Published online May 28, 2012. doi: 10.4329/wjr.v4.i5.220
Revised: March 31, 2012
Accepted: April 7, 2012
Published online: May 28, 2012
AIM: To analyze changes in myocardial glucose metabolism using fluorodeoxyglucose (FDG)-positron emission tomography (PET) in patients treated with adriamycin and to investigate the clinical significance of these changes.
METHODS: Considering that FDG-PET scanning has the ability to show changes in glucose metabolism in the myocardium, we retrospectively analyzed the FDG-PET studies of 18 lymphoma patients treated with adriamycin-based chemotherapy in both the pre- and post-therapy setting. Cardiac contractile parameters such as left ventricular ejection fraction were not available for correlation in all patients due to the short duration and the level of cumulative dose administered in these patients during the time of the follow-up FDG-PET study. The change in myocardial glucose utilization was estimated by change in standard uptake values (SUV) in the myocardium.
RESULTS: We observed a significant change in SUVmean values in the myocardium (defined as more than ± 20% change in cardiac SUVmean between pre- and post-chemotherapy PET) in 12 patients, whereas 6 patients did not show any significant cardiac FDG uptake in both pre- and post-therapy PET scans. Patients were divided into three groups based on the changes observed in myocardial tracer uptake on the follow-up 18F-FDG-PET study. Group A (n = 8): showed an increase in cardiac 18F-FDG uptake in the post-therapy scan compared to the baseline scan carried out prior to starting adriamycin-based chemotherapy. Group B (n = 6): showed no significant cardiac 18F-FDG uptake in post-therapy and baseline PET scans, and group C (n = 4): showed a fall in cardiac 18F-FDG uptake in the post-therapy scan compared to the baseline scan. Mean cumulative adriamycin dose (in mg/m2) received during the time of the follow-up FDG-PET study was 256.25, 250 and 137.5, respectively.
CONCLUSION: Our study shows three different trends in the change in myocardial glucose metabolism in patients undergoing adriamycin-based chemotherapy. A further prospective study with prolonged follow-up of ventricular function is warranted to explore the significance of enhanced FDG uptake as a marker of early identification of adriamycin-induced cardiotoxicity.
- Citation: Borde C, Kand P, Basu S. Enhanced myocardial fluorodeoxyglucose uptake following Adriamycin-based therapy: Evidence of early chemotherapeutic cardiotoxicity? World J Radiol 2012; 4(5): 220-223
- URL: https://www.wjgnet.com/1949-8470/full/v4/i5/220.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i5.220
Anthracycline-based chemotherapeutics such as doxorubicin (i.e., adriamycin) and daunorubicin are some of the most effective agents used in the treatment of cancer; however, their administration is limited by cumulative, dose-related, progressive myocardial damage that may lead to congestive heart failure (CHF)[1-3]. It is well known that adriamycin-induced cardiac failure is a cumulative phenomenon which is caused by steadily accumulating sub-clinical myocardial damage. Adriamycin appears to cause most of its cardiotoxic effects due to the formation of reactive oxygen species or free radicals that interact with mitochondrial membranes leading to damage of these intracellular organelles. Such repeated sub-clinical damage to mitochondria alters metabolism within myocytes and ultimately leads to myocyte apoptosis and necrosis[4-7].
Currently, periodical assessment of left ventricular ejection fraction (LVEF) either by echocardiography and/or radionuclide ventriculography remains the only way of monitoring myocardial function in adriamycin-treated patients. However, it is clear from the above that such contractile parameter monitoring which manifests as late effects clearly lacks the potential to observe early subcellular changes and damage[8-10].
A large-scale study of 630 patients randomized to an adriamycin or placebo arm in three, phase III studies during 1988 to 1992, reported a 5.1% risk of CHF in patients receiving cumulative doses of up to 400 mg/m2, rising to 48% in patients receiving doses of up to 700 mg/m2. However, CHF was reported in patients receiving as little as 300 mg/m2 of adriamycin[11]. Hence, it is clear that although the probability of developing heart failure is proportional to the cumulated dose of adriamycin, there may be significant variation in the tolerance to adriamycin from one individual to the other. Therefore, any observations that can provide clues to future cardiac toxicity are of substantial benefit in individualizing therapy in these patients.
One of the factors identified leading to the variable response to adriamycin is the varied baseline expression of neuregulin (NRG) in different individuals[12]. NRG is a protein secreted by the endocardium and endothelium of cardiac vasculature which binds to erbB2 and erbB4 on cardiac myocytes by a paracrine mode of signaling. NRG-erbB signaling is important in normal development of the myocardium and in adult hearts; it plays a role in myocardial protection, especially in the presence of external insults[13-15]. In cardiac myocyte cultures, the presence of NRG protects against the effects of adriamycin[16]. On the other hand, trastuzumab, an erbB antagonist, is known to increase the cardiotoxic effects of adriamycin[17-21]. Hence, some individuals showing greater tolerance to adriamycin therapy may have better NRG expression compared to individuals who show lower tolerance to adriamycin.
Activation of the NRG-erbB pathway as well as mitochondrial damage after adriamycin therapy can enhance cardiac myocyte glucose utilization as an adaptive pattern in such patients[13,14]. Positron emission tomography (PET) using 18F-fluorodeoxyglucose (FDG) as the radiotracer has established its role in demonstrating myocardial glucose metabolism in vivo[22-26]. In our study we attempted to observe changes in myocardial glucose utilization as reflected by changes in standard uptake values (SUV) in the myocardium of patients treated with adriamycin-based chemotherapy.
We retrospectively analyzed cardiac glucose uptake in the PET scans of 18 lymphoma patients (16 males and 2 females). These patients underwent whole body 18F-FDG PET scans as a routine part of disease evaluation before and after adriamycin-based chemotherapy. PET scans were acquired on a GE Advance system (BGO crystal) 45 min after injection of 10 mCi of 18F-FDG under fasting conditions. 68Ge source was used for attenuation correction. The cardiac uptake on both pre- and post-chemotherapy whole body PET images was identified and separately processed using standard software provided by GE Advance. The cardiac short axis images were used to generate a polar map (Bull’s eye) and to easily exclude the blood background. Cardiac uptake was quantified by calculating the SUVmean (gm/mL) value using this polar map. SUVmean values were used to minimize the region to region variations in myocardial 18F-FDG uptake. To limit the variability in baseline cardiac metabolism, the following exclusion criteria were used: (1) Patients with fasting blood sugar > 150 mg% or < 80 mg%; (2) Patients with h/o diabetes mellitus, coronary artery disease or any other cardiac ailments; and (3) Patients with a difference in fasting blood sugar of ± 10% during the pre- and post-therapy PET scans.
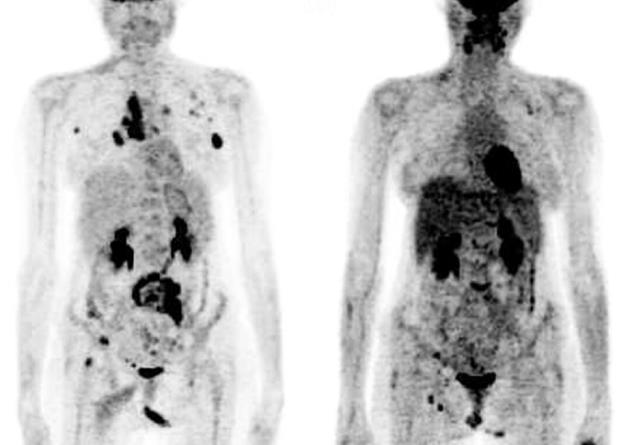
Patients were divided into three groups based on the changes observed in the myocardial tracer uptake in the follow-up 18F-FDG-PET study (Table 1). A cut off value of ± 20% change in cardiac SUVmean between pre- and post-chemotherapy PET scans was considered significant for each patient. Group A: showed an increase in cardiac 18F-FDG uptake in the post-therapy scan compared to the baseline scan carried out prior to starting adriamycin-based chemotherapy (an example is shown in Figure 1). Group B: showed no significant cardiac 18F-FDG uptake in post-therapy and baseline PET scans, group C: showed a fall in cardiac 18F-FDG uptake in the post-therapy compared to the baseline scan.
| Group of patients | n | Mean dose of adriamycin (mg/m2) | Mean duration in days since last chemotherapy to PET scan | Mean difference in cardiac SUVmean (preRx-postRx) |
| Group A | 8 | 256.25 | 15.12 | 4.668 |
| Group B | 6 | 250 | 21.5 | Nil |
| Group C | 4 | 137.5 | 18.5 | -4.138 |
The patients in group A clearly demonstrated increased glucose utilization by cardiac myocytes, whereas group B and group C patients demonstrated non-glucose substrate utilization during metabolism. Unfortunately, cardiac contractile parameters such as LVEF were not available for correlation in these patients due to the short duration and the cumulative dose administered in these patients during the time of the follow-up FDG-PET study. However, it is possible that the metabolic shift preceded the manifestations of cardiotoxicity that could be quantified using contractile dysfunction.
We speculate that it may be possible to identify patients who are susceptible to an adriamycin-induced shift in myocardial metabolism using 18F-FDG-PET. In addition, it is possible to explain the increased glucose utilization as evidenced by greater 18F-FDG trapping within the myocardium post-adriamycin administration, as a marker of cellular alteration preceding the cardiotoxicity cascade. We believe that in group A patients, the dose of adriamycin may have reached an individual limit leading to activation of the NRG-erbB pathway and increased glucose utilization by cardiac myocytes. This hypothesis requires further validation by a larger retrospective or prospective study and correlation with baseline and follow-up cardiac contractile function.
There are certain limitations related to this study: (1) Due to the limited duration of the study, none of the patients developed any obvious signs/symptoms of cardiac failure. However, we must consider that any observable clinical changes in adriamycin-related cardiotoxicity may only appear after a prolonged period of time and may span several years. This was beyond the scope of this retrospective observational study and only a long-term prospective study would provide definitive answers; and (2) As this was a retrospective analysis, no control patients with established cardiotoxicity were available. As clinically observable signs of cardiotoxicity are likely to take longer to appear, such a study population requires to be followed for a longer period in a prospective setting.
Due to the aforementioned two reasons, we propose further prospective studies to evaluate the role of metabolic shift imaging in identifying and monitoring adriamycin-induced cardiac metabolism alterations and cardiotoxicity, which may pave the way for individual-based chemotherapy regimens by optimizing drug dose administration and minimizing toxicity. Such prospective studies, we believe, will allow stringent pre-scan setting, and thus normalize certain variables that can influence cardiac 18F-FDG uptake which may not be possible using a retrospective approach. If the theory proposed in this communication is validated by future studies, it would have a strong potential clinical impact in assessing and preventing cardiac toxicity secondary to adriamycin treatment.
To conclude, we observed significant differences in the pattern of change in myocardial FDG uptake in pre-and post-therapy FDG PET scans in patients treated with adriamycin. Although this study was unable to establish any statistical correlation between the dose of adriamycin and change in cardiac FDG uptake, we speculate that these changes can be ascribed to some unknown individual factors. As it is known that individual response to adriamycin-induced cardiotoxicity differs, we propose further studies to unravel the changes in cardiac metabolism in vivo in response to adriamycin and its impact on the development of adriamycin-induced cardiotoxicity.
Anthracyclines (such as adriamycin) are commonly employed and are effective chemotherapy drugs. However, dose-related cumulative cardiotoxicity is the major side effect of this agent. At present, the assessment of cardiac contractile function using MUGA or ECHO is the standard method for monitoring patients treated with adriamycin. However, contractile parameters of the myocardium show delayed changes in most patients and fail to identify patients with subclinical damage early in the disease process.
Recent reports show that the deleterious action of adriamycin on myocardium is predominantly due to free radical-induced mitochondrial injury. Such injury may lead to altered glucose metabolism in the myocardium. Animal models have demonstrated that metabolic changes induced by adriamycin precede contractile dysfunction. Fluorodeoxyglucose (FDG) positron emission tomography (PET) scanning may be a sensitive tool to identify such metabolic changes.
Recent reports have identified the molecular pathways responsible for cardiac damage caused by adriamycin, as well as cardiac protection pathways such as the neuregulin-erbB pathway. These pathways are being exploited to minimize the cardiotoxic effects of chemotherapy agents and to generate specific cardioprotective mechanisms.
FDG PET scanning may be a potential modality to identify early changes in patients with subclinical cardiotoxicity induced by adriamycin-based chemotherapy. Such developments in screening for cardiotoxicity will help in individualizing the dose of cardiotoxic chemotherapy, and thus maximize the therapeutic advantage of chemotherapy, while limiting its toxic effects in the future.
This is an interesting descriptive study that is hypothesis generating at best. The topic is of high interest and importance to the scientific community.
Peer reviewer: Monvadi Barbara Srichai-Parsia, MD, Department of Radiology and Medicine, NYU School of Medicine, 660 First Avenue, 2nd Floor, NY 11211, United States
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
| 1. | van der Pal HJ, van Dalen EC, Hauptmann M, Kok WE, Caron HN, van den Bos C, Oldenburger F, Koning CC, van Leeuwen FE, Kremer LC. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170:1247-1255. [PubMed] |
| 2. | Panjrath GS, Jain D. Monitoring chemotherapy-induced cardiotoxicity: role of cardiac nuclear imaging. J Nucl Cardiol. 2006;13:415-426. [PubMed] |
| 3. | Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572-1582. [PubMed] |
| 4. | Lebrecht D, Setzer B, Ketelsen UP, Haberstroh J, Walker UA. Time-dependent and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation. 2003;108:2423-2429. [PubMed] |
| 5. | Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900-905. [PubMed] |
| 6. | Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789-1792. [PubMed] |
| 7. | Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: In vivo study. Circulation. 2000;102:572-578. [PubMed] |
| 8. | Corapçioglu F, Sarper N, Berk F, Sahin T, Zengin E, Demir H. Evaluation of anthracycline-induced early left ventricular dysfunction in children with cancer: a comparative study with echocardiography and multigated radionuclide angiography. Pediatr Hematol Oncol. 2006;23:71-80. [PubMed] |
| 9. | Altena R, Perik PJ, van Veldhuisen DJ, de Vries EG, Gietema JA. Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol. 2009;10:391-399. [PubMed] |
| 10. | Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387-1396. [PubMed] |
| 11. | Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869-2879. [PubMed] |
| 12. | Ewer MS, Gibbs HR, Swafford J, Benjamin RS. Cardiotoxicity in patients receiving transtuzumab (Herceptin): primary toxicity, synergistic or sequential stress, or surveillance artifact? Semin Oncol. 1999;26:96-101. [PubMed] |
| 13. | Liu FF, Stone JR, Schuldt AJ, Okoshi K, Okoshi MP, Nakayama M, Ho KK, Manning WJ, Marchionni MA, Lorell BH. Heterozygous knockout of neuregulin-1 gene in mice exacerbates doxorubicin-induced heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H660-H666. [PubMed] |
| 14. | Peng X, Chen B, Lim CC, Sawyer DB. The cardiotoxicology of anthracycline chemotherapeutics: translating molecular mechanism into preventative medicine. Mol Interv. 2005;5:163-171. [PubMed] |
| 15. | De Keulenaer GW, Doggen K, Lemmens K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res. 2010;106:35-46. [PubMed] |
| 16. | Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551-1554. [PubMed] |
| 17. | Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-1672. [PubMed] |
| 18. | Cook-Bruns N. Retrospective analysis of the safety of Herceptin immunotherapy in metastatic breast cancer. Oncology. 2001;61 Suppl 2:58-66. [PubMed] |
| 19. | Perik PJ, de Korte MA, van Veldhuisen DJ, Gietema JA, Sleijfer DT, de Vries EG. Cardiotoxicity associated with the use of trastuzumab in breast cancer patients. Expert Rev Anticancer Ther. 2007;7:1763-1771. [PubMed] |
| 20. | Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215-1221. [PubMed] |
| 21. | Perez EA, Rodeheffer R. Clinical cardiac tolerability of trastuzumab. J Clin Oncol. 2004;22:322-329. [PubMed] |
| 22. | Maddahi J, Schelbert H, Brunken R, Di Carli M. Role of thallium-201 and PET imaging in evaluation of myocardial viability and management of patients with coronary artery disease and left ventricular dysfunction. J Nucl Med. 1994;35:707-715. [PubMed] |
| 23. | Tillisch J, Brunken R, Marshall R, Schwaiger M, Mandelkern M, Phelps M, Schelbert H. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med. 1986;314:884-888. [PubMed] |
| 24. | Eitzman D, al-Aouar Z, Kanter HL, vom Dahl J, Kirsh M, Deeb GM, Schwaiger M. Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography. J Am Coll Cardiol. 1992;20:559-565. [PubMed] |
| 25. | Marshall RC, Tillisch JH, Phelps ME, Huang SC, Carson R, Henze E, Schelbert HR. Identification and differentiation of resting myocardial ischemia and infarction in man with positron computed tomography, 18F-labeled fluorodeoxyglucose and N-13 ammonia. Circulation. 1983;67:766-778. [PubMed] |
| 26. | Voipio-Pulkki LM, Nuutila P, Knuuti MJ, Ruotsalainen U, Haaparanta M, Teräs M, Wegelius U, Koivisto VA. Heart and skeletal muscle glucose disposal in type 2 diabetic patients as determined by positron emission tomography. J Nucl Med. 1993;34:2064-2067. [PubMed] |