Published online Oct 28, 2012. doi: 10.4329/wjr.v4.i10.421
Revised: October 2, 2012
Accepted: October 9, 2012
Published online: October 28, 2012
The routine and potential future applications of equilibrium radionuclide angiocardiography/multigated acquisition (MUGA) in clinical decision making are explored in this review. The non-invasive nature of the test, less operator dependence, lower radiation dose and ease of performing, even in ill patients, are important considerations in clinical cardiology practice. Two important routine uses of this modality in day-to-day clinical practice include the following: serial assessment of left ventricular ejection fraction (LVEF) in patients receiving cardiotoxic chemotherapy, and determination of accurate LVEF in patients with intractable heart failure. Other potential utilities of MUGA that could be translated into clinical practice include determination of regional LVEF, obtaining information about both right and left ventricle in suitable patients as a part of first pass angiocardiography, identification of diastolic dysfunction in patients with heart failure with preserved LVEF, and demonstration of dyssynchrony prior to cardiac resynchronisation, specifically by MUGA single photon emission tomography.The last two indications are particularly important and evolving at this point.
- Citation: Mitra D, Basu S. Equilibrium radionuclide angiocardiography: Its usefulness in current practice and potential future applications. World J Radiol 2012; 4(10): 421-430
- URL: https://www.wjgnet.com/1949-8470/full/v4/i10/421.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i10.421
Multigated acquisition (MUGA) of the cardiac blood pool or equilibrium radionuclide angiocardiography (ERNA) is an established modality in nuclear cardiology practice. The 99m-technetium radio-labelled red blood cells (RBCs) delineate the left ventricular (LV) cavity with high precision and aid in accurate evaluation of LV function. With the advent and routine use of gated myocardial perfusion studies (MPS) and highly improved cardiac computed tomography and magnetic resonance imaging, a number of previous applications of MUGA have almost gone into oblivion or add little to the practical management of cardiac patients at present. Use of the widely available echocardiogram with or without colour Doppler has been highly popular among internists because of its availability, ease of use and low cost. Even though the routine use of MUGA scan has reduced among the user community, its lower operator dependence and high reproducibility are of great advantage and can make this technique pivotal in the evaluation of certain cardiac conditions in routine clinical practice[1,2].
In this review, we have summarized the clinical usefulness of MUGA in today’s cardiology along with some evolving applications. The review explores the role of MUGA in precise estimation of LV ejection fraction (LVEF) (both global EF and regional EF), its relevance as a part of first pass radionuclide angiocardiography (FRNA), identification of diastolic dysfunction in patients of heart failure with preserved ejection fraction (HF-PEF) and also in determining the synchronicity of LV myocardium in the decision making of cardiac resynchronisation therapy.
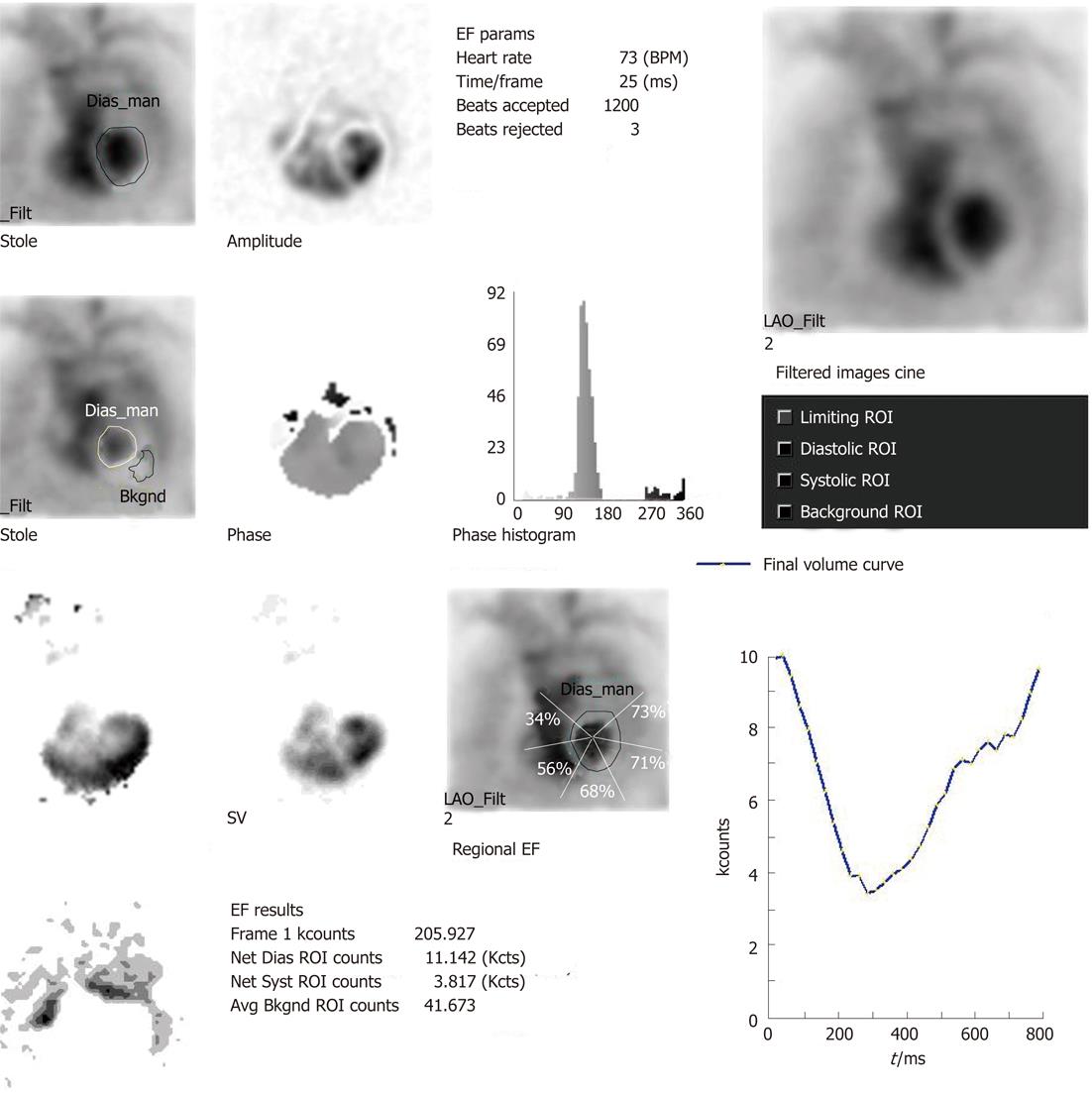
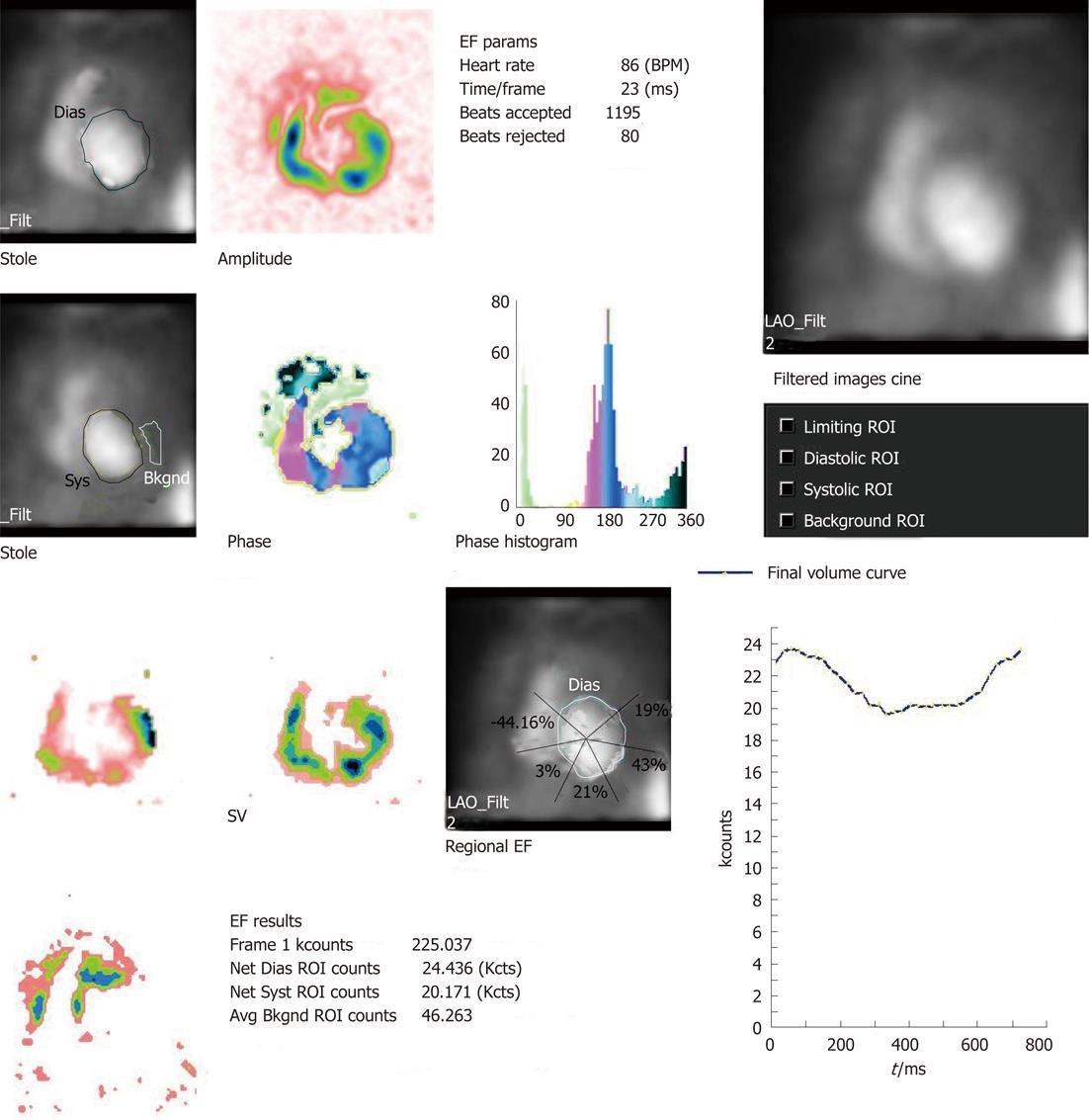
MUGA study, also referred to as radionuclide ventriculogram, uses 99m-technetium labelled RBC (99m-Tc RBC) to provide a relatively accurate and reproducible assessment of LVEF. One prerequisite of electrocardiographic (ECG) gating is less than 10% premature ventricular contraction and hence an ECG rhythm strip is taken before the study. The patient is injected with approximately 20 mCi of 99m-Tc RBC. The tagging is undertaken by different methods (e.g., in vivo/in vitro). The radiolabeled RBCs achieve a state of equilibrium after around 15-20 min and the patient is imaged after an interval of 30 min from injection with a gamma camera. ECG gating is carried out using R wave as a trigger. The cardiac cycle is divided into 16 or 32 frames. The correct frame rate is required to obtain a proper temporal sampling (to obtain the peaks and valleys of the cardiac cycle) and statistical sampling (to obtain proper count statistics). Usually three standard views of the heart are obtained: anterior, left anterior oblique and best septal view (in which the interventricular septum is best visualised delineating both right and LV blood pool)[3]. The scan takes approximately 20 min to be completed. The review and analysis of the data are undertaken both by qualitative and quantitative mode and by the cinematic display on the computer screen of the images with regard to the ventricular contraction and wall motion. After the initial visual assessment, a region of interest (ROI) is drawn around the LV blood pool. This ROI would generate LVEF, regional EF and various other parameters including parametric images with the aid of software analysis (the details of the parameters are described in the following sections)
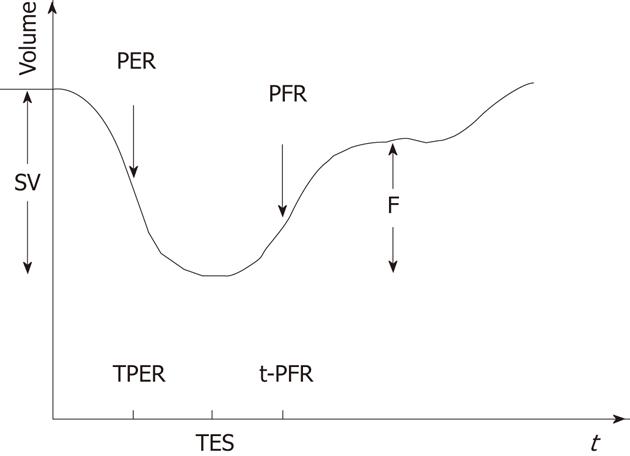
Substantial clinically important information can be obtained from a resting MUGA study. Both the global and regional EF of LV can be calculated with high precision with the help of MUGA study. In addition, MUGA provides information about the size, position and rotation of the heart and proximal great vessels. One can also get an idea about the individual chamber size [LV, right ventricular (RV) and atria]. With regard to EF, usually a normal LVEF at rest is considered to be 50% or more. An important advantage of MUGA study is the knowledge of regional wall motion of LV and regional EF in addition to global LVEF. Regional values can be abnormal even when global ejection fraction is normal, as happens in the setting of a dyskinetic segment of myocardium. The left ventricular time activity curve (LV TAC) gives a fair estimate of contractile pattern of LV and diastolic function. A number of parametric images can be displayed which give added insight into the data described above. A stroke volume (SV) image is obtained by subtracting the end systole image from the end diastole (ED) image. SV is the amount of blood ejected out of LV per contraction. The paradox image is the atrial SV and is generated by subtracting the diastolic image from the systolic one. In this case, all negative pixels are set to zero. The phase display consists of both an image and a graph. The phase display shows us the contractile pattern of myocardium at a given point of time. The images are usually based on colour or gray scale. It helps in determination of synchronicity of LV myocardium. The amplitude image is also a colour scale/gray scale display of the magnitude of contraction of myocardium and obtained from MUGA study. Two more important parameters obtained from the LV TAC are peak filling rate (PFR) and time to peak filling rate (t-PFR). These two parameters are important considerations for diagnosing diastolic dysfunction (Figures 1-4). The clinical relevance of each parameter has been described in respective sections.
The coronary angiogram has been the gold standard for studying LV function[3] but it is an invasive procedure with high radiation burden. The facilities are not widely available and thus are expensive. Instead, the echocardiogram is a widely available tool. It is easy to perform and does not impart any radiation to the patients. The procedure can be performed even at the bed side in a sick patient as well. However, the modality is highly operator dependent and often gives inaccurate results in some specific population groups like obese and female subjects. MUGA is non-invasive in nature, easy to perform, imparts a smaller radiation burden to patients (0.3-0.52 rem equivalent in a standard MUGA study)[3] and is less operator dependent. An entire MUGA study is completed by one hour. So, it can be a reasonable alternative in clinical practice. The use of gating devices with R wave on ECG (corresponding to ED)[4] has helped in assimilating both the electrical and mechanical events of LV in a single study. Apart from routine use of LVEF determination, a MUGA study can provide diastolic parameters, mostly the PFR and t-PFR derived from the LV time activity curve. However, it is widely known that variations in heart rate affect the diastolic portion of the time activity curve from which the PFR and t-PFR values are obtained (heart rate variations do not affect the systolic portion of the curve and therefore do not affect the calculation of ejection fraction). When a physician wishes to obtain this diastolic information the acquisition must include a narrow acceptance window of +/- 10%-15% around the average heart rate R-R interval. The acquisition time will increase proportionally with the amount of heart rate irregularity, which is required for an accurate PFR and t-PFR.
The determination of synchronicity of LV myocardium is of paramount importance in clinical decision making prior to cardiac resynchronisation therapy (CRT). Recent studies show MUGA can also help in this scenario[5] with acceptable precision as compared to speckle tracking echocardiogram. MPS can also provide information about diastolic parameters[6,7] and synchronicity of LV myocardium[8,9] in today’s practice of cardiology but MPS is more expensive and it is a relatively prolonged procedure (resting MPS takes at least 2 h to be completed) compared to a MUGA study. The following sections will elaborate the strength and shortfalls of each modality in details.
Before we proceed further in the discussion, we summarise the main applications of MUGA in a tabular format (Table 1).
| Older applications | Recent applications | Evolving applications for routine use | Commonly employed modality in current practice | |
| Chamber orientation | + | +/- | - | Echocardiogram |
| Chamber size | + | +/- | - | Echocardiogram |
| Rhythm abnormalities | + | - | - | Electrophysiologic studies |
| Global EF | ++ | ++ | Contrast angiogram, 2D echocardiogram | |
| Regional EF | ++ | ++ | MUGA | |
| Wall motion abnormalities | ++ | +/- | - | Gated myocardial perfusion scans |
| Diastolic function evaluation | - | + | ++ | Echo-Doppler with E/A measurements |
| Assessment of synchrony | - | - | ++ | Speckle tracking echocardiogram |
Serial evaluation of LVEF in patients receiving cardiotoxic chemotherapeutic medications like anthracycline antibiotics (doxorubicin, daunorubicin, epirubicin etc), is one of the most commonly utilized indications for MUGA study. The applications of MUGA in this setting has been extensively summarised in an article by Lu[10]. It is observed that these medicines have cumulative dose dependent adverse effects on cardiac myocytes and may lead to cardiomyopathy induced congestive heart failure (CHF) due to superoxide mediated cell damage mechanisms[11-13]. Swain et al[13] have shown that the incidence of CHF is 5%, 26% and 48% in patients who received cumulative doses of 400 mg/m2, 550 mg/m2 and 700 mg/m2 of body surface of doxorubicin, respectively. Around 450-500 mg/m2 cumulative dose is considered a “dangerous dose” for inducing cardiotoxicity. All patients, however, are not vulnerable to the side effects in similar fashion. It depends on an individual’s susceptibility to cardiotoxic anthracyclines. Hence, the beneficial effects of the anthracyclines are not to be curtailed abruptly at a dose of 450-500 mg/m2 in all patients. This has to be customised on a case to case basis, based on available cardiac parameters, mostly by measuring LVEF. The indication to discontinue doxorubicin treatment is a fall of LVEF by 10% or more and/or LVEF being 30% or less. MUGA has excellent reproducibility in determining LVEF and hence forms the basis of its serial application in the clinical setting. The determination of LVEF by MUGA does not require a particular geometric conformation of LV as required by echocardiogram or contrast angiogram and hence the assessment by MUGA is more accurate.
Guidelines for monitoring adult patients on doxorubicin are described in Table 2[14]. The monitoring of paediatric populations receiving anthracyclines is different and an echocardiogram is usually the preferred modality due to no radiation to the patients[15,16]. However, when mediastinal irradiation is necessary, the preferred approach has been described in Table 3.
| Baseline MUGA (before starting of chemotherapy or before 100 mg/m2 dose | If LVEF is ≤ 30% - no doxorubicin |
| If baseline LVEF is > 30% and < 50% | MUGA to perform before each dose |
| If baseline LVEF is ≥ 50% | MUGA to perform at the dose of 250-300 mg/m2, 400-450 mg/m2 and thereafter before each higher dose |
| If the fall of LVEF from previous study is ≥ 10% or if LVEF is ≤ 30% | Discontinue doxorubicin |
| If mediastinal irradiation is < 1000 cGy | Perform echocardiogram every alternate dose of doxorubicin when dose is < 300 mg/m2 and perform echocardiogram before each dose if the dose of chemotherapeutic agent is ≥ 300 mg/m2 |
| If mediastinal irradiation is > 1000 cGy | Perform echocardiogram before each dose |
| When the cumulative dose of chemotherapy crosses 400 mg/m2 | Perform MUGA before any additional dose |
| Discontinue doxorubicin | If the fall of LVEF is ≥ 10% from previous study or LVEF is < 55% |
| Follow up | MUGA at 1st year of completion of therapy and then echocardiogram yearly till next 3 years and a repeat MUGA at 5th year along with electrocardiogram yearly |
Apart from systolic function, diastolic parameters can also be a predictor of depressed myocardial functions for patients on chemotherapy[16] and will be discussed in the relevant section.
The therapeutic decision for patients with intractable cardiac failure is crucial. Patients with end-stage heart failure require surgical management such as heart transplantation[17]. The determination of LVEF in its near exact value is thus crucial to the central decision regarding performing a surgical procedure or managing the patients conservatively. MUGA, being the most dependable methodology to determine the LVEF with minimal inter-observer variation, is definitely the method of choice[1]. Patients with chronic heart failure (HF) with LVEF < 35% are often candidates for CRT on the basis of the duration of QRS complexes on ECG[17]. There is an evolving concept of demonstration of dyssynchrony that is of paramount importance. This will be discussed under the subheading of “evaluation of dyssynchrony prior to CRT”.
First pass radionuclide angiography (FPRNA) is useful in (1) evaluating cardiac shunts and (2) obtaining RVEF. This is performed by injecting the radiopharmaceutical in bolus form and analysing the usable data during the initial transit of the radionuclide bolus through the central circulation. In order to obtain RVEF, the injection should be prolonged to attain an equilibrium blood pool phase in the right ventricle[4]. The details of the FPRNA are beyond the purview of this review; however, it is important to note that FPRNA can be an addendum to a standard MUGA study. However, MUGA remains as the standard modality to evaluate the LVEF due to higher count rate[18].
MUGA is unique to demonstrate the regional EF of LV myocardium with reasonable precision[19]. The change in background corrected count in regions of LV, directly proportional to the change in blood volume, is used to determine the regional EF. Thus the regional EF is calculated from the background corrected time activity curve generated from a standard resting MUGA study. This method obviates the geometric assumptions needed for invasive angiographic method of EF measurement and hence becomes more reproducible in serial studies. Due to automated data processing, patient analysis can be performed within a few minutes after data acquisition. The role of cardiac MR in determining regional EF has emerged in recent times and this appears to be more accurate in the post myocardial infarction (MI) state. The role of the 2D echocardiogram and planar MUGA study in post MI is dubious due to loss of 3D information[20]. However, cardiac MR is an expensive investigation and has limitations (e.g., in patients with claustrophobia and metallic implants). The data on comparative assessment of MUGA and CMR is not available at the moment. Regional EF determinations may prove useful in assessing the natural progression of coronary artery disease, or assessing changes resulting from pharmacologic, surgical or physiological interventions.
Diastolic function can be conceptually described by two distinct parameters like relaxation and compliance. Systolic dysfunction and low ejection fraction have been implicated for various HF patients. The concept of isolated diastolic dysfunction[21] is emerging. Growing evidence is showing a clinical entity in a large number of patients with cardiac failure who have preserved EF but have an impaired diastolic function as the aetiology of heart failure. This entity is termed as heart failure with preserved ejection fraction: HF-PEF[21]. It is estimated that approximately 50% of the heart failure population has a normal LVEF[22]. In heart failure caused by diastolic dysfunction the pathophysiology, treatment and prognosis differ from that seen in heart failure caused by systolic dysfunction. Hence, it is important to assess quantitatively the diastolic properties of the left ventricle as well as the systolic properties of the left ventricle in the management of patients with heart failure. The current diagnostic modality routinely used for assessment of diastolic dysfunction is Doppler echocardiography which allows non-invasive evaluation of ventricular diastolic filling[23]. The trans-mitral velocity curves reflect the relative pressure gradient between the left atrium and left ventricle throughout the diastolic filling period. The progression of diastolic dysfunction in disease states can be assessed by Doppler flow velocity curves. This test gives information about early filling to atrial filling ratio (E/A), deceleration time (dT) and isovolumetric relaxation time (IVRT)[24,25].
Non-invasive and invasive procedures require mathematical assumptions about geometry of the ventricles to quantify ventricular function. Such assumptions work well when ventricular shape is maintained. When the shape of the left ventricle is distorted by infarction, severe hypertrophy or marked dilatation, the accuracy of such geometric approaches is questionable[26]. Echocardiography is operator dependent, and for this reason, following up the patients with serial echocardiography may not give accurate information[27]. A mitral inflow pattern of abnormal relaxation (early filling less than atrial filling; prolonged IVRT; prolonged dT) is commonly associated with coronary artery disease, ischemic cardiomyopathy, hypertension, LV hypertrophy and aging[28]. Alterations in loading conditions e.g. reduced preload or increased after-load can also change a normal pattern to an abnormal filling pattern. As relaxation becomes further delayed, it impinges on the early filling phase, resulting in an increase in left atrial pressure (LAP). This increased LAP causes the filling pattern to appear normal as E/A becomes > 1[26,28]. This transition zone between abnormal relaxation and restrictive filling is termed pseudo-normalization[28] and is characterized by normal diastolic filling values. In order to differentiate between normal and “pseudo-normal”, evaluation of the trans-mitral flow at peak valsalva (or any manoeuvre that reduces preload such as reverse trendelenburg or nitroglycerin) and/or evaluation of pulmonary venous flow is advocated. ERNA results have higher reproducibility because there are no geometric assumptions and do not have the disadvantage of being operator dependent. Also, with the emergence of newer software and high data storage capabilities for storing high frame data, the possibility of employing MUGA in the clinical context of diastolic dysfunction is emerging and appears more feasible for routine use.
Several studies demonstrated a good correlation between 2D echocardiogram and ERNA for a reliable determination of the diastolic parameters[29,30].
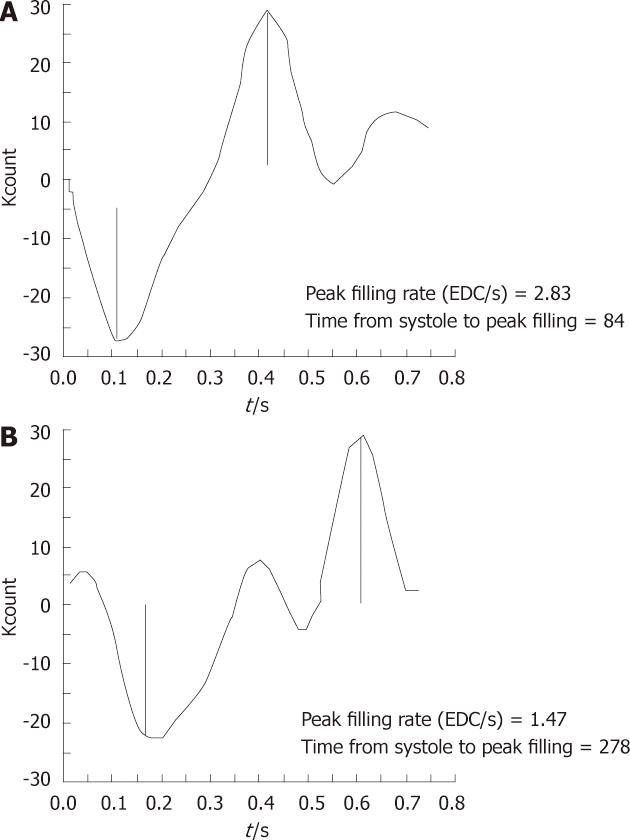
It has been observed that, for diastolic function assessment, a 32 frame gated study[4] is more valuable as compared to the more commonly performed 16 or 24 frame gated studies due to better temporal resolution. The LV TAC is generated by first Fourier harmonics (Figure 2). In a patient with diastolic dysfunction, there will be prolongation of IVRT, delay in onset of rapid filling, decrease in slope of rapid filling phase and exaggerated atrial kick[31]. Quantitatively, peak diastolic filling rate can be obtained from the first derivative of the diastolic portion of the LV TAC. The lower normal limit of PFR is 2.50 end diastolic volume per second (EDV/s). In addition, t-PFR can also be expressed in milliseconds and is expected to be less than 180 ms in a normal subject. The relative contribution of atrial filling to LV filling may be quantified as the ratio of the atrial peak to the peak of the rapid filling phase on the first derivative curve. Ratios of less than 1:4 are normal[3] (Figure 3).
The PFR is calculated by taking the first derivative of the time activity curve. The first major positive peak in the first derivative curve corresponds to the point in the time activity curve at which counts are increasing at their fastest rate. It is expressed as EDV/s. The second major positive peak in the first derivative curve corresponds to the most rapidly increasing count rate during atrial systole and has been referred to as the atrial filling rate (AFR). The PFR and AFR have been shown to correspond to the E and A waves of the Doppler echocardiographic mitral velocity waveform.
Others[31] have studied various parameters in the evaluation of diastolic function by the radionuclide technique. The parameters were PFR, t-PFR, atrial contribution to filling and IVRT. Prolongation of isovolumetric time, delay and/or decrease in early rapid filling and exaggeration of atrial contribution to filling are typical disturbances of normal filling pattern. They may occur alone or in combination (Figure 4).
In addition, the LV TAC can differentiate between a restrictive cardiomyopathy and constrictive pericarditis. The clinical presentations of both these entities are confusing and it is important to differentiate between the aetiology as the treatment plans are different. The walls of the heart become rigid in restrictive cardiomyopathy leading to less compliance of LV during its filling. Constrictive pericarditis is usually a sequela of infection/inflammation of the pericardium. It can also occur subsequent to heart attack or surgery. If early diastolic filling is delayed in a MUGA study, it indicates restrictive disease whereas if it is very rapid, it is suggestive of constrictive disease[3].
CRT, also known as biventricular pacing, is a definitive therapy for patients with intractable heart failure. The selection of patients for CRT is crucial and it depends upon the fulfilment of the following criteria[17]: (1) HF of New York Heart Association grading of heart failure grade III/IV; (2) LVEF < 35% and (3) prolonged QRS (≥ 120 ms) on ECG. A wide QRS complex is a surrogate marker for mechanical dyssynchrony used to select CRT patients. Baseline QRS duration is a good marker of inter-ventricular dyssynchrony, but left intra-ventricular dyssynchrony, which is a more accurate predictor of CRT response, does not correlate with baseline QRS duration[32]. With the use of these selection criteria it is shown that 30% of patients do not show an improvement in their heart failure status even after CRT. So, it is not the prolonged QRS, but rather demonstration of dyssynchrony, that is more important in selecting patients for CRT. A handful of studies have shown that speckle tracking echocardiogram/tissue Doppler imaging is a good modality to demonstrate dyssynchrony by showing lateral to septal delay[33]. A recent multicentre trial has demonstrated inconsistencies in predicting the outcome of CRT on the basis of echocardiographic parameters[34]. Hence, MUGA scan, MUGA single-photon emission computed tomography (SPECT) in particular, is equally efficient in demonstrating dyssynchrony in the LV myocardium and help in taking decisions for CRT. In particular the phase image in MUGA is mostly helpful in diagnosing dyssynchrony. Each phase angle corresponds to a temporal event and thus provides information on synchronous or dyssynchronous patterns. The mean and SD of LV Ø (SD Ø), derived from first harmonic phase analysis and the phase histogram of the ventricular time activity curve in ERNA, have been applied to characterize synchrony[35]. Novel, objective measures of regional contraction and global mechanical synchrony, the synchrony (S) and entropy (E) parameters have been developed and applied to planar ERNA as a tool for evaluation and management of HF patients. S expresses the efficiency of contraction within a region of interest (ROI). S can estimate the contraction potential if the ROI is synchronized. E measures the degree of randomness within the ROI, from 0, with synchronous motion and a single Ø, to 1 with fully dyssynchronous contraction[35,36]. It is designed to differentiate between forms of extremely variable regional dyssynchrony. In preliminary clinical protocols normal values were established and these measures were shown to enhance CRT patient selection, to predict and quantitate CRT outcomes, to optimize CRT pacemaker lead placement based on location of the latest contracting segment, to assess synchrony in HF patients with narrow QRS, and to measure RV synchrony[35,36]. With the phase image from which they are derived, the latest contracting segment can be localized and CRT pacemaker location optimized. Additionally, the importance of RV synchrony can be evaluated over a spectrum of cardiac pathology. As noted by the authors, further studies assessing the ability of these parameters to predict CRT outcome are required and application of the method to SPECT ERNA could add greater resolution and accuracy.
This is an upcoming modality and increasingly more studies have been published in this domain in recent times. The advantage of this technique is the absence of the necessity of a background subtraction in generating the LV TAC, as in the case of planar MUGA study, thus being less liable to manual error[37]. Automatic or semi-automatic programmes which are inherently volumetric and consider LV as a 3D object are the current standard. The timing of the scan is similar to that of planar study i.e., 30 min after the injection of radiolabeled blood cells. Instead of planar acquisition for standard MUGA study, gamma camera heads are rotated around the patient’s body to obtain data from different angles. Usually 180 degree acquisition is performed. The R wave gating and beat window acceptance is kept similar to that of a standard resting MUGA study. The data are then processed with an optimum filtered back projection method to provide short axis oblique slices. With the help of software, different parameters are obtained, including LV volume curve, LVEF, RVEF, LV emptying, RV emptying, LV volumes as well as synchronicity of LV myocardium (i.e., phase histogram)[3]. LVEF obtained from SPECT MUGA study is 7-10 units higher than that obtained from planar MUGA study due to complete removal of all activity from left atrium. This factor must be considered for applying the SPECT MUGA LVEF values in the evaluation of chemotherapy patients where standards have been established using planar techniques[38]. The RV parameters and wall motions are better analyzed on SPECT MUGA. It is a useful modality for the assessment of LV and RV activation sequence and identification of the sites of atrio-ventricular nodal bypass tracks, as well as LV and RV arrhythmias[38]. The newer application of SPECT MUGA is to determine the synchronicity of LV myocardium in HF patients as described earlier for guiding them for CRT[37].
In conclusion, the data supporting potential applications of MUGA in diagnosing diastolic dysfunction and LV dyssynchrony are emerging at present; these could be translated into clinical practice. Due to higher inter-observer reproducibility and precision, MUGA is more reliable and easy to apply method for the estimation of LVEF especially in patients receiving cardiotoxic chemotherapy and in patients with intractable heart failure as compared to commonly practiced 2D echocardiogram.
Professor Gopinathan Nair PG of Department of Nuclear Medicine and Professor Cherian G of Department of Cardiology, Narayana Hrudayalaya Institute of Medical Sciences, Bangalore for providing guidance and encouragement in initial drafts; Dr. Kurlagiri R, Department of Nuclear Medicine, Narayana Hrudayalaya Institute of Medical Sciences, Bangalore for help in the draft of diastolic dysfunction.
Peer reviewer: Monvadi Barbara Srichai-Parsia, Department of Radiology and Medicine, NYU School of Medicine, 660 First Avenue, 2nd Floor, New York, NY 11211, United States
S- Editor Cheng JX L- Editor O’Neill M E- Editor Xiong L
| 1. | van Royen N, Jaffe CC, Krumholz HM, Johnson KM, Lynch PJ, Natale D, Atkinson P, Deman P, Wackers FJ. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843-850. [PubMed] |
| 2. | Nousiainen T, Vanninen E, Jantunen E, Puustinen J, Remes J, Rantala A, Hartikainen J. Comparison of echocardiography and radionuclide ventriculography in the follow-up of left ventricular systolic function in adult lymphoma patients during doxorubicin therapy. J Intern Med. 2001;249:297-303. [PubMed] |
| 3. | Corbett JR, Akinboboye OO, Bacharach SL, Borer JS, Botvinick EH, DePuey GE, Ficaro EP, Hansen CL, Henzlova MJ, Van Kriekinge S. ASNC imaging guidelines for nuclear cardiology procedure. Equilibrium radionuclide angiocardiography. USA: Amer Soc Nucl Card 2008; . |
| 4. | Murphy P, Port S. Radionuclide evaluation of left ventricular function. Diagnostic Nuclear Medicine, 4th ed. Philadelphia: Lippincott Williams and Winkins 2003; 239-271. |
| 5. | Gill JB, Moore RH, Tamaki N, Miller DD, Barlai-Kovach M, Yasuda T, Boucher CA, Strauss HW. Multigated blood-pool tomography: new method for the assessment of left ventricular function. J Nucl Med. 1986;27:1916-1924. [PubMed] |
| 6. | Kumita S, Cho K, Nakajo H, Toba M, Uwamori M, Mizumura S, Kumazaki T, Sano J, Sakai S, Munakata K. Assessment of left ventricular diastolic function with electrocardiography-gated myocardial perfusion SPECT: comparison with multigated equilibrium radionuclide angiography. J Nucl Cardiol. 2001;8:568-574. [PubMed] |
| 7. | Akincioglu C, Berman DS, Nishina H, Kavanagh PB, Slomka PJ, Abidov A, Hayes S, Friedman JD, Germano G. Assessment of diastolic function using 16-frame 99mTc-sestamibi gated myocardial perfusion SPECT: normal values. J Nucl Med. 2005;46:1102-1108. [PubMed] |
| 8. | Chen J, Henneman MM, Trimble MA, Bax JJ, Borges-Neto S, Iskandrian AE, Nichols KJ, Garcia EV. Assessment of left ventricular mechanical dyssynchrony by phase analysis of ECG-gated SPECT myocardial perfusion imaging. J Nucl Cardiol. 2008;15:127-136. [PubMed] |
| 9. | Henneman MM, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C, van der Wall EE, Schalij MJ, Garcia EV, Bax JJ. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med. 2007;48:1104-1111. [PubMed] |
| 10. | Lu P. Monitoring cardiac function in patients receiving doxorubicin. Semin Nucl Med. 2005;35:197-201. [PubMed] |
| 11. | Harvey RA, Champe PC, editors ; Lippincott’s illustrated reviews: Pharmacology. 4th ed. Philadelphia: Lippincott Williams and Winkins, 2008. Chapter 39. Anticancer drugs; p. 461. . |
| 12. | Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789-1792. [PubMed] |
| 13. | Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869-2879. [PubMed] |
| 14. | Schwartz RG, McKenzie WB, Alexander J, Sager P, D'Souza A, Manatunga A, Schwartz PE, Berger HJ, Setaro J, Surkin L. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med. 1987;82:1109-1118. [PubMed] |
| 15. | Steinherz LJ, Graham T, Hurwitz R, Sondheimer HM, Schwartz RG, Shaffer EM, Sandor G, Benson L, Williams R. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Childrens Cancer Study Group. Pediatrics. 1992;89:942-949. [PubMed] |
| 16. | Ganz WI, Sridhar KS, Forness TJ. Detection of early anthracycline cardiotoxicity by monitoring the peak filling rate. Am J Clin Oncol. 1993;16:109-112. [PubMed] |
| 17. | Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977-2016. [PubMed] |
| 18. | Maddahi J, Berman DS, Matsuoka DT, Waxman AD, Stankus KE, Forrester JS, Swan HJ. A new technique for assessing right ventricular ejection fraction using rapid multiple-gated equilibrium cardiac blood pool scintigraphy. Description, validation and findings in chronic coronary artery disease. Circulation. 1979;60:581-589. [PubMed] |
| 19. | Maddox DE, Wynne J, Uren R, Parker JA, Idoine J, Siegel LC, Neill JM, Cohn PF, Holman BL. Regional ejection fraction: a quantative radionuclide index of regional left ventricular performance. Circulation. 1979;59:1001-1009. [PubMed] |
| 20. | Masci PG, Dymarkowski S, Rademakers FE, Bogaert J. Determination of regional ejection fraction in patients with myocardial infarction by using merged late gadolinium enhancement and cine MR: feasibility study. Radiology. 2009;250:50-60. [PubMed] |
| 21. | Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-259. [PubMed] |
| 22. | Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905-918. [PubMed] |
| 23. | Salerno M. Multi-modality imaging of diastolic function. J Nucl Cardiol. 2010;17:316-327. [PubMed] |
| 24. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463. [PubMed] |
| 25. | Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107-133. [PubMed] |
| 26. | Alnabhan N, Kerut EK, Geraci SA, McMullan MR, Fox E. An approach to analysis of left ventricular diastolic function and loading conditions in the echocardiography laboratory. Echocardiography. 2008;25:105-116. [PubMed] |
| 27. | Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086-1119. [PubMed] |
| 28. | Brun P, Tribouilloy C, Duval AM, Iserin L, Meguira A, Pelle G, Dubois-Rande JL. Left ventricular flow propagation during early filling is related to wall relaxation: a color M-mode Doppler analysis. J Am Coll Cardiol. 1992;20:420-432. [PubMed] |
| 29. | Muntinga HJ, van den Berg F, Knol HR, Niemeyer MG, Blanksma PK, Louwes H, van der Wall EE. Normal values and reproducibility of left ventricular filling parameters by radionuclide angiography. Int J Card Imaging. 1997;13:165-171; discussion 173. [PubMed] |
| 30. | Bonow RO, Bacharach SL, Green MV, Kent KM, Rosing DR, Lipson LC, Leon MB, Epstein SE. Impaired left ventricular diastolic filling in patients with coronary artery disease: assessment with radionuclide angiography. Circulation. 1981;64:315-323. [PubMed] |
| 31. | Spirito P, Maron BJ, Bonow RO. Noninvasive assessment of left ventricular diastolic function: comparative analysis of Doppler echocardiographic and radionuclide angiographic techniques. J Am Coll Cardiol. 1986;7:518-526. [PubMed] |
| 32. | Bleeker GB, Schalij MJ, Molhoek SG, Verwey HF, Holman ER, Boersma E, Steendijk P, Van Der Wall EE, Bax JJ. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544-549. [PubMed] |
| 33. | Conca C, Faletra FF, Miyazaki C, Oh J, Mantovani A, Klersy C, Sorgente A, Pedrazzini GB, Pasotti E, Moccetti T. Echocardiographic parameters of mechanical synchrony in healthy individuals. Am J Cardiol. 2009;103:136-142. [PubMed] |
| 34. | Marcus GM, Rose E, Viloria EM, Schafer J, De Marco T, Saxon LA, Foster E. Septal to posterior wall motion delay fails to predict reverse remodeling or clinical improvement in patients undergoing cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2208-2214. [PubMed] |
| 35. | O'Connell JW, Schreck C, Moles M, Badwar N, DeMarco T, Olgin J, Lee B, Tseng Z, Kumar U, Botvinick EH. A unique method by which to quantitate synchrony with equilibrium radionuclide angiography. J Nucl Cardiol. 2005;12:441-450. [PubMed] |
| 36. | Botvinick EH. Scintigraphic blood pool and phase image analysis: the optimal tool for the evaluation of resynchronization therapy. J Nucl Cardiol. 2003;10:424-428. [PubMed] |
| 37. | Bartlett ML, Srinivasan G, Barker WC, Kitsiou AN, Dilsizian V, Bacharach SL. Left ventricular ejection fraction: comparison of results from planar and SPECT gated blood-pool studies. J Nucl Med. 1996;37:1795-1799. [PubMed] |
| 38. | Botvinick EH, O'Connell JW, Badhwar N. Imaging synchrony. J Nucl Cardiol. 2009;16:846-848. [PubMed] |