Published online Apr 28, 2012. doi: 10.4329/wjr.v4.i4.179
Revised: March 5, 2012
Accepted: March 12, 2012
Published online: April 28, 2012
AIM: To investigate contrast-enhanced computed tomography (CECT) for discriminating esophageal squamous cell carcinoma (ESCC) from normal esophagus and evaluating outcomes within tumors after chemoradiotherapy (CRT).
METHODS: Sixty-four patients with surgical ESCC served as group A, and underwent thoracic contrast-enhanced scan with 16-section multidetector row CT 1 wk before surgery. Thirty-five patients with advanced ESCC receiving 4-wk CRT and showing response to CRT served as group B, and underwent CT scans similar with group A 4 wk after completion of CRT. In group A, differences in CT attenuation values (in HU) between the preoperative ESCC and background normal esophageal wall (delta CT1), or between different background normal esophageal walls (delta CT2) were compared. Furthermore, delta CT1 between group A and B was also compared.
RESULTS: In group A, mean delta CT1 was higher than delta CT2 (23.86 ± 10.59 HU vs 6.24 ± 3.06 HU, P < 0.05). When a delta CT1 of 10.025 HU was employed at a cut-off value to discriminate ESCC from normal esophagus, a sensitivity of 89.1% and specificity of 90.6% were achieved. Mean delta CT1 was lower in group B than in group A (9.25 ± 10.86 vs 23.86 ± 10.59, P < 0.05), and a delta CT1 of 15.45 HU was obtained at a cut-off value to assess the CRT changes with a sensitivity of 76.6% and specificity of 77.1%.
CONCLUSION: CECT might be a clinical technique for discriminating ESCC from normal esophagus, and evaluating outcome in the tumors treated with CRT.
- Citation: Li R, Chen TW, Wang LY, Zhou L, Li H, Chen XL, Li CP, Zhang XM, Xiao RH. Quantitative measurement of contrast enhancement of esophageal squamous cell carcinoma on clinical MDCT. World J Radiol 2012; 4(4): 179-185
- URL: https://www.wjgnet.com/1949-8470/full/v4/i4/179.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i4.179
Esophageal carcinoma is one of the most frequent causes of death from digestive systemic malignant tumors, and the squamous cell carcinoma is the frequent histological type[1]. Tumor resection is a well established curative treatment protocol for patients with nonmetastatic esophageal squamous cell carcinoma (ESCC)[2]. However, some patients with advanced ESCC have primary cancer associated with systemic spread at diagnosis, and the outcome of surgery alone for these patients was not satisfying[3,4]. In the process of the tumor cells spreading through the bloodstream to distant tissues, tumor angiogenesis plays a key role[5,6]. For patients with advanced esophageal carcinoma, chemoradiotherapy (CRT) has been established as an effective treatment which is widely performed in clinical settings[7].
Several imaging procedures, such as endoscopy and endoscopic ultrasound (EUS), have been used to assess response to neoadjuvant chemotherapy and radiation therapy by comparison of tumor volume between pre- and post-CRT imaging[8]. However, these methods are limited by their inability to traverse a malignant stricture occurring in 20-30% esophageal carcinoma patients and by their operator dependency[9,10]. EUS may also have a potential risk of perforation[11]. As a noninvasive imaging technique, computed tomography (CT) is the most common approach for evaluating cancers, and contrast-enhanced CT (CECT), which can overcome the limitations of endoscopy and EUS, has been clinically applied to detect esophageal primary tumors and lymph node or distant metastasis, and to assess the response to neoadjuvant chemotherapy and radiation therapy[12-14].
Furthermore, tumor angiogenesis is characterized by an increase in tumor blood vessel count, and this process will impact on CECT[15-19]. We presume that the level of CT enhancement might be interpreted as an indicator of tumor angiogenesis. To the best of our knowledge, few articles have focused on the CT attenuation value in esophageal tumor and background normal esophagus on CECT in patients treated with or without CRT. Thus, the objective of this study was to investigate the feasibility of CECT to quantitatively distinguish esophageal tumor from background normal esophagus, and for assessing therapeutic outcome in patients with cancer who received CRT in a clinical settings.
The institutional ethics committee of our hospital approved this study, and written informed consent was obtained from each participant prior to the study.
According to the therapeutic strategy, there were two groups - group A and B - in our study. Patients were enrolled into group A according to the following inclusion criteria: (1) they had ESCC initially confirmed by endoscopic biopsy; (2) the mass was clearly visible on CECT images; (3) the patients did not receive any tumor-related treatment such as radiotherapy, or chemotherapy prior to the CT examination; and (4) there were no contraindications to tumor resection for therapy with thoracotomy. Patients were enrolled into group B if ESCC was pathologically confirmed, if there were contraindications to tumor resection for therapy with thoracotomy, if they received CRT and showed a response to CRT, if they underwent CECT at least 4 wk after the therapy, and if the mass was clearly visible on CECT images.
From January to November 2010, 64 consecutive patients (53 men and 11 women; mean age, 61.51 years; age range, 37-79 years) with endoscopic biopsy proven ESCC, who met the inclusion criteria, were enrolled into group A. In this group, the mean coverage of the tumor along the z-axis was 5.33 ± 2.85 cm (range 2.54-8.42 cm). The tumors were located in the lower thoracic portion of the esophagus in 10 patients, in both the midthoracic and lower thoracic portion in 17, in the midthoracic portion in 27, in both the upper thoracic and midthoracic portion in 8, and in upper thoracic portion in 2. One week after the CECT scan, all patients underwent tumor resection with thoracotomy. According to the postoperative pathology, all the surgical margins were not involved by this carcinoma.
During the same period, 35 patients (29 men, 6 women; mean age 56.75 years; age range from 47 to 76 years) with unresectable ESCC, who had already completed a CRT schedule for at least 4 wk, served as group B. The mean coverage of the tumor along the z-axis was 3.79 ± 2.13 cm (range 1.33-6.91 cm). The tumors were located in the lower thoracic portion of esophagus in 9 patients, in both the midthoracic and lower thoracic portion in 4, in the midthoracic portion in 7, in both the upper thoracic and midthoracic portion in 4, and in the upper thoracic portion in 11. CRT consisted of simultaneous radiotherapy and chemotherapy. For radiotherapy, the patients were irradiated using a 10-MV linear accelerator photon beam at a daily dose of 2 Gy, which was continued daily 5 times per week for 4 wk, to a total dose of 40 Gy. The target included the primary tumor and the enlarged regional lymph nodule. The chemotherapy schedule, which was initiated on day 1 of radiotherapy, consisted of cisplatin (7 mg/m2 per day) by intravenous administration and 5-fluorouracil (350 mg/m2 per day) by continuous intravenous infusion for 5 d[20,21]. All patients showed a therapeutic response to CRT, which was assessed 4 wk after the completion of CRT according to the therapeutic criteria defined by the World Health Organization[22].
Patients in groups A and B underwent spiral thoracic enhanced scans with a 16-section multidetector row CT (MDCT) system (Aquilion 16 CFX Edition, Toshiba Medical System, Japan) 1 wk before tumor resection and 4 wk after completion of CRT, respectively. Each patient received 200-400 mL water as oral esophageal negative contrast material immediately before the examination. A 19-gauge cannula was placed into an antecubital fossa vein after the patient lay supine on the scanner table. Eighty milliliters of a nonionic contrast medium (Ultravist 300, Iopamidol, Schering, Germany) containing 300 mg of iodine per milliliter was administered intravenously as a bolus with a flow rate of 2.5-3.0 mL/s using an automatic injector (MEDRAD Vistron CT Injection System, Medrad company, USA). Enhanced CT was performed 35 s after the initiation of the bolus contrast injection using the following scanning parameters: 120 kV, 100 mAs, 0.5 s rotation time, a pitch of 0.938, 400 mm field of view, 7 mm section thickness, and 512 mm × 512 mm matrix. Each scan was performed during a breath-hold to minimize the movement of the esophagus. All data were reconstructed with a 1 mm section thickness at 1 mm intervals, and were then transferred to an image processing workstation (Vitrea 2.0 vital images, Minnesota, USA).
Image data in groups A and B were retrospectively reviewed on the image processing workstation by an experienced radiological professor (the corresponding author with 13 years of experience in thoracicoabdominal radiology), and an experienced radiologist (the first author with 4 years of experience in thoracicoabdominal radiology) by consensus to keep the accuracy of analysis focusing on the difference in attenuation values (∆CT, in Hounsfield units) between the tumor and background normal esophageal wall (∆CT1, in Hounsfield units) in group A and B, or between background normal esophageal walls (∆CT2, in Hounsfield units) in group A.
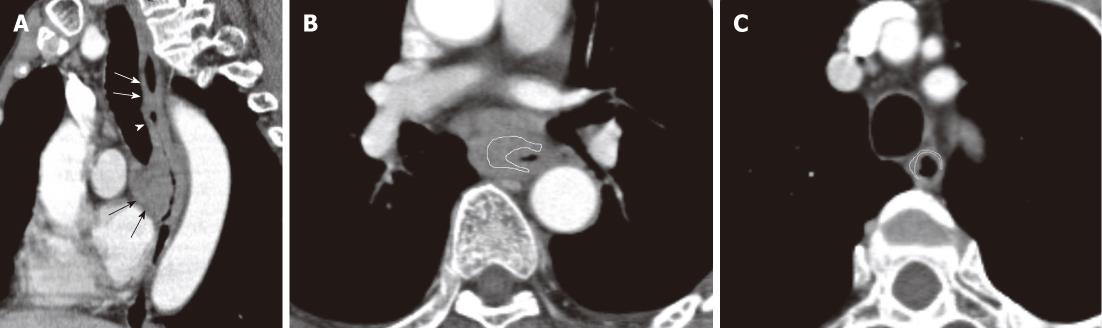
In order to measure the attenuation of the tumor in groups, reconstructed axial images (Figure 1A) were used to reformat the oblique-sagittal images at 1.0 mm intervals with a slice thickness of 1.0 mm to display the extension of esophageal carcinoma. Standard mediastinal window images (window width, 400 HU; window level, 60 HU) were used for displaying the images. Based on the extension of the tumor on the oblique-sagittal view, ten contiguous transverse sections corresponding to the maximal segment of tumors were selected for the representative tumor sections. A reliable tumor region of interest (ROI) within the representative thickened esophageal wall was manually drawn in the transverse section, and the area of tumor ROI (area range: 36-408 mm2) was more than 60% of that of the entire tumor in the section (Figure 1B). The tumor attenuation value was derived automatically by the software on this image processing workstation. To minimize partial volume averaging with surrounding tissues, care was taken to draw the ROI of the tumor to exclude periesophageal fat and intraluminal gas, and to avoid the necrotic area within the tumor. This previous process and analysis was repeated for each contiguous transverse level, until the ten representative tumor sections had been covered. All ten attenuation values were then averaged across all the sections to be regarded as the representative attenuation values for esophageal carcinomas.
For measuring the attenuation value of background normal esophagus in groups, the ROI of the normal esophagus was determined. In group A, the tumor did not involve the surgical cut edge in all patients confirmed by the postoperative pathology, and the residual portions of esophagus after surgery were determined as background normal esophagus. According to the postoperative pathology and the reformatted images in the oblique-sagittal plane (Figure 1A), five contiguous axial sections corresponding to the background normal esophagus were randomly selected for each patient in group A. In group B, the portions of background esophagus 5 cm away from the irradiating target were determined as background normal esophagus, and five contiguous axial sections corresponding to the background normal esophagus were also randomly selected. The measurement of CT values in the normal esophagus was similar to that in esophageal carcinoma.
Subsequently, ∆CT1 was calculated by subtracting the referenced attenuation value for the background normal esophageal wall from the representative attenuation value for esophageal carcinomas. ∆CT2 was defined as the standard deviation of the attenuation value for the portions of background normal esophagus, which was in accordance with the difference in attenuation values between background normal esophageal walls.
To clarify the inter-observer agreement on the measurement of ∆CT, we randomly assessed the reproducibility of ∆CT1 measurement. Data from each group was reanalyzed by the other observers (the third author with 3 years of experience in thoracicoabdominal radiology, and the fourth author with 2 years of experience in radiology). We then compared two sets of the measurements, and if good agreement between the replicated measurements was achieved, values of the first set were regarded as the final ∆CT1.
Repeatability between two sets of measurements for ∆CT1 was assessed by Bland and Altman analysis[23]. The mean differences and their 95% CI between two sets of measurements, and 95% limits of agreement for ∆CT1 were determined to evaluate the difference in replicated measurements. The interclass correlation coefficients and their 95% CI were applied to assess the level of agreement. If the interclass correlation coefficient was greater than 0.99, and the mean difference of the replicated measurements was close to zero, good agreement between the replicated measurements was considered to be obtained[23].
By using the statistical software (version 13.0 for Windows, SPSS Inc., Chicago, IL, USA), independent sample Student’s t tests were subsequently performed to compare ∆CT1 and ∆CT2 in group A, and ∆CT1 between group A and B. The probability value of less than 0.05 was considered to indicate a significant difference. If significant difference was proved, receiver operating characteristic (ROC) analysis was then carried out to determine the cutoff of ∆CT1 for discriminating esophageal carcinoma from background normal esophagus, and for assessing the CRT change of esophageal carcinoma.
In group A, the mean CT attenuation value of background normal esophagus was 53.77 ± 7.04 HU (range, 30.98 to 68.62 HU). The mean CT attenuation value of esophageal carcinoma was 77.62 ± 9.13 HU (range, 63.18 to 106.23 HU) for the initial measurement. The first set of mean ∆CT1 was 23.86 ± 10.59 HU (range, -1.85 to 44.86 HU). For the repeated measurement, mean CT attenuation value of esophageal carcinoma was 77.61 ± 9.11 HU (range, 63.06 to 105.86 HU), and the repeated set of mean ∆CT1 was 23.83 ± 10.60 HU (range, -2.56 to 47.02 HU).
In group B, the mean CT value of background normal esophagus was 55.09 ± 7.30 HU (range, 37.68 to 71 HU). For the initial measurement, the mean CT value of esophageal cancer and mean ∆CT1 were 64.35 ± 12.89 HU (range from 34.07 to 94.82 HU) and 9.25 ± 10.86 HU (range from -10.02 to 35.67 HU), respectively; and for the replicated measurement, the mean CT attenuation value of esophageal cancer and mean ∆CT1 were 64.25 ± 12.99 HU (range from 33.98 to 64.26 HU) and 9.16 ± 10.84 HU (range from -10.39 to 34.24), respectively. A high level of repeatability of ∆CT1 measurements was achieved in groups (Table 1).
| Group | Mean differences of replicated measurements | 95% Interobserver correlation coefficient | ||
| Differences between two sets of measurements | 95% CI of the difference | 95% limits of agreement | ||
| A (HU) | -0.2 ± 9.03 | -17.8 to 17.5 | -18.26 to 17.86 | 0.9913 (0.9817 to 0.9933) |
| B (HU) | -1.2 ± 12.6 | -26 to 23.5 | -26.4 to 24 | 0.9956 (0.9911 to 0.9978) |
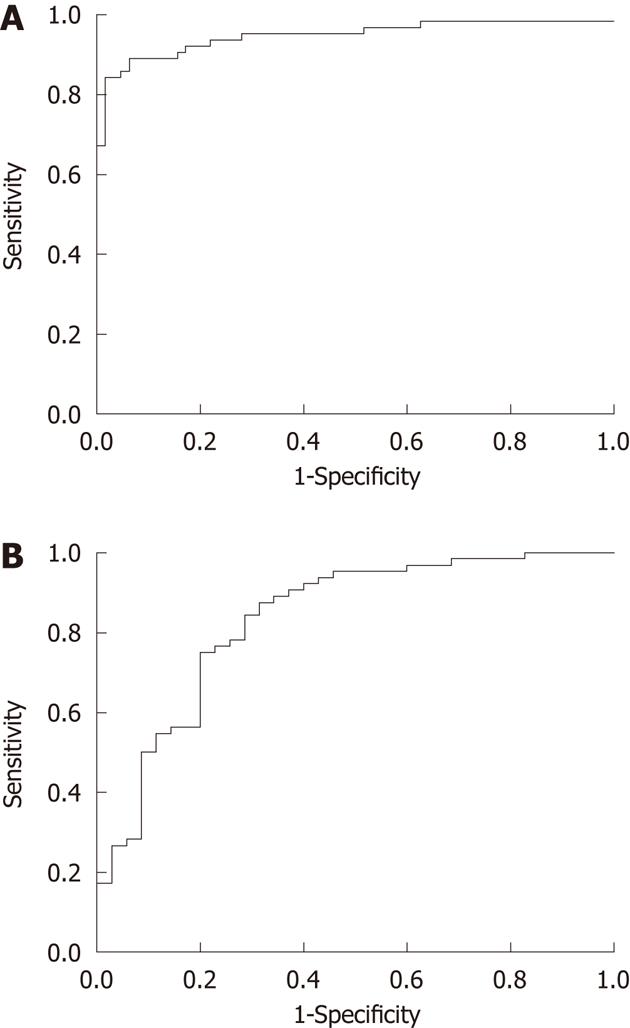
In patients with esophageal carcinoma in group A, the mean ∆CT1 was 23.86 ± 10.59 HU, and mean ∆CT2 was 6.24 ± 3.06 HU (range, 2.39 to 18.66 HU). ∆CT1 was significantly higher than ∆CT2 in group A (P < 0.0001). To discriminate the visual difference of esophageal carcinoma from that of background normal esophageal walls, the ROC curve analysis (Figure 2A) was performed between ∆CT1 and ∆CT2, and an area under the curve of 0.948 (95% CI: 0.906 to 0.99, P < 0.0001) was observed. By using 10.025 HU of ∆CT1 as the cut-off value, the ROC curve showed a sensitivity of 89.1%, a specificity of 90.6%, a positive predictive value of 90.4%, a negative predictive value of 89.2%, and an accuracy of 89.8%.
In patients treated with and without CRT, mean ∆CT1 was 23.86 ± 10.59 HU and 9.25 ± 10.86 HU in group A and group B, respectively. Due to the treatment, mean ∆CT1 was markedly decreased in group B compared with that in group A (P < 0.0001). To assess the therapeutic change, the ROC curve analysis (Figure 2B) was also performed between ∆CT1 in groups, and an area under the curve of 0.833 (95% CI: 0.746 to 0.920, P < 0.0001) was observed. By using 15.45 HU of ∆CT1 as the cut-off value, the ROC curve showed a sensitivity of 76.6%, a specificity of 77.1%, a positive predictive value of 64.29%, a negative predictive value of 85.96%, and an accuracy of 76.77%.
In this study, an unenhanced CT scan was not performed prior to contrast-enhanced scan to control the patient radiation dose by lowering scan time. A 16-section MDCT was used to perform the enhancement data acquisitions, which has better collimation of X-ray beams and newer filter design compared with single section spiral CT[24,25]. As shown in our study, the measurement of difference in contrast enhancement between esophageal carcinoma and background normal esophageal wall might be a reproducible technique, because good agreement between replicated measurements of the difference was obtained. Thus, we used a contrast-enhanced scan with 16-section MDCT in the present study.
Clinically, the results of our study showed that the contrast-enhanced attenuation value within ESCC was significantly higher than that in the background normal esophageal wall. Our findings were consistent with those obtained by triple-phase dynamic CT (23.86 ± 10.59 HU vs 28.3 ± 17.1 HU)[12]. Our findings may be explained by the fact that ESCC is typically hypervascular[26,27], and the process of developing a new arterial vessel supply and the formation of tumor microvessels in the tumors could result in a marked increase of enhanced attenuation value.
Because of a significantly higher difference in CT enhancement between the tumor and background normal esophagus vs between background normal esophageal walls, the difference in CT enhancement between the tumor and background normal esophagus illustrated by ∆CT1 could be used as a criterion to differentiate tumor from background normal esophagus. A threshold value of ∆CT1 was obtained by performing ROC analysis. Our findings suggested that the cut-off ∆CT1 of 10.025 HU had high sensitivity, specificity, positive predictive value, negative predictive value and accuracy at more than 85%. Therefore, a ∆CT1 value of 10.025 HU may be used as a criterion to discriminate the microcirculation of ESCCs from that of the background normal esophagus.
Another finding in our study is that the difference in contrast enhancement between the tumor and background normal esophageal wall was significantly lower in patients treated with CRT than without CRT. These phenomena might be attributed to the cytotoxic effects of X-rays on the vascular endothelium cells within squamous cell carcinoma[28], and the tumor vascularity may shrink after CRT, resulting in lower CECT. We used ROC analysis to evaluate the therapeutic change within tumors and our findings suggested that the cut-off ∆CT1 of 15.45 HU had sensitivity, specificity, negative predictive value and accuracy at more than 75%. Hence, a ∆CT1 value of 15.45 HU could be used as a criterion to evaluate therapeutic changes in tumors treated by CRT.
Our research has limitations. Firstly, measurement of CT enhancement is a semi-quantitative method for assessing the tumor vascularity, and is significantly constrained by the impact of patient cardiac output and central blood volume. To try our best to overcome this limitation, we measured the extent of CT enhancement within the tumor by subtracting the attenuation value of background normal esophageal walls from that of esophageal tumors, which may help to avoid the confounding influence of cardiac output and central blood volume. Another limitation is that normal esophagus was more subject to partial volume averaging with adjacent tissue or air, which may influence the accuracy of the measurement of CT enhancements in the esophageal wall. To minimize partial volume averaging, the measurements of CT enhancement were analyzed on thin-section and magnifying images.
The cut-off value of difference in CT enhancement between ESCC and background normal esophagus (∆CT1 = 10.025 HU) could be used to quantitatively discriminate tumor from normal esophagus, and the cut-off value of difference in ∆CT1 between the tumors treated with and without CRT (15.45 HU) could be used to assess the outcomes of CRT in vivo in clinical settings. Recently, fully automatic methods for 2D and 3D segmentation of liver structures from CT scans were developed to obtain high accuracy for demonstrating the liver volume, hepatic tumor and vessel morphology[29,30]. Automated methods for 3D segmentation of esophagus from CT scans had also developed to obtain high accuracy for showing the anatomy of esophagus[31]. Based on the difference in CT enhancement between ESCC and background normal esophagus, we hope to develop the techniques of automatic segmentation for depicting the profile of ESCC for surgical planning and to determine the therapeutic outcomes of CRT, which will be performed in our future study.
Esophageal squamous cell carcinoma (ESCC) is one of the most frequent causes of death from digestive systemic malignant tumors. In the process of the tumor cells spreading through the bloodstream to distant tissues, tumor angiogenesis plays a key role and is characterized by an increase in tumor blood vessel count, which will impact on contrast-enhanced computed tomography (CECT). However, few articles have focused on the CT attenuation value in esophageal tumor and background normal esophagus on CECT in patients treated with or without chemoradiotherapy (CRT).
CT attenuation values (in HU) of ESCC and background normal esophageal walls were measured on thoracic contrast-enhanced CT data. The differences in CT attenuation values between surgical ESCC and background normal esophageal wall (∆CT1), and between different background normal esophageal walls (∆CT2) were compared for discriminating ESCC from normal esophagus. In addition, the differences in ∆CT1 between patients with ESCC treated with and without CRT was compared for evaluating the CRT outcomes.
The cut-off value of difference in CT enhancement between ESCC and background normal esophagus (∆CT1 = 10.025 HU) could be used to quantitatively discriminate the tumor from normal esophagus, and the cut-off value of difference in ∆CT1 between the tumors treated with and without CRT (15.45 HU) could be used to assess the outcomes of CRT in vivo in a clinical setting.
Based on the difference in CT enhancement between ESCC and background normal esophagus, we hope to develop the techniques of automatic segmentation for depicting the profile of ESCC for surgical planning and for determining the therapeutic outcomes of CRT.
Thoracic contrast-enhanced CT is a valuable procedure to quantitatively measure the difference in CT enhancement between ESCC and background normal esophageal wall. It is hoped that this can be used to develop automatic segmentation techniques for depicting the profile of ESCC for surgical planning and determination of the therapeutic outcomes of CRT.
In this paper, the authors demonstrated that difference in CT enhancement between ESCC and background normal esophagus could quantitatively discriminate the tumor from normal esophagus. The addressed research topic is of great importance in the field and the presented work effectively illustrates the final finding of the study.
Peer reviewer: Sergio Casciaro, PhD, Institute of Clinical Physiology - National Research Council, Campus Universitario Ecotekne, Via Monteroni, 73100 Lecce, Italy
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. |
| 2. | Kranzfelder M, Büchler P, Lange K, Friess H. Treatment options for squamous cell cancer of the esophagus: a systematic review of the literature. J Am Coll Surg. 2010;210:351-359. |
| 3. | Daly JM, Karnell LH, Menck HR. National Cancer Data Base report on esophageal carcinoma. Cancer. 1996;78:1820-1828. |
| 4. | Ohtsu A, Boku N, Muro K, Chin K, Muto M, Yoshida S, Satake M, Ishikura S, Ogino T, Miyata Y. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915-2921. |
| 5. | Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9-19. |
| 6. | Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364. |
| 7. | Urschel JD, Ashiku S, Thurer R, Sellke FW. Salvage or planned esophagectomy after chemoradiation therapy for locally advanced esophageal cancer--a review. Dis Esophagus. 2003;16:60-65. |
| 8. | Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA, Eloubeidi MA. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg. 2005;129:1232-1241. |
| 9. | Mallery S, Van Dam J. Increased rate of complete EUS staging of patients with esophageal cancer using the nonoptical, wire-guided echoendoscope. Gastrointest Endosc. 1999;50:53-57. |
| 10. | Pfau PR, Ginsberg GG, Lew RJ, Faigel DO, Smith DB, Kochman ML. Esophageal dilation for endosonographic evaluation of malignant esophageal strictures is safe and effective. Am J Gastroenterol. 2000;95:2813-2815. |
| 11. | Wallace MB, Hawes RH, Sahai AV, Van Velse A, Hoffman BJ. Dilation of malignant esophageal stenosis to allow EUS guided fine-needle aspiration: safety and effect on patient management. Gastrointest Endosc. 2000;51:309-313. |
| 12. | Umeoka S, Koyama T, Togashi K, Saga T, Watanabe G, Shimada Y, Imamura M. Esophageal cancer: evaluation with triple-phase dynamic CT--initial experience. Radiology. 2006;239:777-783. |
| 13. | Yoon YC, Lee KS, Shim YM, Kim BT, Kim K, Kim TS. Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection prospective study. Radiology. 2003;227:764-770. |
| 14. | Wu LF, Wang BZ, Feng JL, Cheng WR, Liu GR, Xu XH, Zheng ZC. Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol. 2003;9:219-224. |
| 15. | Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029-1039. |
| 16. | Baillie CT, Winslet MC, Bradley NJ. Tumour vasculature--a potential therapeutic target. Br J Cancer. 1995;72:257-267. |
| 17. | Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641-2658. |
| 18. | Miles KA. Tumour angiogenesis and its relation to contrast enhancement on computed tomography: a review. Eur J Radiol. 1999;30:198-205. |
| 19. | Ahn MJ, Jang SJ, Park YW, Choi JH, Oh HS, Lee CB, Paik HK, Park CK. Clinical prognostic values of vascular endothelial growth factor, microvessel density,and p53 expression in esophageal carcinomas. J Korean Med Sci. 2002;17:201-207. |
| 20. | Doki Y, Takachi K, Ishikawa O, Sasaki Y, Miyashiro I, Ohigashi H, Yano M, Ishihara R, Tsukamoto Y, Nishiyama K. Reduced tumor vessel density and high expression of glucose transporter 1 suggest tumor hypoxia of squamous cell carcinoma of the esophagus surviving after radiotherapy. Surgery. 2005;137:536-544. |
| 21. | Yano M, Yasuda T, Miyata H, Fujiwara Y, Takiguchi S, Monden M. Correlation between histological effects on the main tumors and nodal status after chemoradiotherapy for squamous cell carcinoma of the esophagus. J Surg Oncol. 2005;89:244-250; discussion 250. |
| 22. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. |
| 23. | Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-310. |
| 24. | Greess H, Wolf H, Baum U, Lell M, Pirkl M, Kalender W, Bautz WA. Dose reduction in computed tomography by attenuation-based on-line modulation of tube current: evaluation of six anatomical regions. Eur Radiol. 2000;10:391-394. |
| 25. | Itoh S, Koyama S, Ikeda M, Ozaki M, Sawaki A, Iwano S, Ishigaki T. Further reduction of radiation dose in helical CT for lung cancer screening using small tube current and a newly designed filter. J Thorac Imaging. 2001;16:81-88. |
| 26. | Chen TW, Yang ZG, Li Y, Li ZL, Yao J, Sun JY. Quantitative assessment of first-pass perfusion of oesophageal squamous cell carcinoma using 64-section MDCT: initial observation. Clin Radiol. 2009;64:38-45. |
| 27. | Chen TW, Yang ZG, Wang QL, Li Y, Qian LL, Chen HJ. Whole tumour quantitative measurement of first-pass perfusion of oesophageal squamous cell carcinoma using 64-row multidetector computed tomography: correlation with microvessel density. Eur J Radiol. 2011;79:218-223. |
| 28. | Sulman EP, Schwartz DL, Le TT, Ang KK, Morrison WH, Rosenthal DI, Ahamad A, Kies M, Glisson B, Weber R. IMRT reirradiation of head and neck cancer-disease control and morbidity outcomes. Int J Radiat Oncol Biol Phys. 2009;73:399-409. |
| 29. | Massoptier L, Casciaro S. A new fully automatic and robust algorithm for fast segmentation of liver tissue and tumors from CT scans. Eur Radiol. 2008;18:1658-1665. |
| 30. | Conversano F, Franchini R, Demitri C, Massoptier L, Montagna F, Maffezzoli A, Malvasi A, Casciaro S. Hepatic vessel segmentation for 3D planning of liver surgery experimental evaluation of a new fully automatic algorithm. Acad Radiol. 2011;18:461-470. |
| 31. | Feulner J, Zhou SK, Hammon M, Seifert S, Huber M, Comaniciu D, Hornegger J, Cavallaro A. A probabilistic model for automatic segmentation of the esophagus in 3-D CT scans. IEEE Trans Med Imaging. 2011;30:1252-1264. |