Published online Apr 28, 2012. doi: 10.4329/wjr.v4.i4.135
Revised: March 23, 2012
Accepted: March 30, 2012
Published online: April 28, 2012
AIM: To highlight sonographic and clinical characteristics of scar endometrioma with special emphasis on size-related features.
METHODS: Thirty women (mean age 30.6 years, range 20-42 years) with 33 scar endometriomas (mean diameter 27.1 mm, range 7-60 mm) were consecutively studied by Sonography and Color Doppler examination prior to surgery. Pathological examination was available in all cases.
RESULTS: The most frequent (24 of 33 nodules, 74%) sonographic B-mode aspect of endometrioma was that of an inhomogenously hypoechoic roundish nodule with fibrotic changes (in the form of hyperechoic spots or strands), a peripheral inflammatory hyperechoic ring, spiculated margins and a single vascular pedicle entering the mass at the periphery. On average, 1.6 cesarean sections were recorded per patient (range 1-3). The median interval between the last cesarean section and admission to hospital was 36 mo (range 12-120 mo) and the median duration of symptoms before admission was 25.7 mo (range 0.5-80 mo). 13 patients had 13 large endometriomas (≥ 30 mm) with a mean lesion diameter of 41.3 ± 9.02 mm (range 30-60 mm). Seventeen women had 20 small endometriomas with a mean lesion size of 18.2 ± 5.17 mm (range 7-26 mm). The mean interval between the last cesarean section and admission to hospital (66.0 mo vs 39.6 mo, P < 0.01) and the mean duration of symptoms before admission (43.0 mo vs 17.4 mo, P < 0.01) were significantly longer in patients with large endometriomas; in addition, a statistically significant higher percentage of patients with large implants had undergone previous inconclusive diagnostic examinations, including either computed tomography/magnetic resonance imaging/fine needle biopsy/laparoscopy (38.4% vs 0%, P < 0.05). On sonography, large endometriomas showed frequent cystic portions and fistulous tracts (P < 0.02), loss of round/oval shape (P < 0.04) along with increased vascularity (P < 0.04).
CONCLUSION: Endometrioma near cesarean section scar is an often neglected disease, but knowledge of its clinical and sonographic findings may prevent a delay in diagnosis that typically occurs in patients with larger (≥ 3 cm) endometriomas.
- Citation: Francica G. Reliable clinical and sonographic findings in the diagnosis of abdominal wall endometriosis near cesarean section scar. World J Radiol 2012; 4(4): 135-140
- URL: https://www.wjgnet.com/1949-8470/full/v4/i4/135.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i4.135
Subcutaneous endometriosis near cesarean delivery scar (so-called “scar endometrioma”) is a rare form of extrapelvic endometriosis, occurring in 0.03% to 1.5% of all women who have had cesarean deliveries[1-6]. Although for extrapelvic endometriosis it has been suggested that multipotential mesenchymal cells may undergo metaplasia into endometriosis, in the case of endometrioma near a Pfannenstiel incision, the most likely explanation is inadvertent transportation of endometrial cells during cesarean section[7-9]. An iatrogenic origin has also been proposed in the case of endometriotic implants occurring after hysterectomy[9], appendectomy[3], laparoscopic trocar tract[10], needle tract amniocentesis[11] and perineal episiotomy incision[12].
On a clinical basis, the most typical finding is a palpable small mass near the cesarean section scar becoming painful with menses[3,13,14].
However, the comparative rarity of scar endometrioma may represent a major factor explaining why the correct diagnosis is frequently overlooked by both clinicians[15] and radiologists[13,16]. Moreover, endometriosis of the abdominal wall may be a diagnostic challenge since a variety of pathologic conditions (a suture granuloma, an incisional hernia, a primary or metastatic cancer) should be taken into account in the differential diagnosis[13,16].
The aim of this study was to report on a large series of scar endometriomas by highlighting the clinical and sonographic characteristics of this neglected disease.
Thirty women (mean age 30.6 years, range 20-42 years) with 33 scar endometriomas were consecutively seen at the Ultrasound Unit between June 1999 and April 2010. All of the patients underwent wide surgical excision and pathologic analysis of all surgical specimens was available.
In each patient the following parameters were determined: (1) historical data: number of cesarean sections, time from both last cesarean section and onset of symptoms before admission, known pelvic endometriosis, inconclusive previous diagnostic exams other than sonography [computed tomography (CT), magnetic resonance imaging (MRI), fine needle biopsy (FNB), laparoscopy], previous pelvic/abdominal surgery related to painful symptoms, characteristics of pain (cyclic with menses vs continuous); (2) clinical data: physical examination at entry (palpability of nodule/s); and (3) sonographic and color doppler findings: lesion size, site, echotexture, margins, presence of vascular pedicle/s, presence of central vascularity and values of resistive index (RI) of arterial flow.
In all patients, sonographic examination was carried out by a single operator (Francica G) with high frequency probes (7.5 MHz up to 12 MHz) with the sonographic machines available over the study time period (Logic 500, Logic 700 Expert Series, Logic 7, Logic 9, GE Healthcare, Milwaukee, WI, USA). After 2004, only high-frequency wide band electronic transducers along with spatial compound and tissue harmonic imaging modalities were used.
Color Doppler parameters were set to detect low blood flow velocities (PRF 500-1000 Hz, Wall filter 50 Hz, High filters for color-vs-echo priority and color persistence); RI of arterial flow (if detectable) was expressed as the mean of 2 measurements.
For the purpose of comparison, a large scar endometrioma was considered if the widest nodule diameter was equal to or greater than 30 mm.
Fisher’s exact test and χ2 test for categorical data and the unpaired t-test for continuous variables were used for statistical analysis. P values < 0.05 were considered statistically significant.
The institutional ethics committee of the author’s hospital approved the study design, and written consent to participate in the study was obtained from all patients.
The 33 endometriomas had a mean diameter of 27.1 mm (range 7-60 mm); twenty-six nodules (78.7%) were located between subcutaneous fat and the muscular sheath; in three cases (9.1%) both the subcutaneous and muscular plane were infiltrated; two endometriomas (6.1%) were purely subcutaneous and two more nodules (6.1%) were entrapped in the muscular layer of the abdominal wall. Endometriomas did not show a preferential distribution along the incision scar: 16 nodules were located on the right, 14 on the left and 3 on the midline.
Thirteen patients (43%) had 13 large endometriomas (≥ 30 mm) with a mean lesion diameter of 41.3 ± 9.02 mm (range 30-60 mm). Seventeen women (56.7%) had 20 small endometriomas with a mean lesion size of 18.2 ± 5.17 mm (range 7-26 mm). One subject had a 10-mm satellite nodule near the largest endometrioma of this series (60 mm).
On average, 1.6 cesarean sections were recorded per patient (range 1-3). The median interval between the last cesarean section and admission to hospital was 36 mo (range 12-120 mo) and the median duration of symptoms before admission was 25.7 mo (range 0.5-80 mo). A history of previous pelvic endometriosis was rarely reported (two cases, 6.6%). At entry, abdominal wall lesions were palpable in all cases but one, an obese patient with a 7-mm nodule. Cyclic pain with menses associated with a palpable mass was recorded in 24 cases (80%); in the remaining 6 patients, pain was described as continuous.
In Table 1, the clinical and demographic findings are shown according to endometrioma size.
| Clinical data | L-SE (13 cases with 13 nodules) | S-SE (17 cases with 20 nodules) | P |
| Mean age (yr) (range) | 31.3 (22-39) | 30.8 (20-42) | NS |
| No. of cesarean sections (range) | 1.8 (1-3) | 1.5 (1-3) | NS |
| Time since last cesarean section (mo) | 66.0 ± 29.5 | 39.6 ± 18.0 | < 0.01 |
| Onset of symptoms (mo before admission) | 43.0 ± 29.0 | 17.4 ± 20.0 | < 0.01 |
| Known pelvic endometriosis | 2 | 1 | NS |
| Previous pelvic/abdominal surgery | 2 | 0 | NS |
| Inconclusive previous diagnostic exams1 | 5 | 0 | < 0.05 |
| Continuous pain | 4 | 2 | NS |
| Cyclic pain | 8 | 14 | NS |
Patients with endometriomas larger than 3 cm had a longer mean interval between the last cesarean section and admission to hospital (66.0 mo vs 36 mo, P < 0.01) and mean duration of symptoms before admission (43.0 mo vs 17.4 mo, P < 0.01) when compared with patients bearing smaller endometriomas. In addition, a statistically significant higher percentage of patients with large endometriomas (38.4% vs 0%, P < 0.05) had undergone inconclusive diagnostic examinations elsewhere [CT (1 case), MRI (2 cases)/FNB (1 case)/laparoscopy (twice in 1 case)] that were aimed at clarifying the origin of lower abdominal pain.
Although not statistically significant, patients with larger endometriomas more often complained of continuous pain associated with a palpable mass (33.3%) than cases with small lesions (12.5%). In addition, surgical interventions for “bowel adhesions” that had not ameliorated patients’ symptoms were recorded in 2 cases (16.6%) with large implants. At the time of writing, no relapse of endometriosis was recorded in either group.
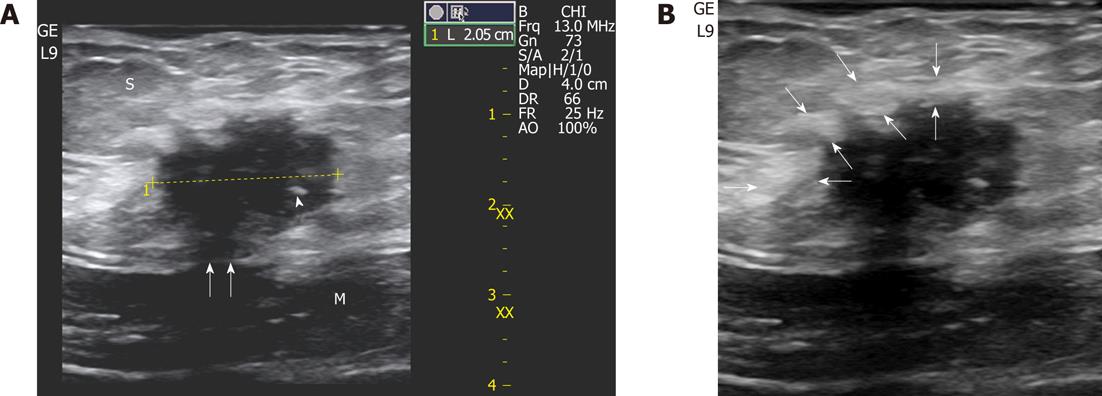
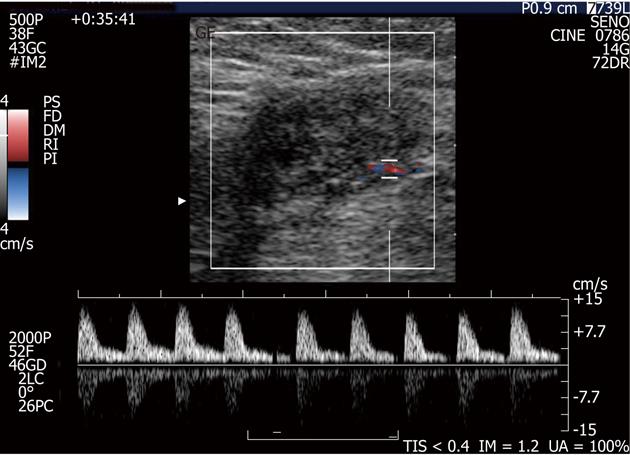
The most frequent B-mode aspect of endometrioma was that of an inhomogenously hypoechoic roundish nodule with fibrotic changes (in the form of hyperechoic spots or strands), a peripheral hyperechoic ring (complete or incomplete), spiculated margins, and a single vascular pedicle entering the mass at the periphery (Figures 1 and 2) with a mean RI of 0.78. This sonographic pattern was identified in 24 of 33 scar endometriomas (74%).
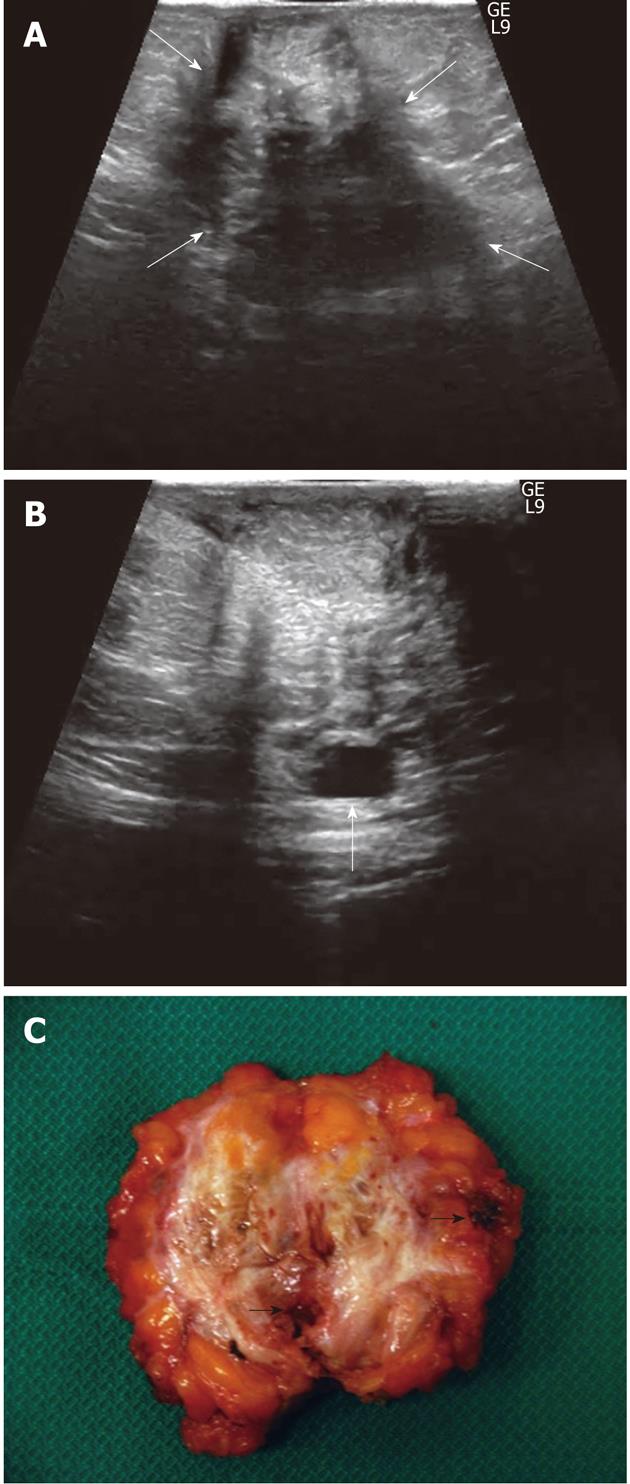
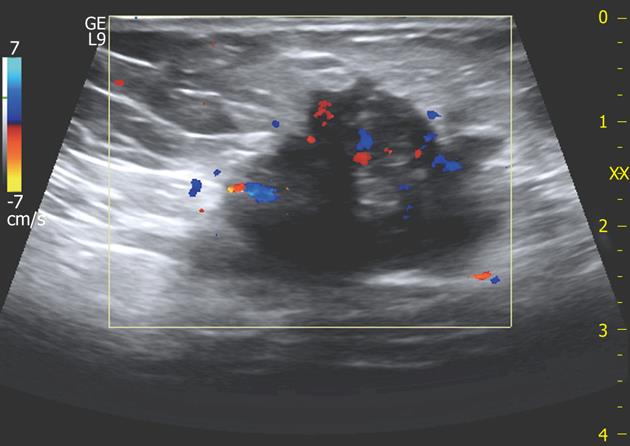
In the remaining 9 nodules (26%), all greater than 3 cm, a more elongated and irregular shape along with a more heterogenous echostructure and the presence of internal small cystic areas and/or fistulous tracts towards either the skin or the muscle (Figures 3 and 4) were observed. Multiple vascular pedicles associated with increased central vascularisation were also observed (Figure 5). In two small endometriomas (7 and 14 mm) Doppler signals were not demonstrated.
To sum up, large endometriomas showed frequent cystic portions and fistulous tracts (P < 0.02), loss of round/oval shape (P < 0.04) along with increased vascularity (P < 0.04) (Table 2).
| L-SE (13 nodules) | S-SE (20 nodules) | P | |
| US findings | |||
| Size (mean ± SD) | 41.3 ± 9.02 | 18.2 ± 5.17 | |
| Echotexture | |||
| Hypoechoic with hyperechoic spots/strands | 11 | 19 | NS |
| Cystic portions and/or fistulous tract | 7 | 1 | < 0.02 |
| Peripheral hyperechoic ring (absent/present) | 1/12 | 7/13 | NS |
| Margins (spiculated-infiltrating) | 9 | 14 | NS |
| Loss of oval or round shape | 4 | 0 | < 0.04 |
| Color doppler findings | |||
| Resistive index (mean ± SD) | 0.75 ± 0.08 | 0.66 ± 0.16 | NS |
| Multiple vascular pedicles | 8 | 2 | < 0.04 |
| Central vascularisation | 8 | 2 | <0.04 |
During the same time period, seven surgically-proved lesions near cesarean section scars were correctly identified as non-endometrioma nodules (1 metastasis, 2 incisional hernias, 1 abscess, 2 chronic inflammation, and 1 desmoid tumor).
Endometriosis of the abdominal wall arising on a Pfannenstiel incision represents an often neglected disease[17] which is more prevalent than previously reported, especially if one considers the increasing cesarean section rate in Western countries[18,19].
Sonographic and Doppler characteristics of scar endometrioma described in the literature[14,20,21] are very different from the usual sonographic appearance of pelvic endometriosis: classic-appearing adnexal endometriomas are cystic-like masses with round shape, regular margins, thick walls, and homogeneous low-level internal echoes[13,22,23]. In contrast, scar endometriomas present as solid masses, inhomogeneously hypoechoic with a fibrotic component appearing as either tiny hyperechoic spots or thick hyperechoic strands and irregularly, often frankly spiculated margins; vascularity may be appreciated by Color Doppler in most of the masses, whereas cystic changes have been rarely reported[14,20,21]. Moreover, in most cases perilesional hyperechoic rings (due to inflammatory reaction triggered by monthly haemorrhage in adjacent tissues) complete or incomplete may be appreciated[14,21].
The present study confirmed the peculiar clinical and sonographic presentation of these lesions, but also demonstrated that large lesions (≥ 30 mm) displayed some clinical and sonographic characteristics which are different from the above-mentioned classic pattern.
Indeed, when compared to patients with smaller lesions, women with a large endometrioma showed a medical history characterized by a longer interval between both the last cesarean delivery and the onset of painful symptoms before hospital admission. Of note, they tended to complain of continuous pain instead of cyclic pain with menses and to more often undergo more expensive imaging examinations (CT/MRI) and invasive diagnostic (FNB/laparoscopy) and therapeutic (e.g., surgery for bowel adhesions) procedures without obtaining either a correct diagnosis or resolution of their illness.
In addition, implant size ≥ 30 mm was associated with peculiar sonographic features of endometriomas that showed more frequent small cystic areas, fistulous digitations and loss of the usual round/oval shape. Color Doppler examination showed multiple vascular pedicles entering the mass from different points and an abundant central vascularisation in contrast with the single vascular pedicle and scarce or absent intralesional vessels seen in smaller scar endometriomas. A likely explanation for these phenomena may rely on the longstanding cyclic bouts of haemorrhage facilitating both accumulation of larger fluid collections and loss of the initial nodular shape of the lesions due to inflammatory and fibrotic changes in the surrounding tissues triggered by haemorrhage itself.
The development of a fistulous tract may be regarded as a consequence of the infiltrative course of this chronic inflammatory process, a feature that further contributes to the irregular shape of endometriomas. An increase in vasculature density, which can be detected more easily on Color Doppler, is another obvious result of increasing nodule size over time.
If one considers that 43% of cases included in the present series had large endometriomas (≥ 3 cm) at the time of diagnosis, this pathologic entity is a truly overlooked diagnosis with several consequences. Firstly, the masses in the abdominal wall are allowed to grow, a condition that brings about a change in the characteristics of both pain (from cyclic to continuous) and sonographic findings (more irregularly-shaped, heterogeneous lesions with cystic areas, fistulous tracts, and more abundant vascularisation). In turn, this entails widening of the differential diagnosis gamut between benign and malignant conditions, thus baffling physicians and radiologists further. Secondly, patients are bound to undergo more frequent inconclusive diagnostic work-ups with costly (i.e., CT/MRI) and sometimes invasive exams (i.e., FNB/laparoscopy). Thirdly, unnecessary abdominal surgery may be performed to relieve painful abdominal symptoms.
In addition, although the malignant transformation of abdominal wall endometrioma has not been clearly elucidated, owing to its rarity, such an eventuality should always be considered[24,25]; therefore early detection and prompt treatment are mandatory.
Although scar endometrioma is a challenging diagnosis, only the first three cases in the present series were misdiagnosed (suture granulomas and sarcoma) at the beginning of this study. Since then every occupying-space lesion in the abdominal wall near a cesarean section incision in a patient with chronic abdominal pain (either cyclic or continuous) was considered a likely endometrioma, and all 27 subsequent patients were correctly diagnosed prior to operation. Moreover, seven non-endometrioma nodules near cesarean section scars were correctly identified during the same time period. Detailed clinical history and accurate physical and sonographic examination were the clues to the high diagnostic accuracy achieved; furthermore, none of the cases underwent CT, MRI and FNB in the diagnostic work-up and surgery, which is the sole form of effective therapy in abdominal wall endometriosis[6,9,26], was not delayed. Although CT and MRI features of scar endometrioma have been described, they often provide only aspecific findings[22,27,28]; furthermore, in the Author’s opinion, evaluation of disease extent in musculo-cutaneous planes of the abdominal wall (one of the main alleged reasons to perform CT and/or MRI) may be assessed nowadays by electronic wide-band high frequency sonographic probes as accurately as by CT and/or MRI. Some authors have proposed FNB to be a valuable diagnostic tool for scar endometrioma[20,29,30], however, inconclusive data have also been obtained[31]. This was true for one of the studied patients who underwent FNB with an inconclusive diagnosis several months before admission.
In conclusion, the clinical scenario (palpable mass near cesarean section scar and pain, especially if cyclic with menses) along with a careful sonographic examination of the entire abdominal wall of the lower quadrants, taking into account all the suggestive sonographic features, even if they might differ according to endometriotic implant size, are sufficient for a confident preoperative diagnosis of abdominal wall endometrioma.
Endometriotic implants in the abdominal wall near a cesarean section scar represent a rare but often misdiagnosed form of extrapelvic endometriosis.
The classic sonographic presentation of endometrioma (i.e., an inhomogenously hypoechoic roundish nodule with fibrotic changes, a peripheral hyperecohoic ring, spiculated margins and scarce internal vascularisation) may vary according to nodule size. In fact, large endometriomas show frequent cystic portions and fistulous tracts, loss of round/oval shape along with increased vascularity.
The author reported the experience in 33 scar endometriomas by highlighting the clinical and sonographic characteristics of this neglected disease. The research is well written and clearly presented. It is a rare entity and of interest to radiologists.
Peer reviewers: Ragab Hani Donkol, Professor, Radiology Department, Aseer Central Hospital, 34 Abha, Saudi Arabia; Jacob Sosna, MD, Section Chief, CT, Director of Research and Imaging Laboratories Department of Radiology, Hadassah Hebrew University Medical Center, Jerusalem 91120, Israel
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
| 1. | Bergqvist A. Different types of extragenital endometriosis: a review. Gynecol Endocrinol. 1993;7:207-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Singh KK, Lessells AM, Adam DJ, Jordan C, Miles WF, Macintyre IM, Greig JD. Presentation of endometriosis to general surgeons: a 10-year experience. Br J Surg. 1995;82:1349-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Wicherek L, Klimek M, Skret-Magierlo J, Czekierdowski A, Banas T, Popiela TJ, Kraczkowski J, Sikora J, Oplawski M, Nowak A. The obstetrical history in patients with Pfannenstiel scar endometriomas--an analysis of 81 patients. Gynecol Obstet Invest. 2007;63:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Minaglia S, Mishell DR, Ballard CA. Incisional endometriomas after Cesarean section: a case series. J Reprod Med. 2007;52:630-634. [PubMed] |
| 6. | Ozel L, Sagiroglu J, Unal A, Unal E, Gunes P, Baskent E, Aka N, Titiz MI, Tufekci EC. Abdominal wall endometriosis in the cesarean section surgical scar: a potential diagnostic pitfall. J Obstet Gynaecol Res. 2012;38:526-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328:1759-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 532] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 8. | Steck WD, Helwig EB. Cutaneous endometriosis. Clin Obstet Gynecol. 1966;9:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 66] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Patterson GK, Winburn GB. Abdominal wall endometriomas: report of eight cases. Am Surg. 1999;65:36-39. [PubMed] |
| 10. | Kodandapani S, Pai MV, Mathew M. Umbilical laparoscopic scar endometriosis. J Hum Reprod Sci. 2011;4:150-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Kaunitz A, Di Sant'Agnese PA. Needle tract endometriosis: an unusual complication of amniocentesis. Obstet Gynecol. 1979;54:753-755. [PubMed] |
| 12. | Yackovich FH, Bender GN, Tsuchida AM. Case report: peri-anal episiotomy scar endometrioma imaged by CT and sector endoluminal ultrasound. Clin Radiol. 1994;49:578-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21:193-216; questionnaire 288-294. [PubMed] |
| 14. | Francica G, Giardiello C, Angelone G, Cristiano S, Finelli R, Tramontano G. Abdominal wall endometriomas near cesarean delivery scars: sonographic and color doppler findings in a series of 12 patients. J Ultrasound Med. 2003;22:1041-1047. [PubMed] |
| 15. | Blanco RG, Parithivel VS, Shah AK, Gumbs MA, Schein M, Gerst PH. Abdominal wall endometriomas. Am J Surg. 2003;185:596-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Park SB, Kim JK, Cho KS. Sonography of endometriosis in infrequent sites. J Clin Ultrasound. 2008;36:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Nirula R, Greaney GC. Incisional endometriosis: an underappreciated diagnosis in general surgery. J Am Coll Surg. 2000;190:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Black C, Kaye JA, Jick H. Cesarean delivery in the United Kingdom: time trends in the general practice research database. Obstet Gynecol. 2005;106:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2005. Natl Vital Stat Rep. 2006;55:1-18. [PubMed] |
| 20. | Hensen JH, Van Breda Vriesman AC, Puylaert JB. Abdominal wall endometriosis: clinical presentation and imaging features with emphasis on sonography. AJR Am J Roentgenol. 2006;186:616-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Savelli L, Manuzzi L, Donato ND, Salfi N, Trivella G, Ceccaroni M, Seracchioli R. Endometriosis of the abdominal wall: ultrasonographic and doppler characteristics. Ultrasound Obstet Gynecol. 2012;39:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Kinkel K, Frei KA, Balleyguier C, Chapron C. Diagnosis of endometriosis with imaging: a review. Eur Radiol. 2006;16:285-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Asch E, Levine D. Variations in appearance of endometriomas. J Ultrasound Med. 2007;26:993-1002. [PubMed] |
| 24. | Bats AS, Zafrani Y, Pautier P, Duvillard P, Morice P. Malignant transformation of abdominal wall endometriosis to clear cell carcinoma: case report and review of the literature. Fertil Steril. 2008;90:1197.e13-1197.e16. [PubMed] |
| 25. | Yan Y, Li L, Guo J, Zheng Y, Liu Q. Malignant transformation of an endometriotic lesion derived from an abdominal wall scar. Int J Gynaecol Obstet. 2011;115:202-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Koger KE, Shatney CH, Hodge K, McClenathan JH. Surgical scar endometrioma. Surg Gynecol Obstet. 1993;177:243-246. [PubMed] |
| 27. | Amato M, Levitt R. Abdominal wall endometrioma: CT findings. J Comput Assist Tomogr. 1984;8:1213-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Coley BD, Casola G. Incisional endometrioma involving the rectus abdominis muscle and subcutaneous tissues: CT appearance. AJR Am J Roentgenol. 1993;160:549-550. [PubMed] |
| 29. | Douglas C, Rotimi O. Extragenital endometriosis--a clinicopathological review of a Glasgow hospital experience with case illustrations. J Obstet Gynaecol. 2004;24:804-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Dwivedi AJ, Agrawal SN, Silva YJ. Abdominal wall endometriomas. Dig Dis Sci. 2002;47:456-461. [PubMed] |