INTRODUCTION
The main indications for transarterial Embolisation (TAE) are to reduce preoperative and postoperative blood loss in hypervascular tumors, to simplify the excision of tumors, as a palliative measure to reduce pain, blood loss, pyrexia, and hypercalcemia associated with inoperable tumors, and in certain tumors to increase the response to chemotherapy and radiotherapy[1]. Of these indications, most TAEs are performed as a pretreatment procedure to reduce blood loss associated with excision of many metastatic or primary hypervascular lesions. The principle behind TAE of bone tumors, as for other regions, is the precise targeting of the occlusive embolic material to tumor feeding vessels. The aim of TAE is the exclusion of the tumor capillary bed and not the major arterial feeder, as occluding only the major vessel supplying the tumor leads to revascularization of the tumor from other routes[2]. Embolic agents are classified into liquid and particulate. The most critical factor in the choice of an occlusive agent is operator experience. In general, particulate agents are easier to handle and require less operator experience compared to liquid agents[3]. The main concern in TAE is non-target Embolisation and occlusion of a non-target vessel. This can be prevented by careful review of the angiograms obtained immediately before Embolisation and meticulous care during injection of the embolic agent. In some cases, TAE may be contraindicated due to the origin of a vital artery from a tumor feeding artery, e.g., spinal artery. However, overall TAE is a safe procedure.
Embolisation AGENTS
The choice of embolic agent depends on the individual tumor vasculature including vessel calibre, presence of arteriovenous shunts, collateral supply to or from adjacent normal tissues and most important, operator experience[3]. Embolisation agents can be classified based on whether temporary or permanent and on the basis of their physical state as liquid and particulate.
Liquid Embolisation agents include glue [N-butyl cyanoacrylate (NBCA)], absolute alcohol, Ethibloc (Ethicon, Norderstedt, Germany), sodium tetradecyl sulfate, Onyx (Microtherapeutics, Irvine, CA, USA) and particulate agents include Embosphere™ (Biosphere, France), polyvinyl alcohol (PVA)-particles (Contour™, BSIC; Cook Inc., Bloomington, IN, USA) and Gelfoam™ (Pharmacia, USA). In general, liquid embolics result in more tumor necrosis than particulate agents and are advantageous when definitive treatment is desired, but carry an increased risk of non-target Embolisation and catheter gluing, particularly in the case of tissue adhesives (cyanoacrylates) compared with particulate agents[5]. As bone is a non-end organ supplied by multiple arteries, liquid agents may be associated with a greater risk of non-target Embolisation than in other tissues[6]. Available evidence involving the use of liquid embolic agents in TAE of bone tumors is limited; in general, when TAE is performed to devascularize the tumor, there is little advantage over particulate embolics.
Particulate agents are relatively easy to handle. An error in particle size selection, however, may lead to venous efflux with potential for pulmonary embolism[7] when smaller particles are used, or reflux when used in too large sizes or amounts or fast injections.
PVA is available in sizes ranging from 50 to 1000 μm; the commonest size used is in the 300-500 μm range. PVA has several desirable characteristics which makes it a commonly used agent. It is capable of penetrating and occluding the tumor blood supply, and it is relatively inexpensive and easy to handle. However, PVA particles can aggregate and form clumps causing catheter occlusion and some unpredictability in the level of Embolisation.
Embosphere™ (Biosphere, France) are engineered PVA particles; have a more uniform size and are compressible with easier delivery through small catheters[6]. According to some authors, the 300-500 μm sized Embosphere™ particles are first choice agents[3].
Gelfoam or gelatin sponge is a dissolvable sponge-like material; is considered a temporary Embolisation agent; the occluded artery usually recanalizes within a month.
Coils are usually reserved for occlusion of the distal segment of large feeding arteries, prior to superselective TAE of a tumor with particulate agent. This technique known as “backdoor Embolisation” prevents the occlusion of vessels supplying back and anterior abdominal wall muscles and also improves the efficacy of Embolisation by preventing recruitment of collaterals[3]. They have a potential role in the emergency setting with an inexperienced operator or when time is lacking for other forms of Embolisation.
The basic principle in TAE is the occlusion of most of the capillary tumor bed. The occlusion of only the major tumor feeding arteries is ineffective, because of numerous collaterals in hypervascular bone tumors[2].
When large intervertebral-, intercostal- or lumbar-feeding tumor arteries are targeted, backdoor Embolisation is recommended. Ideally, surgery must be performed within 3 d of Embolisation in order to avoid revascularization. Besides non-target Embolisation, a more common complication is “post-Embolisation” syndrome. This presents with pain at the target site, fever, headache and malaise. This syndrome is encountered more commonly in hepatic and renal Embolisation.
PRIMARY BONE TUMORS
Aneurysmal bone cyst
Aneurysmal bone cysts (ABCs) are highly vascular benign bone tumors. Management of ABCs depends upon their location and aggressiveness. Aggressive extremity lesions are usually treated with intralesional curettage and bone-grafting[8]. Non-aggressive lesions are followed up as they often show spontaneous regression[9]. Pelvic ABCs are large and their treatment is difficult because of the relative inaccessibility of the lesions, massive intraoperative bleeding related to multiple anastomoses formed by branches of major vessels in this region, proximity to neurovascular structures, and the potential risk to the acetabulum or sacroiliac joint[10]. Preoperative TAE can reduce intraoperative and postoperative blood loss and in some cases Embolisation can induce ossification and maturation of ABCs, avoiding surgery altogether (Figures 1 and 2)[11]. Several studies support preoperative TAE as an effective adjunct to surgery. Yildirim et al[12] reported two cases of pelvic ABCs treated with TAE as an adjunct to curettage and bone grafting. They performed Embolisation with PVA 500-700 μm in diameter following catheterization of the main feeding artery arising from the left inferior epigastric which was followed by curettage and bone grafting. There was no evidence of recurrence at 6-mo follow-up. Embolisation of peripheral ABCs has also been reported. Börüban et al[1] described four cases of peripheral ABCs; in three cases, Embolisation was followed by surgery and in one case of a distal femur lesion, Embolisation achieved cure. As regards the Embolisation agent, encouraging results have been reported with the use of NBCA. Marushima et al[13] reported a case of thoracic ABC treated with NBCA which was stable at 3 years following transcatheter Embolisation. Rossi et al[14] reported their experience of using NBCA in the TAE of ABC. They performed 55 Embolisations in 36 patients. The treatment was considered effective in 94% of cases with a follow-up of 2 years.
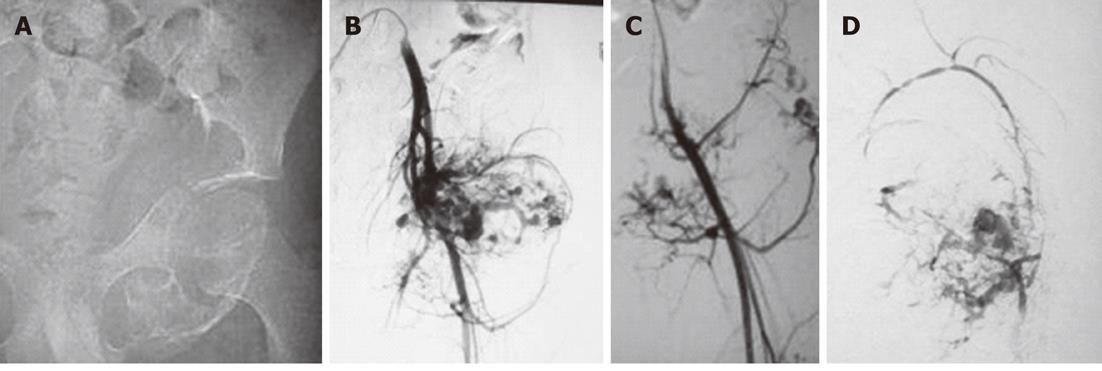
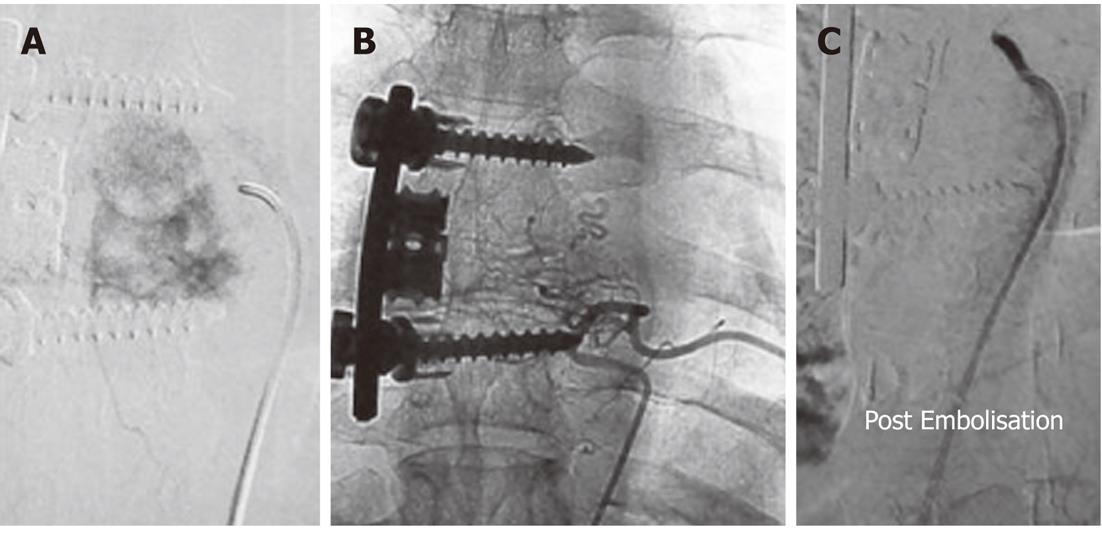
Figure 1 Pre-operative Embolisation of aneurysmal bone cyst of left pelvic bones in a 13-year-old male.
A: An image from a digital subtraction angiography examination of a 13-year-old male showed an expansile lesion involving the left inferior pubic ramus and ischium with no cortical breech or extraosseous soft tissue component. The lesion proved to be an aneurysmal bone cyst on histopathology; B: Angiogram obtained after cannulation of left common iliac artery revealed marked vascularity of the lesion with multiple tumor feeders arising from both the internal and external iliac artery; C: Selective left common femoral angiogram showed the tumour supply from this artery; D: Post-Embolisation angiogram after injection of 95% alcohol selectively into the major feeder arising from both the internal and external iliac arteries showed a marked reduction in tumor blush.
Figure 2 Embolisation of left distal femoral aneurysmal bone cyst in a 42-year-old male.
A: Forty-two-year-old male with an expansile lytic lesion involving the left medial femoral condyle with histopathologic confirmation of aneurysmal bone cyst. Left common femoral angiogram revealed a prominent arterial feeder arising from the superficial femoral artery at the level of the adductor canal; B: Angiogram obtained after selective cannulation of the arterial feeder revealed the marked vascularity of the lesion; C: Embolisation with polyvinyl alcohol particles 300-500 μm was performed following selective cannulation of the feeder. Post-Embolisation angiogram demonstrated marked reduction in tumor blush.
Giant cell tumor
Giant cell tumors (GCTs) are benign locally aggressive and highly vascularized bone tumors. The most common sites of origin are distal femur, proximal tibia and distal radius. Surgery is the treatment modality of choice for these long bone GCTs; however, local recurrence does occur. The spine is rarely involved and the most common location in the spine is the sacrum. Similar to the complexity of surgery for pelvis ABCs, sacral GCTs are difficult lesions to treat surgically. Radiotherapy is contraindicated because of its potential to induce malignant transformation[15]. Several studies have reported Embolisation as an alternative treatment method for non-resectable tumors[16-18]. Börüban et al[1] reported a recurrent GCT lesion in the proximal fibula treated with TAE. Preoperative Embolisation resulted in 80% devascularization and surgery was performed without severe bleeding (Figures 3 and 4). Hosalkar et al[19] reported a series of nine cases treated with repeated TAE (a mean of 4.8 treatments per patient). They employed various embolic techniques and demonstrated a substantial improvement in pain scores and lack of tumor progression in seven of nine cases over a follow-up of 8.96 years. Lin et al[20] reported 18 patients with sacral GCTs managed with TAE over a 26-year period. Embolisations were performed at 2 to 4 mo intervals with the end point being the lack of a hypervascular mass. The results indicated local recurrence rates of 31% at 10 years and 43% at 15 and 20 years, respectively. GCTs may also occur in the areas of the spine other than the sacrum and studies have demonstrated the efficacy of TAE in these circumstances[21]. Tsuchiya et al[22] reported a case of a GCT of the atlas which was treated with combined surgical and preoperative Embolisation of the right vertebral artery.
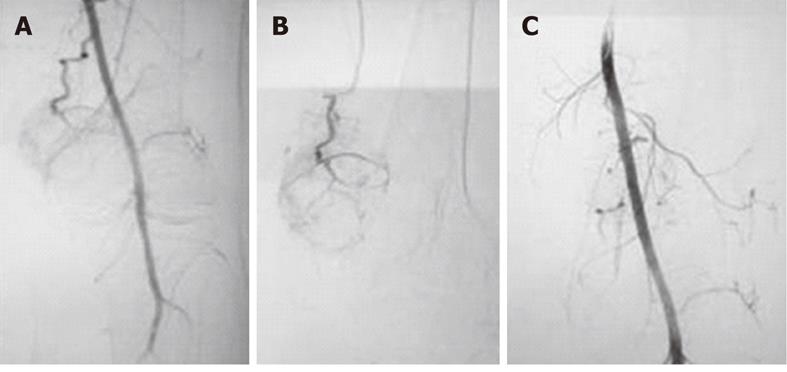
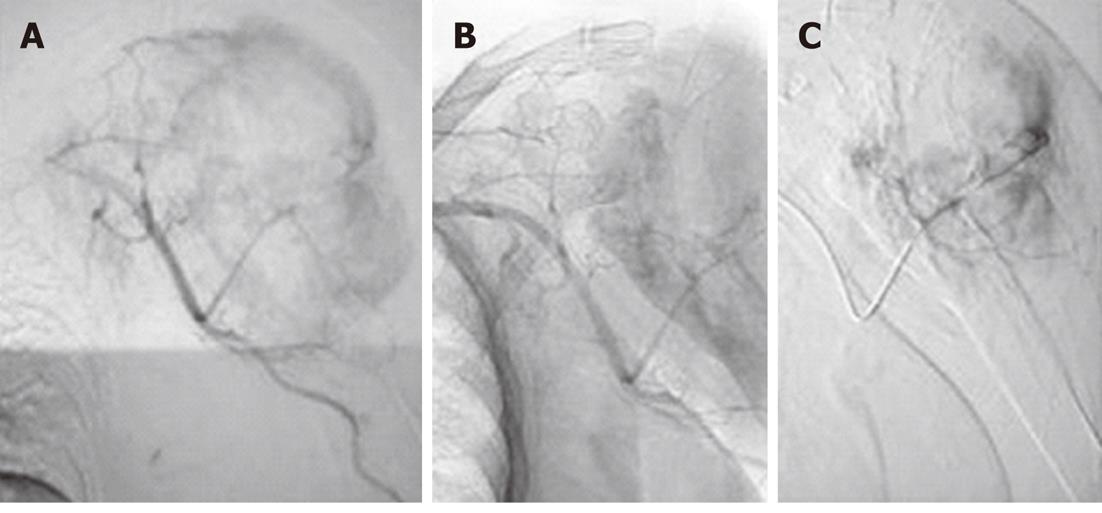
Figure 3 Embolisation of left proximal tibial giant cell tumor in a 29-year-old male.
A: Twenty-nine-year-old male with an eccentric expansile lytic lesion of the left proximal tibial epiphysis extending to the subarticular surface which proved to be a giant cell tumor on histopathology; B: Left common femoral angiogram revealed prominent arterial feeders from the superficial femoral artery with marked tumor blush; C: Angiogram obtained after Embolisation with polyvinyl alcohol particles 300-500 μm shows considerable reduction in tumor blush; D: Postoperative radiographs (AP and lateral) reveal bone cements placed in the defect with multiple screws and fixation plates.
Figure 4 Right iliac giant cell tumor Embolisation in a 25-year-old female.
A: Twenty-five-year old female with a large lytic expansile lesion involving the right iliac wing confirmed to be a giant cell tumor on histopathology. Internal iliac artery angiogram showed marked tumor vascularity from the posterior division; B: A delayed phase image showed marked tumor blush; C: Embolisation was performed with gelfoam. Post-Embolisation angiogram showed the occlusion of a large tumor feeder with reduction in tumor blush.
Osteoblastoma
Osteoblastoma is a benign primary hypervascular tumor, most commonly originating in the spinal column. Surgical resection is the treatment of choice. However, complete resection is often complicated by extensive intraoperative bleeding. TAE reduces intraoperative bleeding, making a complete surgical resection feasible with a reduction in post-operative complications. Silva et al[23] reported successful preoperative TAE of two cases of osteoblastoma in children. Trübenbach et al[24] reported preoperative TAE in three cases of cervical osteoblastoma. Surgical resection could be performed in all cases without significant bleeding and the postoperative course was uneventful.
Vertebral hemangioma
Vertebroplasty for vertebral hemangiomas is generally indicated for lesions without neurological deficit. When patients present with spinal pain or cord compression with neurological deficit, radiation or decompression surgery is the treatment of choice. Massive hemorrhage is often encountered during surgery from these highly vascular lesions. Several authors have shown TAE to be a useful adjunctive method to reduce perioperative blood loss (Figure 5). Jayakumar et al[25] reported 12 patients with symptomatic vertebral hemangiomas in whom particulate Embolisation was performed. Eleven of the 12 patients subsequently underwent decompressive laminectomy. On follow-up at 8 mo, 11 patients showed improvement. Ng et al[26] reported a case of symptomatic thoracic vertebral hemangioma treated with preoperative TAE with NBCA.
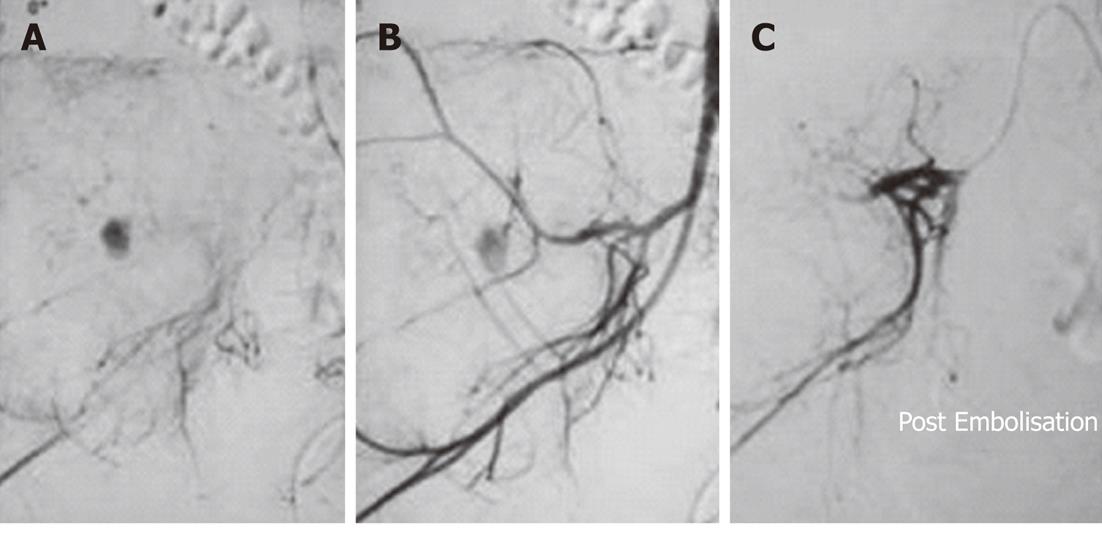
Figure 5 Pre-operative trans-arterial Embolisation of a thoracic vertebral hemangioma in a young male.
A: Twenty-three-year-old male with pain in the upper back for 3 mo. Radiographs revealed an expansile lytic lesion of the left transverse process of the sixth thoracic vertebra. Computed tomographic-guided biopsy of the lesion confirmed it to be a hemangioma. A flush aortogram with an angiographic pigtail placed at the level of the aortic arch identified the tumor feeding artery arising from the sixth left posterior intercostal artery which was selectively cannulated; B: Selective angiogram obtained from the feeder revealed marked tumor blush; C: Angiogram obtained after Embolisation with gelfoam showed almost complete loss of tumor blush.
Osseous arteriovenous malformations
Primary osseous arteriovenous malformations (AVMs) are rare with most lesions involving the maxilla or mandible[27]. There are several reports of TAE or transvenous Embolisation of osseous AVMs. Katzen et al[28] reported TAE of an AVM of the tibia with remission of symptoms at 1 year of follow-up.
Osteosarcoma
Surgery along with adjunctive systemic chemotherapy is the standard treatment for osteosarcomas, however, transarterial chemoEmbolisation in combination with limb salvage surgery has been shown to yield encouraging results (Figure 6). Chu et al[29] reported their experience with transarterial chemoEmbolisation in 32 patients with osteosarcomas who subsequently underwent limb salvage surgery. They employed a triple-drug regimen; following infusion of the chemotherapeutic agents, the vessels were embolized with a variety of embolic agents. They demonstrated a reduced incidence of local recurrence. TAE without chemotherapy has also been shown to be effective as an adjunct to surgery[30,31].
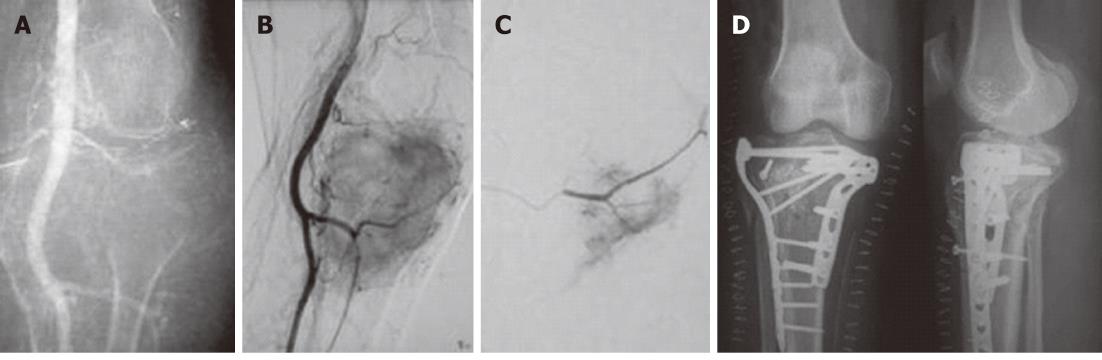
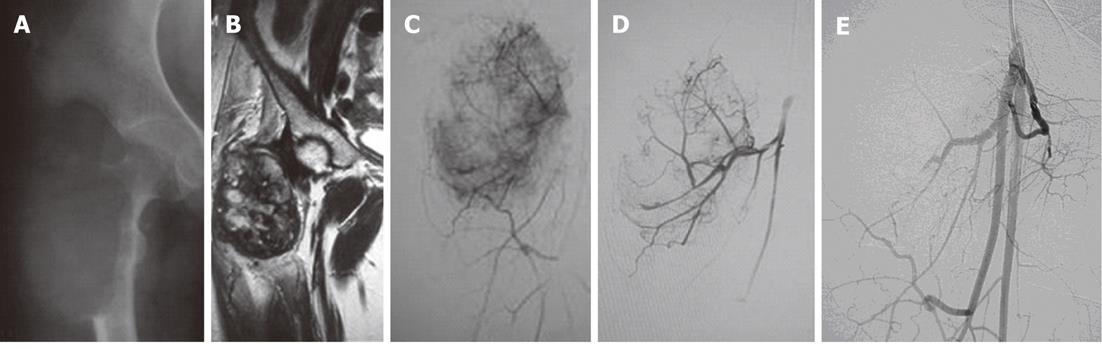
Figure 6 Telengectiatic osteosarcoma in a young male managed preoperatively by trans-arterial embolisation.
A: Twenty-year-old male with a large eccentric expansile lytic lesion of the right femoral metaphysis which was confirmed to be a telangiectatic osteosarcoma on histopathology; B: Magnetic resonance imaging was performed for tumor staging. The lesion was well circumscribed with no involvement of neurovascular bundle, no satellite lesions and no extension to the hip joint; C: Right common femoral angiogram revealed tumor supply from profunda femoris artery; D: Selective angiogram revealed marked tumor blush; E: Post-Embolisation angiogram after injection of polyvinyl alcohol particles 300-500 μm showed marked reduction in tumor blush.
Other primary bone lesions
Isolated case reports of TAE in other rare bone pathologies have been reported. Findik et al[32] reported preoperative TAE of a rib hemangiopericytoma. Sanchez-Mejia et al[33] reported a case of sacral epithelioid angiosarcoma treated with TAE.
Bone metastases
There are several indications for TAE in bone metastases: preoperative TAE to reduce vascularity and intraoperative blood loss; palliation of bone pain, fever, hypercalcemia and other rheological features; to enhance sensitivity to chemotherapy or radiotherapy. The aim is to improve patient quality of life. Barton et al[34] in their review of the literature found that the most common indication for Embolisation in bone tumors is bone metastases from thyroid (Figure 7) and renal carcinoma (RCC) (Figure 8) which are hypervascular in 65%-70% of cases. Forauer et al[35] reported a retrospective review of 21 patients presenting for palliative Embolisation of painful RCC skeletal metastases. Thirty separate Embolisations were performed and it was concluded that Embolisation can provide effective palliation in patients with renal skeletal metastases who otherwise have therapeutic options. Van Tol et al[36] reported a significant initial reduction in serum thyroglobulin levels suggesting reduction of tumor bulk in five patients with large thyroid carcinoma skeletal metastases in whom they performed TAE in combination with radioiodine therapy. Guzman et al[37] reported preoperative TAE in 24 patients with hypervascular vertebral metastases. They concluded that the procedure is safe and facilitates surgical excision. Sun et al[38] reported the effectiveness of preoperative Embolisation to reduce blood loss during surgical repair of bone metastases from RCC. They recruited 16 patients with bone metastases for preoperative Embolisation and concluded that preoperative TAE reduced intraoperative blood loss with no adverse effects on healing. Few studies have demonstrated the effectiveness of TAE in hepatocellular carcinoma (HCC) bone metastases. Hansch et al[39] reported a case of TAE of an unusual HCC to the humerus. Uemura et al[40] compared the relative efficacy of TAE, TAE with external radiotherapy and external radiotherapy alone in 39 metastatic bone lesions from HCC. They concluded that TAE alone provides immediate pain relief, whereas a combination of TAE and external radiotherapy is required for permanent pain relief.
Figure 7 Preoperative embolisation in a metastatic lesion from thyroid carcinoma in an elderly male.
A: Sixty-two-year-old male with papillary carcinoma of thyroid and an expansile lytic lesion involving the left proximal humerus; B: Left axillary arteriogram revealed marked tumour vascularity; C: Post-Embolisation angiogram after injection of polyvinyl alcohol particles 300-500 μm showing 90% reduction in tumour vascularity.
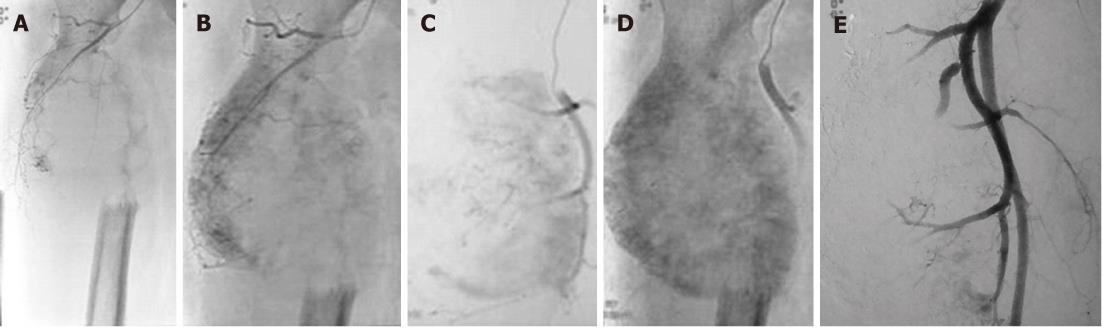
Figure 8 Preoperative embolisation in a metastatic lesion from renal cell carcinoma in an elderly male.
A: Fifty-two-year-old male with renal cell carcinoma (RCC) and a large destructive lesion of the right femoral metaphysis extending to involve the proximal diaphysis. Preoperative Embolisation was planned to reduce tumour vascularity. Metastases from thyroid carcinoma and RCC are highly vascular; B and C: Right common femoral angiogram revealed marked tumor vascularity; D: Delayed phase image shows profound tumor vascularity; E: Embolisation was performed with 300-500 μm polyvinyl alcohol particles and marked reduction (90%) in tumour vascularity was achieved.