Published online Aug 28, 2011. doi: 10.4329/wjr.v3.i8.199
Revised: July 16, 2011
Accepted: July 23, 2011
Published online: August 28, 2011
This review aims to familiarize the radiologist with the common types of sinus surgery including their indications and techniques. We also illustrate how surgeons interpret 3D sinus anatomy when evaluating computed tomography (CT) studies. Preoperative evaluation by CT is mandatory for all patients undergoing functional endoscopic sinus surgery (FESS). In the past decade in particular, CT of the paranasal sinuses has become a roadmap for FESS. The radiologist’s goal is to report on five key points: the extent of sinus opacification, opacification of sinus drainage pathways, anatomical variants, critical variants, and condition of surrounding soft tissues of the neck, brain and orbits. We present a systematic approach to the use of coronal, axial, and sagittal images in CT evaluation before FESS.
- Citation: Cashman EC, MacMahon PJ, Smyth D. Computed tomography scans of paranasal sinuses before functional endoscopic sinus surgery. World J Radiol 2011; 3(8): 199-204
- URL: https://www.wjgnet.com/1949-8470/full/v3/i8/199.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i8.199
Chronic rhinosinusitis is one of the most common chronic diseases in the United States and is now the most common indication for sinus surgery. Chronic rhinosinusitis is estimated to occur in 14% of the population, however the degree of accuracy of many reported cases of chronic rhinosinusitis is difficult to ascertain[1]. To support a clinical diagnosis of chronic rhinosinusitis, in accordance with the American Academy of Otolaryngology and Head and Neck Surgery (AAO-HNS) criteria, at least two major or one major and two minor symptoms are required (Table 1). Although the diagnosis of chronic rhinosinusitis is largely a clinical one, the final diagnosis should be confirmed by objective measures and, in 2003, the AAO-HNS amended the existing 1996 diagnostic criteria for chronic rhinosinusitis, citing confirmatory radiographic, nasal endoscopic or findings on physical examination necessary to confirm a diagnosis of chronic rhinosinusitis.
| Major criteria | Minor criteria |
| Purulence in nasal cavity | Headache |
| Facial pain, pressure, congestion, fullness | Fever (all nonacute) |
| Nasal obstruction, blockage, discharge, purulence | Halitosis |
| Fever (acute rhinosinusitis only) | Fatigue |
| Hyposmia/anosmia | Dental pain |
| Cough | |
| Ear pain and fullness |
Congenital anomalies and normal anatomical variants in this region, while rare, are important as they have pathological consequences and may lead to difficulties intraoperatively. Therefore, appropriate radiological imaging with accurate interpretation of normal and aberrant anatomy plays a vital role in the diagnosis and safe surgical management of these patients.
Open approaches to the maxillary sinus were first described as early as the 18th century. The Caldwell-Luc procedure was described in 1893 by George Caldwell and further elucidated in France by Henri Luc in 1897. Some of the more recent advances in the movement towards functional endoscopic sinus surgery (FESS), can be attributed to Messerklinger and Stammberger in the early 1980s and 1990s, respectively[2], with the role of computed tomography (CT) in sinus surgery expanding greatly with the advent of image-guided surgery (IGS) in recent years.
FESS confers the advantage of being minimally invasive and allows for sinus air cells and sinus ostia to be opened under direct visualization. The primary goal of FESS is to return the mucociliary drainage of the sinuses to normal function. FESS is most successful in patients who have recurrent acute or chronic infective sinusitis, with success rates as high as 98% cited[3]. Surgical failure when it occurs is usually as a result of postoperative adhesions or when the surgeon fails to address outflow tract of the frontal sinus[3]. Sinus surgery conducted in certain settings may be facilitated by IGS. Such situations could include revision surgery in the absence of recognizable anatomical landmarks, cases where disease extends into the frontal or sphenoid sinus, or disease abutting the skull base. IGS essentially permits the real-time correlation of the operative field to a preoperative imaging data set that reflects the precise location of a surgical instrument in relation to surrounding anatomical landmarks.
The classical indication for FESS is chronic rhinosinusitis but also includes nasal polyposis, antrochoanal polyps (arising from the maxillary antrum), sinus mucoceles, cerebrospinal leak closure, orbital decompression, choanal atresia repair, optic nerve decompression, control of epistaxis and dacryocystorhinostomy (Table 2). Although the most common indication for FESS is failure to respond to conservative management, intraoperative correlation with real-time CT imaging has allowed the use of FESS for a wider range of procedures, including access to skull base malignancies and trans-sphenoidal approaches to the pituitary gland. IGS is also deployed in cases where a dehiscent lamina papyracea has been noted on a preoperative scan or in cases where there is orbital pathology present.
| Chronic sinusitis refractory to medical treatment |
| Recurrent acute sinusitis |
| Nasal polyposis |
| Antrochoanal polyps |
| Sinus mucoceles |
| Excision of selected tumors |
| Cerebrospinal fluid leak closure |
| Orbital decompression (e.g. Graves ophthalmopathy) |
| Optic nerve decompression |
| Dacryocystorhinostomy |
| Choanal atresia repair |
The success of FESS is based on the premise that the symptoms in chronic rhinosinusitis arise largely as a result of blockage of the ostiomeatal complex (OMC), which Mackay and Lund have described as an outflow tract for the maxillary, anterior ethmoid and frontal sinus[4].
Radiologically, it is obstruction of the OMC by inflammation, polyposis, or more rarely, by tumor that is of key interest to the otolaryngologist. This obstruction leads to a defect in mucociliary clearance, resulting in stagnation of secretions and subsequent development of a culture medium facilitating the development of infection, and is one of the most common findings in patients with chronic rhinosinusitis (Figure 1). A recent study by Nouraei et al[5] has noted an obstructed OMC in 53% of patients with chronic rhinosinusitis. CT scanning is extremely useful in confirming a clinical suspicion of chronic rhinosinusitis and classically, features such as significant mucosal thickening, air-fluid levels, OMC obstruction, or polyposis are suggestive of sinogenic disease. However, many of the radiological manifestations of sinus disease are more subtle.
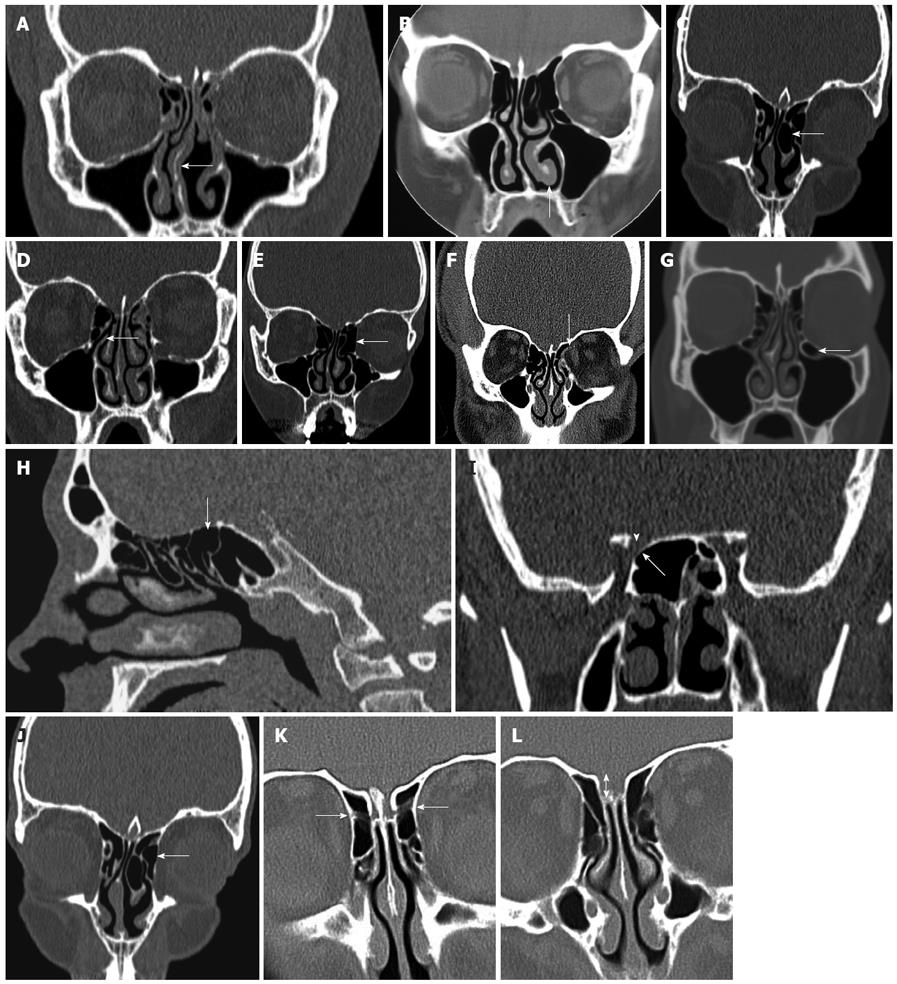
Intraoperatively, on entering nasal cavity, the first structures encountered are the nasal septum and inferior turbinate. The septum consists of quadrangular cartilage extending to the perpendicular plate of the ethmoid bone postero-superiorly and the vomer postero-inferiorly. It is important to recognize septal deviations because these may lead to significant nasal obstruction and limit endoscopic visualization. As appropriate, patients with septal deviations noted preoperatively on CT, may be counseled regarding the need for septoplasty in conjunction with FESS (Figure 2A).
Extending along the inferior nasal wall posteriorly towards the nasopharynx is the inferior turbinate. In patients with an allergic component to their disease, the inferior turbinate may be edematous and in some cases enlarged, to the extent that the patient is likely to benefit from turbinate reduction (Figure 2B). Again, if significant inferior turbinate enlargement is noted on preoperative imaging, patients can be advised of the benefits of turbinate reduction in conjunction with their sinus surgery.
As the endoscope is advanced through the nasal cavity, the next structure encountered is the middle turbinate, which attaches superiorly to the cribriform plate. It is composed of a vertical and horizontal component; the latter is also referred to as the basal lamella, which partitions the anterior and posterior ethmoid air cells. The middle turbinate is a key landmark in FESS. It has a vertical (lying in sagittal plane running from posterior to anterior) and horizontal component (lying in coronal plane, running medial to lateral). The vertical part, referred to as the basal lamella, divides the anterior and posterior ethmoid cells (Figure 3A). Care must be taken when manipulating the middle turbinate because it attaches to the skull base at the cribriform plate and, in addition, the surgeon must be mindful of the importance of preserving the middle turbinate because it serves as an important landmark during revision surgery. A relatively common anatomical variant of sinonasal anatomy is a concha bullosa or pneumatized middle turbinate (Figure 2C). Their cited incidence varies in the literature from 15% to 45%, although they are not thought to have any significant role in the pathogenesis of chronic rhinosinusitis[6,7].
Another rare aberration is a paradoxical middle turbinate. Convexity of the middle turbinate is usually deviated medially towards the septum. When it is paradoxically curved (convexity of bone directed laterally), the inferior end of the turbinate may obstruct and narrow the nasal cavity and middle meatus. Such structures however, have very little relevance surgically.
The next key landmark is the uncinate, an L-shaped bone of the lateral nasal wall, which forms the anterior border of the hiatus semilunaris, or infundibulum, which marks the location of the OMC (Figure 2D). The natural ostium of the maxillary sinus is typically located just posterior to the uncinate process, one third of the distance along the middle turbinate, from its anterior edge. Surgically, the uncinate must be removed to gain access to the ethmoid infundibulum and maxillary sinus ostium. The free edge of the uncinate may be deviated medially, laterally, pneumatized or bent and when such deviations are lateral, they can result in narrowing of the hiatus semilunaris and infundibulum, jeopardizing their patency. A so called “atelectatic” uncinate occurs where the free edge of the uncinate approximates the orbital floor or inferior aspect of the lamina papyracea, and an uncinectomy in this setting may result in damage to orbital contents. Once the uncinate has been resected, the ostium of the maxillary sinus can be visualized and enlarged by a maxillary antrostomy. These steps, uncinectomy and maxillary antrostomies, form the basis of the FESS procedure and are essential for optimal surgical outcome.
The next structure encountered is the ethmoid bulla (Figure 2E). The relationship of the ethmoid bulla with the lamina papyracea laterally, and the floor of the anterior cranial fossa superiorly, should be clarified on preoperative CT. The ethmoid bulla is a reliable surgical landmark, because it is the largest and most constant of the anterior ethmoid cells. It is located just beyond the natural ostium of the maxillary sinus and forms the posterior border of the hiatus semilunaris. Superiorly, the bulla may extend to the ethmoid roof or alternatively, a suprabullar recess may exist above the roof of the bulla.
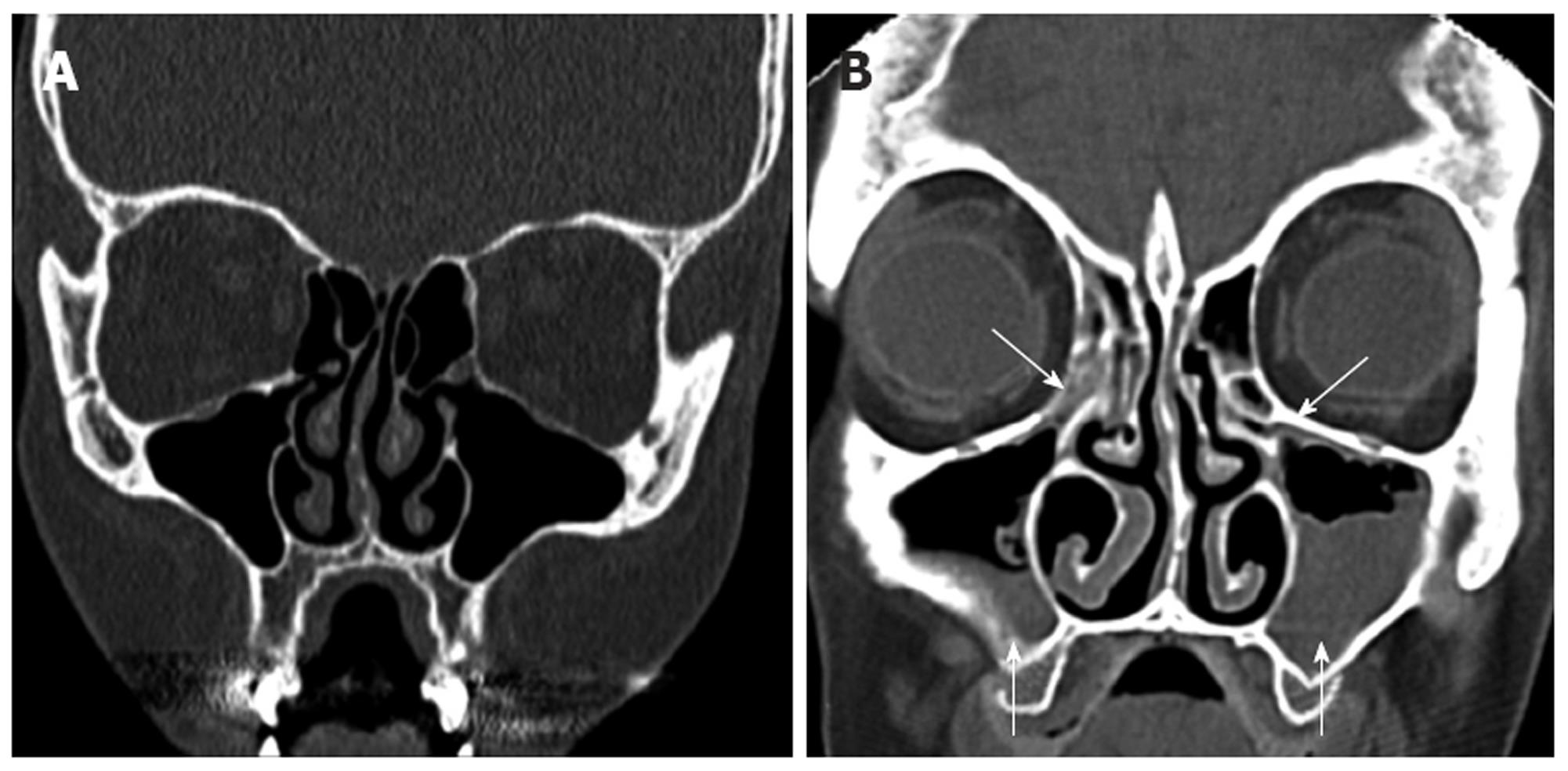
The ethmoid sinus is composed of anterior and posterior groups of air cells, consisting of a variable number of cells (typically 7-15). The close relationship of the orbit and the anterior skull base render these structures vulnerable intraoperatively. An important finding to note on review of imaging of the anterior ethmoid air cells is the presence of a dehiscent lamina papyracea (Figure 2F). In this anatomical variant, the orbital contents are not protected by bone along the lateral orbital margin, and are at increased risk of injury intraoperatively.
Haller cells are ethmoid cells that extend into the floor of the orbit and are more appropriately referred to as infraorbital cells, which vary in size, but when large, can narrow the ostia of the maxillary sinus or ethmoid infundibulum (Figure 2G). Controversy surrounds the exact origin of Haller cells, as to whether they arise from the anterior or posterior ethmoid cells. Their prevalence is thought to be in the region of 10%, although results in the literature vary widely. Haller cells are thought to be implicated as a possible etiological factor in recurrent maxillary sinusitis due to their negative influence on maxillary sinus ventilation by narrowing the infundibulum and maxillary ostium.
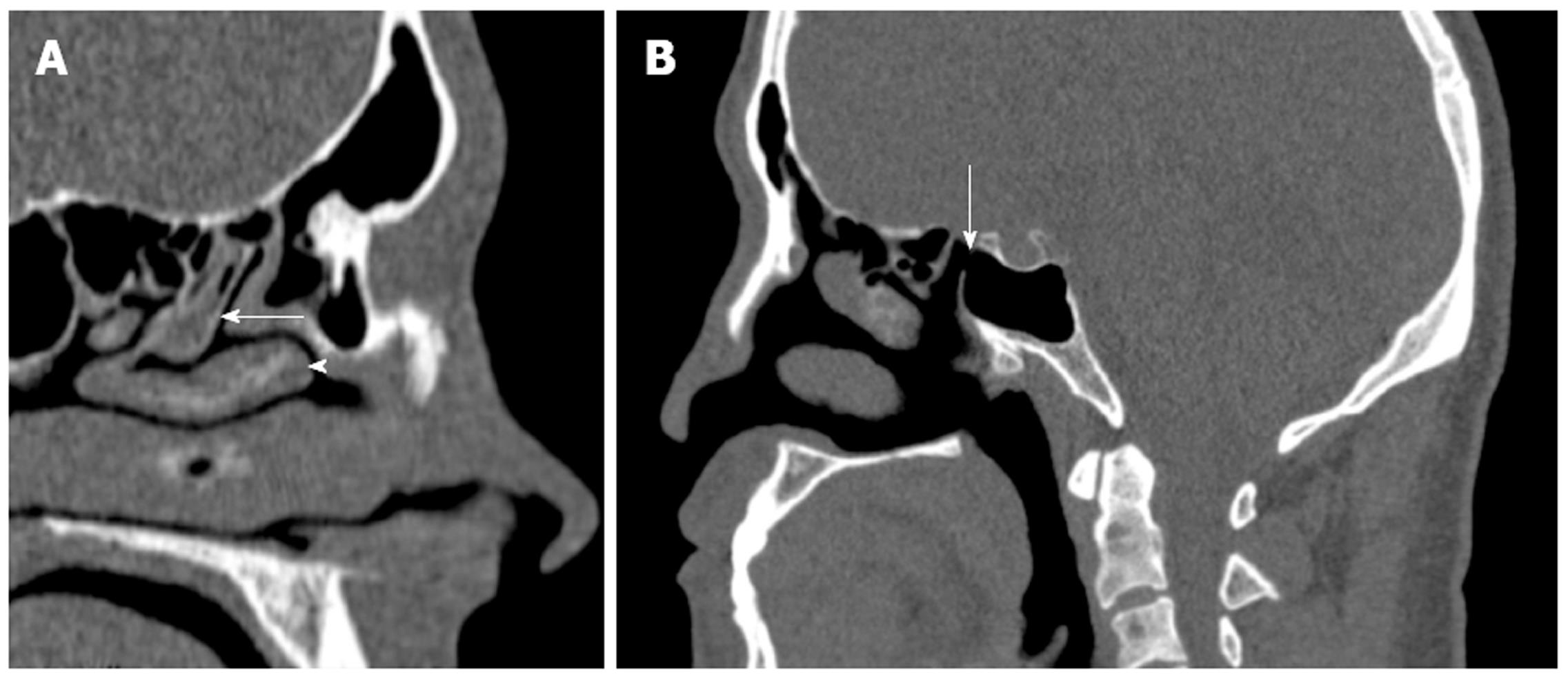
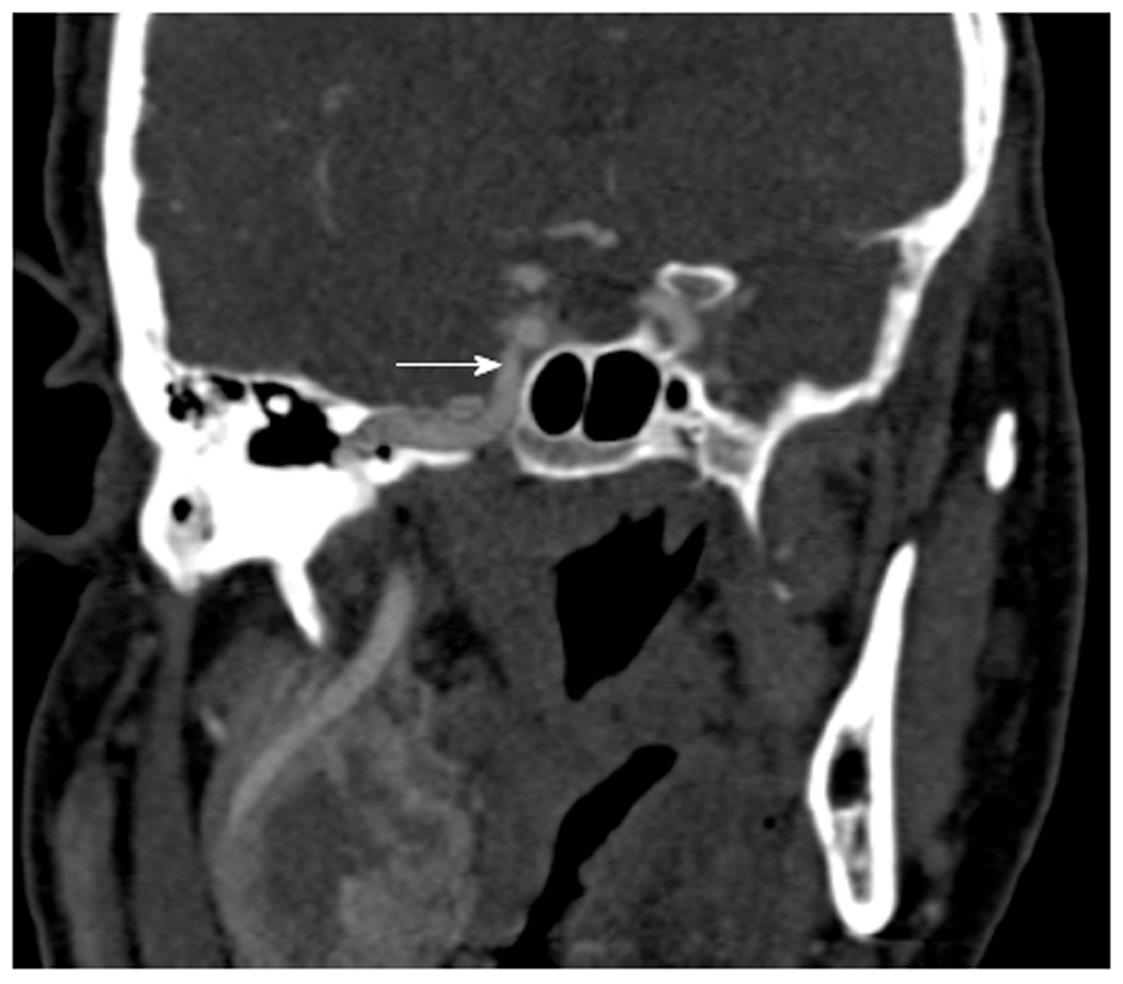
The posterior ethmoidal and sphenoid sinuses are accessed through the anterior ethmoidal air cells. The anatomy of the posterior ethmoid is critically important because of its variable relationship with the sphenoid sinus. Although routine FESS does not ordinarily encompass a posterior ethmoidectomy or entry to the sphenoid sinus, such steps may be indicated if significant pathology is evident on preoperative imaging. Exenteration of the posterior ethmoid results in access to the sphenoid sinus; the most posterior of the sinuses (Figure 2H). The posterior ethmoid cells may extend laterally or superiorly beyond the anterior wall of the sphenoid sinus, and therefore, the surgeon cannot assume that the sphenoid sinus is directly behind the posterior ethmoid cells. Furthermore, the sphenoid sinus is related to several important, potentially hazardous structures, including the internal carotid artery; typically the most posterior-lateral structure within the sphenoid sinus (Figure 4). Another key structure, the optic nerve, produces an anteroposterior indentation in the roof of the sphenoid, and in about 4% of patients, the overlying bone is dehiscent (Figure 2I), and thus the importance of a systematic approach to the use of coronal, axial and sagittal images for CT evaluation[7].
The presence of an Onodi cell, a posterior ethmoid cell extending to the sphenoid sinus, lying medially to the optic nerve, places the nerve at an increased risk of damage during sinus surgery. These cells can, in some cases, even surround the optic nerve and their reported incidence is about 5%[8]. The best orientation for identifying the Onodi cells are axial images, where the course of the optic nerve can be followed past the orbital apex and judged in relation to the posterior ethmoid and sphenoid sinuses. Identification of an Onodi cell preoperatively is paramount because its presence may contribute to an increased risk of injury, of not only the optic nerve, but additionally, the internal carotid artery. Another common anatomical variant are agger nasi cells (Figure 2J), which are located just anterior to the attachment of the middle turbinate and the frontal recess. These represent the most anterior ethmoid cells and are an important surgical landmark, and are identified in as many as 88.5% of preoperative images[9]. On coronal CT they appear inferior to the frontal recess and lateral to the middle turbinate, and on the sagittal plane they are located anterior and inferior to the frontal recess. Surgically, opening these cells usually provides an excellent view of the frontal recess. When there is extensive pneumatization of the agger nasi cells, it may displace the attachment of the middle turbinate medially and superiorly and result in anatomical narrowing of the frontal recess. The exact location of the agger nasi cells is of clinical importance in the pathogenesis of chronic rhinosinusitis, and the close relationship of these cells to the lacrimal bone may explain the presence of epiphora in select patients with sinus disease.
If they are not noted preoperatively, some anatomical variations may contribute to surgical complications along the floor of the anterior cranial fossa. The ethmoid roof is formed by the fovea ethmoidalis of the frontal bone laterally and the cribiform plate of the ethmoid bone medially, and asymmetry in its height may expose the lower side to inadvertent penetration during surgery. The anterior ethmoidal artery crosses the ethmoid sinus and enters the anterior cranial fossa before exiting and re-entering the nasal cavity via the cribiform plate, and this is the site where the vessel is most vulnerable to injury (Figure 2K).
Another important anatomical variation occurring along the ethmoid roof is described by the Keros classification. This measures the vertical height between the cribriform plate and fovea ethmoidalis and the depth is categorized as 1-3 mm (Keros I), 3-7 mm (Keros II) and 7-16 mm (Keros III) (Figure 2L). Clearly, as this bone is thin, an increased vertical height will result in an increased risk of intraoperative damage.
The nasopharynx or postnasal space is evaluated on preoperative CT, although this region is not routinely involved in the FESS procedure. Postnasal space imaging is most commonly indicated for the presence of nasopharyngeal lesions. Extension of nasopharyngeal tumors, especially at the skull base and deep facial planes, is well illustrated on imaging. Magnetic resonance imaging best depicts perineural spread, whereas CT is useful to detect very early skull base erosion[10].
Finally, the sphenoid sinus may be involved in sinogenic disease. Drainage of this sinus is through the sphenoethmoidal recess, although the position of the natural ostium of the sphenoid is highly variable[11] (Figure 3B). The intersphenoid septum, partitioning the sinus, is often deflected to one side attaching to the bony wall covering the carotid artery, which can be avulsed during surgery. The artery may bulge into the sinus in 65%-72% of patients[12], and the thin bone separating the artery and sphenoid sinus may be absent in 4%-8% of cases[13]. Recent studies have re-emphasized the need for multiplanar reconstruction as a routine part of presurgical work-up of this complex anatomical region[14].
Since the introduction of FESS in the United States in 1985, CT has been imperative in the understanding of regional anatomical variation and has been integral in the guidance of surgical procedures. Improvement in both FESS techniques and CT technology has concurrently expanded the indications for sinus surgery. Preoperative CT also can provide data for intraoperative stereotactic guidance systems, which are used to manage complex disease, and for revision surgery. Major complications in FESS, although rare, are potentially catastrophic. A detailed knowledge of normal and aberrant sinonasal anatomy is essential to the success and safety of sinus surgery. CT has very much emerged as the gold standard in preoperative diagnosis and allows for accurate patient selection for FESS, and radiologists should be familiar with the FESS technique and have a systematic approach to reviewing CT scans for normal and aberrant sinus anatomy.
Peer reviewers: Bunyamin Sahin, PhD, Professor, Vice President of Turkish Society for Stereology, Department of Anatomy, Medical School, Ondokuz May’s University, Atakum, Samsun 55139, Turkey; AAK Abdel Razek, MD, Professor, Diagnostic Radiology Department, 62 El Nokri St, Meet Hadr, Mansoura Faculty of Medicine, Mansoura 25512, Egypt; Paul V Puthussery, Assistant Professor, Department of Radiodiagnosis, Government Medical College, Thrissur, Puthussery House, Prasannapuram, Chowara PO, KOCHI 683571, India
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
| 1. | Kaliner MA, Osguthorpe JD, Fireman P, Anon J, Georgitis J, Davis ML, Naclerio R, Kennedy D. Sinusitis: bench to bedside. Current findings, future directions. Otolaryngol Head Neck Surg. 1997;116:S1-20. [PubMed] |
| 2. | Stammberger H, Posawetz W. Functional endoscopic sinus surgery. Concept, indications and results of the Messerklinger technique. Eur Arch Otorhinolaryngol. 1990;247:63-76. [PubMed] |
| 3. | Huang BY, Lloyd KM, DelGaudio JM, Jablonowski E, Hudgins PA. Failed endoscopic sinus surgery: spectrum of CT findings in the frontal recess. Radiographics. 2009;29:177-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Hellmich S. Surgical treatment of sinusitis. Acta Otorhinolaryngol Belg. 1983;37:624-634. [PubMed] |
| 5. | Nouraei SA, Elisay AR, Dimarco A, Abdi R, Majidi H, Madani SA, Andrews PJ. Variations in paranasal sinus anatomy: implications for the pathophysiology of chronic rhinosinusitis and safety of endoscopic sinus surgery. J Otolaryngol Head Neck Surg. 2009;38:32-37. [PubMed] |
| 6. | Lloyd GA, Lund VJ, Scadding GK. CT of the paranasal sinuses and functional endoscopic surgery: a critical analysis of 100 symptomatic patients. J Laryngol Otol. 1991;105:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 77] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Hoang JK, Eastwood JD, Tebbit CL, Glastonbury CM. Multiplanar sinus CT: a systematic approach to imaging before functional endoscopic sinus surgery. AJR Am J Roentgenol. 2010;194:W527-W536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Unal B, Bademci G, Bilgili YK, Batay F, Avci E. Risky anatomic variations of sphenoid sinus for surgery. Surg Radiol Anat. 2006;28:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Jones NS. CT of the paranasal sinuses: a review of the correlation with clinical, surgical and histopathological findings. Clin Otolaryngol Allied Sci. 2002;27:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Dubrulle F, Souillard R, Hermans R. Extension patterns of nasopharyngeal carcinoma. Eur Radiol. 2007;17:2622-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Aygun N, Zinreich SJ. Imaging for functional endoscopic sinus surgery. Otolaryngol Clin North Am. 2006;39:403-16, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Sethi DS, Stanley RE, Pillay PK. Endoscopic anatomy of the sphenoid sinus and sella turcica. J Laryngol Otol. 1995;109:951-955. [PubMed] |
| 13. | Sethi DS, Pillay PK. Endoscopic management of lesions of the sella turcica. J Laryngol Otol. 1995;109:956-962. [PubMed] |
| 14. | Beale TJ, Madani G, Morley SJ. Imaging of the paranasal sinuses and nasal cavity: normal anatomy and clinically relevant anatomical variants. Semin Ultrasound CT MR. 2009;30:2-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |