authors thank Chantal Gautier for her precious help.
A recent randomized phase II trial has demonstrated that conventionally fractionated radiotherapy and aromatase inhibitors could be safely delivered concurrently as adjuvant therapy in breast cancer patients. There is however no data in the literature regarding the use of aromatase inhibitors concurrently with hypofractionated radiotherapy, which is frequently proposed as an alternative to standard fractionation in elderly patients treated with a breast conservative strategy.
The optimal sequence for adjuvant endocrine therapy and radiation therapy (RT) remains undetermined. In vitro data reported that aromatase inhibitors sensitize breast cancer cells to ionizing radiation. This suggested possible additive effects for the combined treatment. This increased sensitivity might translate into greater toxicity.
Although prospective confirmation is warranted, this is the fist study to report how aromatase inhibitors are tolerated when given concurrently with hypofractionated radiotherapy, and how those can affect on cosmetic outcome. Acute high-grade toxicities were reported in 1.5% of patients. With a median follow-up of 34 mo, no grade 3 or greater delayed toxicity was observed.
This study suggests that treatment with aromatase therapy could safely continued during the irradiation process in patients receiving hypofractionated radiotherapy. However, multiple other factors can influence the severity of acute and late reactions. Those should be carefully considered when choosing the optimal technique for adjuvant radiation therapy.
Hypofractionated RT refers to the use of a lower number of fractions, each fraction delivering a higher dose than the standard schedule (> 2 Gy per fraction). It has demonstrated non-inferiority for adjuvant treatment of breast cancer in post-menopausal patients with good local control and acceptable toxicity.
The authors report on a retrospective study assessing the acute and late effects of aromatase inhibitor therapy concurrently with hypofractionated radiotherapy in breast cancer patients. They concluded that aromatase inhibitor was well tolerated. While numerous other factors influence the severity of acute and late reactions, the study was not designed to assess cardiac toxicity. Further prospective assessment is therefore recommended.
Peer reviewers: Ioannis G Valais, PhD, Department of Medical Instrument Technology, Technological Educational Institution of Athens, Ag Spyridonos and Dimitsanis, Egaleo, 12210 Athens, Greece; Volker Rudat, Professor, Department of Radiation Oncology, Saad Specialist Hospital, PO Box 30353, Al Khobar 31952, Saudi Arabia
S- Editor Cheng JX L- Editor O’Neill M E- Editor Xiong L
| 1. | Lin NU, Winer EP. Advances in adjuvant endocrine therapy for postmenopausal women. J Clin Oncol. 2008;26:798-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, Dimitrov NV, Wolmark N, Wickerham DL, Fisher ER. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1020] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 3. | Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. Swedish Breast Cancer Cooperative Group. J Natl Cancer Inst. 1996;88:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 230] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Dalberg K, Johansson H, Johansson U, Rutqvist LE. A randomized trial of long term adjuvant tamoxifen plus postoperative radiation therapy versus radiation therapy alone for patients with early stage breast carcinoma treated with breast-conserving surgery. Stockholm Breast Cancer Study Group. Cancer. 1998;82:2204-2211. [PubMed] |
| 5. | Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1162] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 6. | Kaufmann M, Jonat W, Hilfrich J, Eidtmann H, Gademann G, Zuna I, von Minckwitz G. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol. 2007;25:2664-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Boccardo F, Rubagotti A, Puntoni M, Guglielmini P, Amoroso D, Fini A, Paladini G, Mesiti M, Romeo D, Rinaldini M. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol. 2005;23:5138-5147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 333] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 8. | Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60-62. |
| 9. | Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, Hilfrich J, Kwasny W, Menzel C, Samonigg H. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455-462. |
| 10. | Azria D, Larbouret C, Cunat S, Ozsahin M, Gourgou S, Martineau P, Evans DB, Romieu G, Pujol P, Pèlegrin A. Letrozole sensitizes breast cancer cells to ionizing radiation. Breast Cancer Res. 2005;7:R156-R163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (2)] |
| 11. | Ishitobi M, Komoike Y, Motomura K, Koyama H, Nishiyama K, Inaji H. Retrospective analysis of concurrent vs. sequential administration of radiotherapy and hormone therapy using aromatase inhibitor for hormone receptor-positive postmenopausal breast cancer. Anticancer Res. 2009;29:4791-4794. [PubMed] |
| 12. | Azria D, Belkacemi Y, Romieu G, Gourgou S, Gutowski M, Zaman K, Moscardo CL, Lemanski C, Coelho M, Rosenstein B. Concurrent or sequential adjuvant letrozole and radiotherapy after conservative surgery for early-stage breast cancer (CO-HO-RT): a phase 2 randomised trial. Lancet Oncol. 2010;11:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Kirova YM, Campana F, Savignoni A, Laki F, Muresan M, Dendale R, Bollet MA, Salmon RJ, Fourquet A. Breast-conserving treatment in the elderly: long-term results of adjuvant hypofractionated and normofractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Chargari C, Kirova YM, Laki F, Savignoni A, Dorval T, Dendale R, Bollet MA, Fourquet A, Campana F. The impact of the loco-regional treatment in elderly breast cancer patients: hypo-fractionated exclusive radiotherapy, single institution long-term results. Breast. 2010;19:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1204] [Cited by in RCA: 1277] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 16. | International Commission on Radiation Units and Measurements. ICRU report 50: Prescribing, recording, and reporting photon beam therapy. Bethesda: ICRU 1999; . |
| 17. | Pavy JJ, Denekamp J, Letschert J, Littbrand B, Mornex F, Bernier J, Gonzales-Gonzales D, Horiot JC, Bolla M, Bartelink H. EORTC Late Effects Working Group. Late effects toxicity scoring: the SOMA scale. Radiother Oncol. 1995;35:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 213] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Campana F, Kirova YM, Rosenwald JC, Dendale R, Vilcoq JR, Dreyfus H, Fourquet A. Breast radiotherapy in the lateral decubitus position: A technique to prevent lung and heart irradiation. Int J Radiat Oncol Biol Phys. 2005;61:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Buzdar A, Howell A, Cuzick J, Wale C, Distler W, Hoctin-Boes G, Houghton J, Locker GY, Nabholtz JM. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7:633-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 290] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Chargari C, Toillon RA, Macdermed D, Castadot P, Magné N. Concurrent hormone and radiation therapy in patients with breast cancer: what is the rationale. Lancet Oncol. 2009;10:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Bollet MA, Kirova YM, Antoni G, Pierga JY, Sigal-Zafrani B, Laki F, Campana F, Dendale R, Salmon R, Cottu P. Responses to concurrent radiotherapy and hormone-therapy and outcome for large breast cancers in post-menopausal women. Radiother Oncol. 2007;85:336-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 778] [Article Influence: 38.9] [Reference Citation Analysis (0)] |