CASE REPORT
A 70-year-old man, who had been generally healthy, noted malaise, yellowing of the bulbar conjunctiva, and brown urine, and consulted a local clinic. He was diagnosed with obstructive jaundice and was admitted to our hospital. Blood chemistry tests showed that ALT was 202 IU/L (normal < 31 IU/L), ALP was 3023 IU/L (normal < 314 IU/L), T-bilirubin was 16.1 mg/dL (normal <1.0 mg/dL), D-bilirubin was 10.9 g/dL (normal < 0.3 mg/dL), C reactive protein (CRP) was 5.9 mg/dL (normal < 0.2 mg/dL), and WBC was 16300/μL (normal < 9000 μL), indicating jaundice, increases in hepatic and biliary tract enzymes and inflammatory reactions. Abdominal ultrasonography (US) showed a hypoechoic solid mass in the middle of the common bile duct with dilation of the intrahepatic duct and enlargement of the gallbladder. Abdominal CT confirmed findings similar to those on US and showed swelling of lymph nodes around the mass and in the abdominal cavity. Narrowing over about 20 mm of the common bile duct was noted on endoscopic retrograde cholangiopancreatography (ERCP), and a diagnosis of adenocarcinoma was made following biopsy. On the basis of these findings, a diagnosis of inoperable common bile duct cancer was made, and a plastic stent (10Fr× 40 mm, Zimmon Biliary Stent, Wilson-Cook Medical) was inserted endoscopically to drain the bile, and chemotherapy (TS-1: oral 5-fluorouracil derivative) was initiated. Abdominal pain and jaundice appeared approximately 6 mo after the beginning of chemotherapy. A diagnosis of stent occlusion and cholangitis was made, and the plastic stent was removed and substituted for an SEMS (80 × 10 mm, WallFlex® Biliary RX uncovered Stent, Boston Scientific) endoscopically. CT images obtained 5 mo later showed no abnormal findings in the right hepatic artery (Figure 1). After 9 mo, abdominal pain and jaundice recurred, and the patient was hospitalized again. T-bilirubin was 3.7 mg/dL, CRP was increased at 9.4 mg/dL, and CT and ERCP showed dilation of the intrahepatic bile duct, indicating cholangitis, but, at this point, a small pseudoaneurysm of the right hepatic artery, measuring 9 × 6 mm, protruding into the common bile duct lumen and in contact with the SEMS was overlooked (Figure 2). On the 2nd hospital day, an endoscopic naso-biliary drainage (ENBD) (7.2Fr. pigtail, Hanako Medical Co., Tokyo) and a plastic stent (7Fr × 100 mm, Zimmon Biliary Stent, Wilson-Cook Medical) were placed into the SEMS (stent-in-stent placement). On CT performed on the 6th hospital day, the pseudoaneurysm had enlarged to 21 × 11 mm (Figure 3). US performed on the same day showed marked dilation of the common bile duct to a diameter of 24 mm and consequent extrinsic compression of the portal vein. The SEMS was displaced toward the liver, and hypoechoic solid components filling the space between the SEMS and bile duct and a pseudoaneurysm showing cystic growth in the lumen of the bile duct were observed. Only the apex of the pseudoaneurysm was in contact with the SEMS (Figure 4). The pseudoaneurysm and hypoechoic solid components filling the dilated bile duct were examined on contrast-enhanced ultrasonography (CEUS) using Sonazoid, and the maximum diameter and neck diameter of the pseudoaneurysm were found to be 23 mm and about 14 mm, respectively. However, there were no Sonazoid bubbles detected in the bile duct other than the pseudoaneurysm, and the hypoechoic solid components filling the bile duct were judged to be debris (Figure 5). While no hemorrhage from the bile duct was noted during the disease course, the pseudoaneurysm tended to enlarge, and TAE was performed on the 12th hospital day. On angiography, the left and right hepatic arteries were found to arise from the superior mesenteric artery, and the pseudoaneurysm was confirmed to be located in the right hepatic artery, as suggested by computed tomography (CT). The aneurysm was located upstream of the common bile duct cancer at the upper third of the SEMS and was separated from the ends of the stent. A micro-catheter was led into the pseudoaneurysm in the right hepatic artery, two 3D shape coils, two 2D shape (GDC™ Detachable Coils, Boston Scientific) were placed, and seven coils (IDC™Detachable Coils, Boston Scientific) were then placed in the right hepatic artery on the distal and proximal sides of the pseudoaneurysm using the isolation method, and the absence of contrast medium influx into the pseudoaneurysm was confirmed (Figure 6). There was no sign of partial hepatic infarction after the procedure on lab data and images. Since no blood flow signal of the pseudoaneurysm was noted on subsequent Doppler US, the ENBD was removed, and the patient was discharged on the 20th hospital day. Chemotherapy has continued to the present.
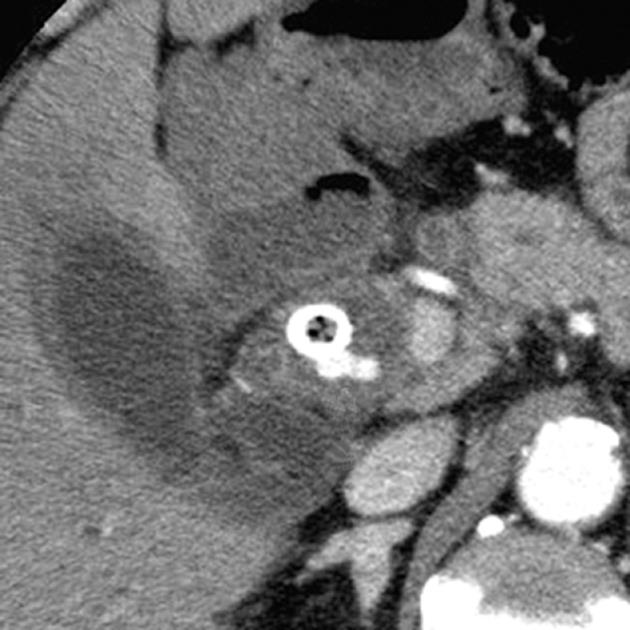
Figure 1 Computed tomography image obtained 5 mo after self-expandable metallic stent insertion showed no abnormal findings of the right hepatic artery (arrow).
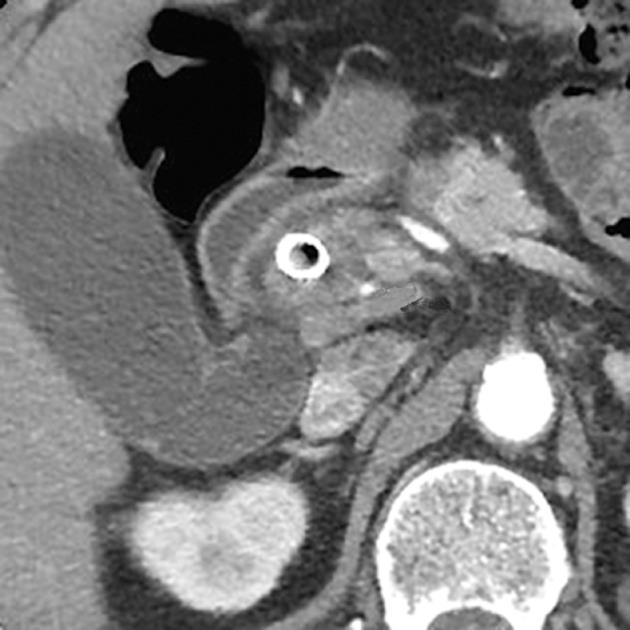
Figure 2 Computed tomography showed a small pseudoaneurysm of the right hepatic artery, measuring 9 × 6 mm, protruding into the common bile duct lumen (arrow) 9 mo after self-expandable metallic stent insertion.
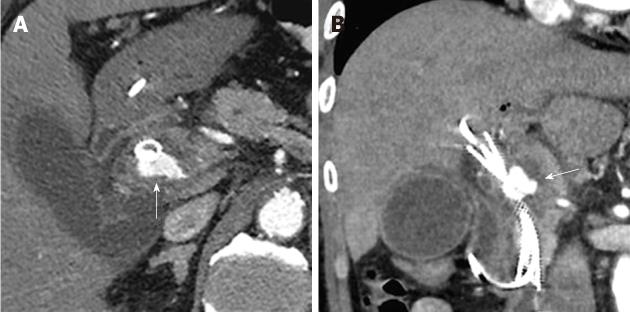
Figure 3 On the 2nd hospital day, an endoscopic naso-biliary drainage and a plastic stent were placed into the self-expandable metallic stent.
On the 6th hospital day, axial (A) and coronal (B) plane arterial phase computed tomography showed enlargement of the pseudoaneurysm (arrow).
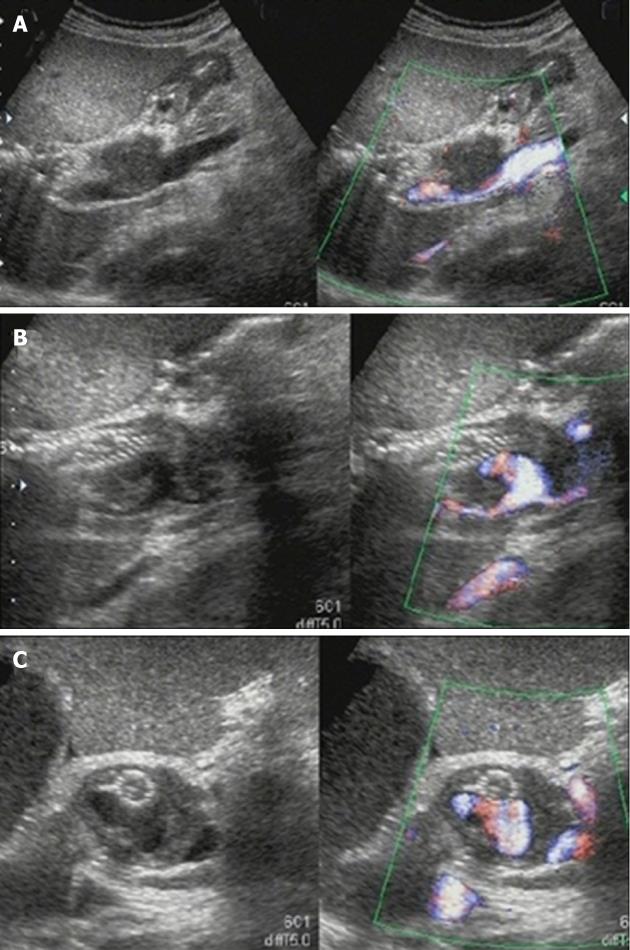
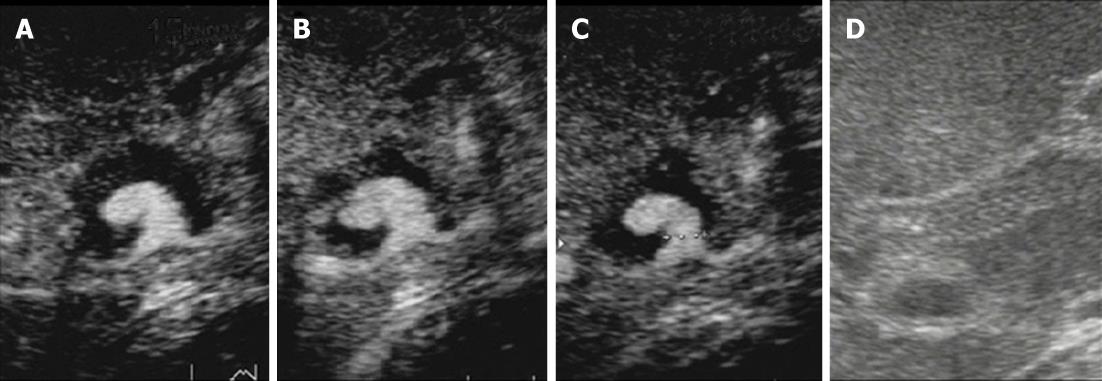
Figure 4 Color Doppler ultrasonography on the 6th hospital day.
A: Ultrasonography showed marked dilation of the common bile duct to a diameter of 24 mm and consequent extrinsic compression of the portal vein; B:The self-expandable metallic stent (SEMS) was displaced toward the liver, and hypoechoic solid components filling the space between the SEMS and bile duct and a pseudoaneurysm showing cystic growth in the lumen of the bile duct was observed; C: Only the apex of the pseudoaneurysm was in contact with the SEMS.
Figure 5 The pseudoaneurysm and hypoechoic solid components filling the dilated bile duct were examined on contrast-enhanced ultrasonography.
A-C: Images obtained at 15 s (A), 55 s (B) and 127 s (C) after injection of Sonazoid (0.5 mL) via a left cubital venous line showed no Sonazoid bubbles in the common bile duct other than in the pseudoaneurysm; D: Monitor B-mode ultrasonography image.
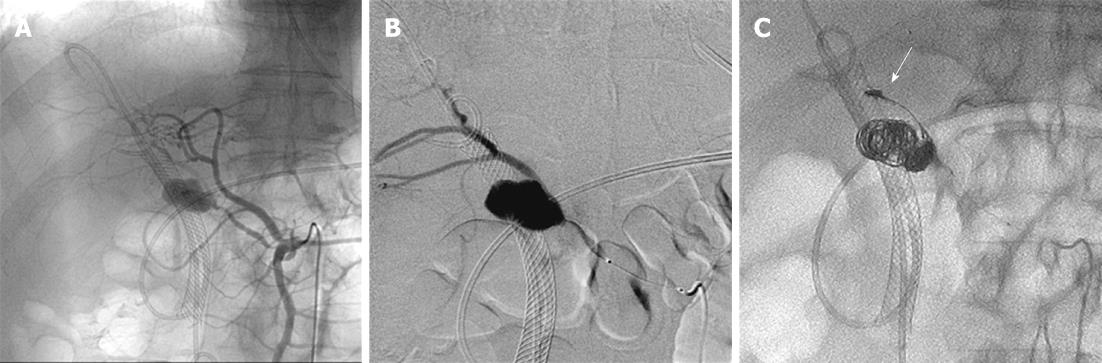
Figure 6 Transcatheter arterial embolization performed on the 12th hospital day.
A, B: On angiography, the left and right hepatic arteries were found to arise from the superior mesenteric artery, and the pseudoaneurysm was confirmed to be located in the right hepatic artery; C: One IDC coil was placed in the right hepatic artery on the distal side of the pseudoaneurysm (arrow) after the pseudoaneurysm was framed using several types of coils. Seven coils were placed in the right hepatic artery on the distal and proximal sides of the pseudoaneurysm using the isolation method.
DISCUSSION
The incidence of serious complications associated with SEMS placement in the bile duct has been reported to be 7%-24%[1,2], and cholangitis, pancreatitis, acute cholecystitis, liver abscess, common bile duct perforation, duodenal perforation, and hemobilia are frequent complications. These complications occur relatively early after the placement of an SEMS, are caused by the procedure of stent placement and re-occlusion due to sludge, and are often resolved by conservative treatment. However, hemobilia occasionally leads to a serious outcome. Generally, hemobilia associated with SEMS placement is considered to be caused by damage to the artery or liver parenchyma due to percutaneous transhepatic puncture prior to SEMS placement or vascularization due to infection. Green et al[3] reported in a review of 222 recent cases of hemobilia, that the hemorrhage was caused by medical procedures involving the hepatobiliary system such as percutaneous transhepatic biliary drainage in 147. Moreover, pseudoaneurysms were reported to have been noted in 1.7%-16.9% of cases with hemobilia associated with surgical procedures of the hepatobiliary system[4,5].
To date, there have been a few reports of pseudoaneurysm caused by stents including plastic stents rather than by complications of endoscopic or percutaneous transhepatic procedures, however, those caused by SEMS are very rare[6,7]. Monroe et al[6] noted a pseudoaneurysm of the pancreaticoduodenal artery 3 wk after the endoscopic placement of an SEMS, and reported that it was caused by necrosis of the arterial wall due to compression by the SEMS. Rai et al[7] reported a case that developed obstructive jaundice 6 mo after percutaneous transhepatic placement of an SEMS, and underwent endoscopic placement of a plastic stent in the SEMS for bile drainage. The stent re-occluded after several months, and, when the plastic stent alone was removed, a pseudoaneurysm of the right hepatic artery ruptured. They concluded that inflammation around the bile duct and adhesion between the SEMS and hepatic artery contributed to the formation of the right hepatic artery pseudoaneurysm.
In our patient, a plastic stent was placed for obstructive jaundice due to inoperable middle common bile duct cancer, and was replaced with an SEMS endoscopically due to the recurrence of jaundice after 6 mo. CT performed the next day showed no abnormal findings in the right hepatic artery, but CT performed 9 mo after stent re-occlusion first disclosed a pseudoaneurysm in the right hepatic artery. On US and CT, the pseudoaneurysm was about 20 mm in diameter, protruded into the markedly dilated lumen of the common bile duct, and was displaced with only its apex being in contact with the external wall of the SEMS. In addition, US suggested the presence of hypoechoic solid components in the space between the external wall of the SEMS and common bile duct, apparently filling the pseudoaneurysm. However, on CEUS, no Sonazoid bubbles were noted in the hypoechoic area around the pseudoaneurysm, thus, the solid components were judged to be sludge or debris rather than tumor invasion. Moreover, the pseudoaneurysm was located near the center of the SEMS and was distant from its ends, suggesting that the possibility of its formation due to trauma or compression of the arterial wall by the metal at the tip of the SEMS was low. From a review of the disease course, the pseudoaneurysm in the right hepatic artery in our patient was unlikely to have been caused by placement of the plastic stent or SEMS or invasion of the primary cancer to the hepatic artery. Also, no pancreatitis associated with the endoscopic procedure or stent placement was noted, excluding pancreatitis as a cause of pseudoaneurysm formation. Therefore, in our patient, the pseudoaneurysm was considered to have developed due to the promotion of erosion of the hepatic artery wall in contact with the common bile duct by the concurrence of factors including marked dilation of the common bile duct due to the accumulation of sludge and debris and the spread of inflammation to surrounding tissues from recurring cholangitis associated with obstructive jaundice. This etiology of pseudoaneurysms resembles that reported by Monroe et al[6], however, there has been no report of a hepatic artery pseudoaneurysm in a patient undergoing endoscopic placement of an SEMS.
More than 60% of unruptured pseudoaneurysms of the hepatic artery are either asymptomatic and detected incidentally or are symptomatic but present non-specific findings[8,9]. Of ruptured pseudoaneurysms, 10% are asymptomatic and symptoms such as hemobilia, hematemesis, hematochezia, and abdominal pain are noted in 64%, 30%, 14% and 20%, respectively[10]. The possibility of hepatic artery pseudoaneurysm rupture is 21%-80%[11-14], and the mortality rate due to their rupture is high at 21-43%[13,15], thus, appropriate imaging diagnosis and treatment before rupture are desired.
Due to the recent development of imaging modalities, high-performance ultrasound devices, multi-detector row CT (MDCT), and magnetic resonance angiography have become available for the diagnosis of pseudoaneurysms[15-17]. Kim et al[16] compared the usefulness of MDCT and Doppler US for the early detection of hepatic artery pseudoaneurysms, which is a complication of liver transplantation from a living donor, and reported MDCT as more effective, probably because US is affected by gas in the digestive tract and the examiner’s skill. CEUS, on the other hand, is advantageous in that it is free of radiation exposure or iodine allergy and can also be used in patients with renal dysfunction. The use of CEUS in patients with hepatic artery pseudoaneurysm has not been reported. However, with the use of contrast media such as Sonazoid, its diagnostic utility for hepatic artery pseudoaneurysms may not only be improved, but may also contribute to treatment planning by allowing delineation of the morphology of the lesion, measurement of its neck, determination of the presence or absence of mural thrombi, and detailed and real-time evaluation of the environment around the lesion. According to the report by Green et al[3] concerning treatments for hemobilia including that due to pseudoaneurysms, 43% were treated conservatively, 36% by TAE, and 20% surgically, and the rate of surgical treatment appears to be decreasing due to the development of interventional radiology. We also treated our patient with TAE. Embolization can be performed either by covered-stent insertion[18,19] or by simultaneous embolization of both the proximal and distal sides of the pseudoaneurysm[20]. In our patient, because of the width of the neck with a diameter of 14 mm and the mural structure of the pseudoaneurysm, consisting only of a delicate fibrous capsule[18], the internal wall of the aneurysm was framed first using a 3D shape coil, and then a 2D shape coil, a conventional platinum coil, was placed so that it would entwine with the 3D coil to prevent escape of the 2D coil out of the pseudoaneurysm. Thereafter, seven IDC coils were placed in the right hepatic artery on the distal and proximal sides of the pseudoaneurysm, and the procedure was completed by confirming the absence of blood flow in the pseudoaneurysm. It was very important for the safe execution of the procedure that the responsible artery could be identified as a branch of the hepatic artery that arose from the SMA by CT, and that the shape and size of the pseudoaneurysm and diameter of its neck could be determined by CEUS before TAE. The success rate of TAE in controlling hemobilia has been reported to be 80%-100%[3], and the mortality rate due to TAE for pseudoaneurysm of the hepatic artery has been reported to be 25% lower than that due to surgery[10]. Therefore, surgery should be selected for large pseudoaneurysms and patients in whom TAE has been unsuccessful or is contraindicated.
In conclusion, in our patient, prophylactic TAE was performed for a hepatic artery pseudoaneurysm without hemobilia. In addition, CEUS was very useful for delineation of the pseudoaneurysm on examination before TAE. A lethal pseudoaneurysm or hemobilia may occur during long-term placement of an SEMS in the bile duct. While an SEMS reduces the incidence of jaundice and cholangitis, it may cause specific refractory complications that have not been evaluated sufficiently. Also, if a stent is placed over a long period, many problems including biocompatibility of the stent material and structure remain to be evaluated. The accumulation of cases based on long-term follow-up is necessary.