INTRODUCTION
Budd-Chiari syndrome (BCS) consists of disorders causing hepatic venous outflow obstruction either at the level of the hepatic veins (HV) or at the inferior vena cava (IVC). This leads to increased hepatic sinusoidal pressure and portal hypertension[1]. Two forms of the disease are recognized: an acute form and chronic disease. Acute disease, which is commonly associated with the use of oral contraceptive pills, pregnancy or underlying thrombotic disorder, is more prevalent in the west[2-7]. As indicated by the various studies with regard to BCS in India, membranous/fibrous obstruction of the IVC, HV or both is more common as compared to isolated hepatic vein involvement[8-11]. The majority of cases of BCS with IVC obstruction have membranous obstruction of the IVC[12]. Membranous obstruction of the IVC and/or HV which was once considered to be congenital is nowadays considered to be organized thrombus[13]. This is a possible explanation for the majority of Indian patients with BCS having a chronic course.
Clinical presentation ranges from minimal or no symptoms to ascites with portal hypertension, splenomegaly, variceal bleeding or varicose veins. Imaging plays an important role in management of the disease, right from the diagnosis to follow up. Previously, surgical porto-systemic shunting was considered to be the standard treatment for patients having BCS with stable liver function; whereas liver transplantation was reserved for those having fulminant liver failure or end stage chronic liver disease[14-19]. Of late, various studies and clinical trials have shown good midterm outcome following radiological interventions consisting of recanalization of stenosed segment of hepatic vein and/or IVC or creation of transjugular intrahepatic portosystemic shunt (TIPS)[20-24].
Restoring normal hepatic venous outflow by opening/dilating one of the stenosed HVs and/or IVC is considered the most physiological way of treating BCS. However, percutaneous dilatation may be attempted in selected patients. This may not be possible in patients having all the HVs replaced by small collaterals. However, this subset of patients may need TIPS for alleviation of symptoms and liver transplantation remains the ultimate treatment. In this article we will discuss the various imaging findings and radiological procedures carried out for the management of BCS.
ETIO-PATHOLOGY
BCS mostly occurs in young adults and the majority of patients have an underlying hematologic abnormality. Amongst various thrombotic disorders, myeloproliferative disease is the most common abnormality identified[23]. Other common conditions associated with BCS are JAK2 V617F mutation, Factor V Leiden mutation, oral contraceptive pills, pregnancy, antiphospholipid antibodies, paroxysmal nocturnal hemoglobinuria, and protein C and S deficiency[23]. Multiple causes may also be present in the same patient. BCS may present as acute and fulminant forms or as chronic disease. Membranous occlusion of IVC and HV, presenting as the chronic form of the disease, is more common in Indians (Figure 1)[8-12]. However, there are a few studies documenting prevalence of hypercoagulable states in Indian patients; thus there is a change of spectrum of BCS in India[25,26]. BCS may also be seen in patients with metastatic involvement of the IVC and HV secondary to kidney, liver, or suprarenal gland tumors (Figure 1).
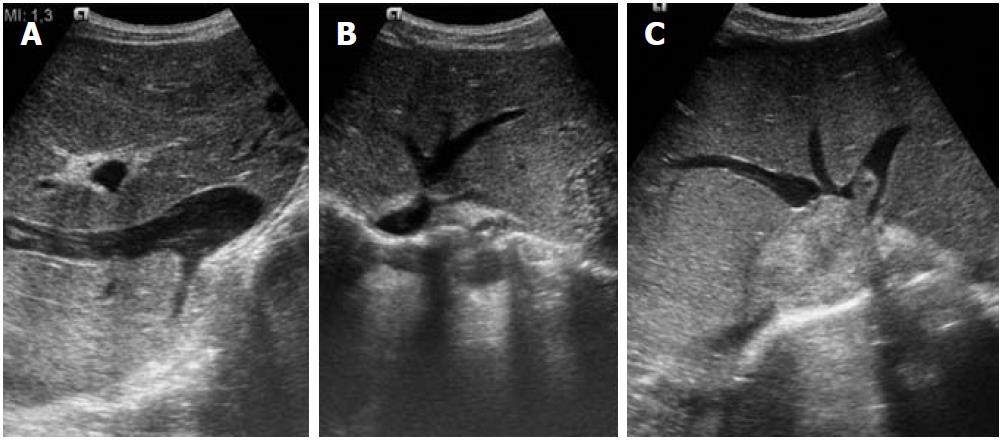
Figure 1 Ultrasound images.
A: Inferior vena cava (IVC) occlusion; B: Hepatic venous web causing outflow obstruction; C: Secondary Budd-Chiari syndrome due to IVC thrombus extending into the hepatic veins.
The parenchymal changes in BCS are secondary to obstruction of the hepatic vein, which leads to increase in sinusoidal pressure, hepatic congestion and portal hypertension. Due to this increase in sinusoidal pressure there is reduction in hepatic perfusion leading to ischemia and necrosis of hepatocytes especially in perivenular zones; hence, leading to hepatic fibrosis, nodular regenerative hyperplasia (NRH) and cirrhosis.
IMAGING
Imaging modalities commonly used for evaluation of BCS consist of ultrasound (US) and color Doppler examination; these may be further complemented by computed tomography (CT) and magnetic resonance imaging (MRI). BCS may have varied imaging findings depending upon the form of disease (acute or chronic). In the acute phase of the disease, the liver is enlarged and swollen with presence of ascites. Sometimes the liver may have a heterogeneous appearance secondary to hemorrhage and infarction. The HV are thrombosed with varying echogenicity within the lumen and no appreciable flow in Doppler. However, in chronic BCS the imaging findings are myriad; they may range from chronic membranous occlusion of IVC and/or HV to complete replacement of native HV by extensive intrahepatic collaterals (Figure 2). These intrahepatic collaterals are small and tortuous having a comma-shaped appearance which is an essential finding on imaging. The caudate lobe is mostly spared as it has a separate vein draining into the IVC leading to enlargement of the caudate lobe. In appropriate settings, visualization of a caudate vein having a caliber of more than 3 mm may be diagnostic[27]. The Doppler waveform of these HV with membranous occlusion or intrahepatic collaterals is invariably monophasic as opposed to the normal patent HV which show a triphasic spectral pattern due to transmitted atrial pulsations (Figure 3). Other imaging findings correspond to hepatic fibrosis, NRH, cirrhosis and portal hypertension, appearing as coarse hepatic echotexture with prominent splenoportal axis and splenomegaly.
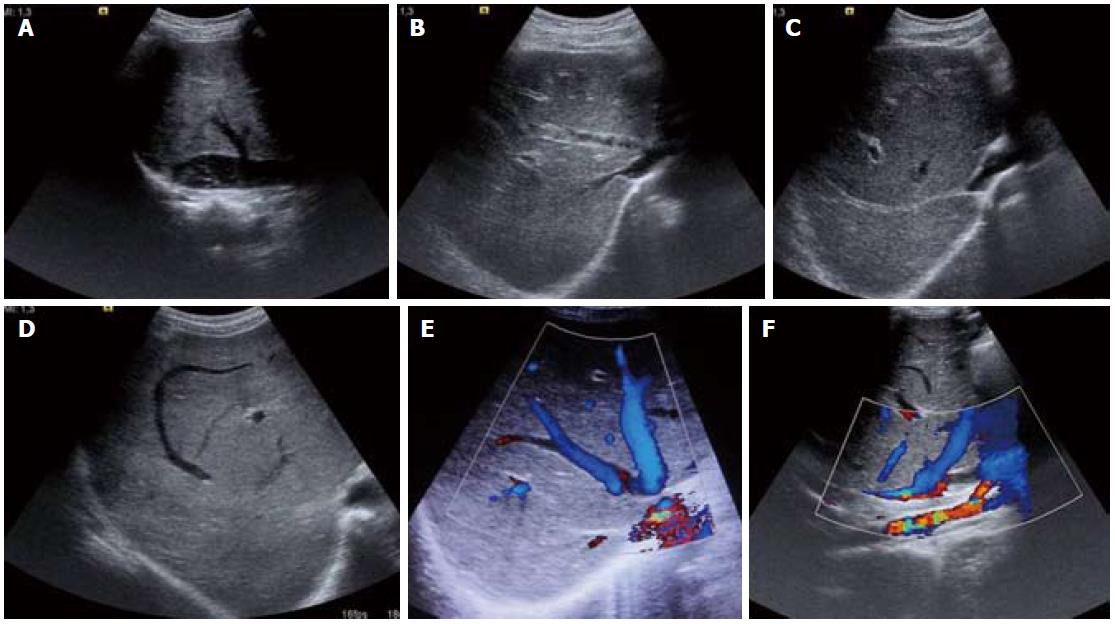
Figure 2 Ultrasound images (A-D) and color Doppler images (E, F).
A: Inferior vena cava web with intra-luminal floating thrombus; B: Partial thrombus within the middle hepatic vein and osteal narrowing of the right hepatic vein; C: Fibrosed right hepatic vein; D: Comma-shaped intrahepatic collaterals; E, F: Web at hepatic vein ostium and intrahepatic collaterals.
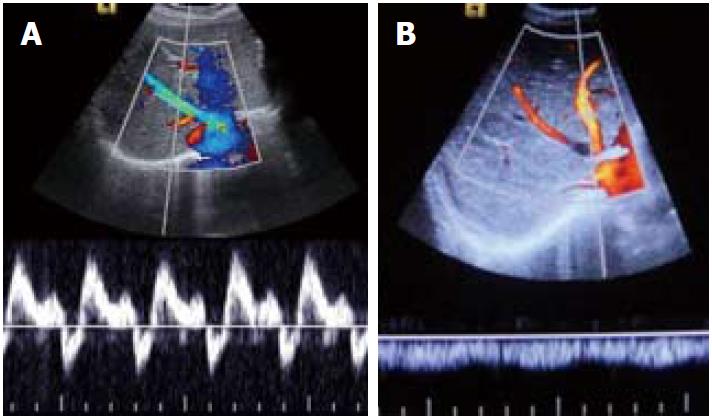
Figure 3 Spectral Doppler images.
A: Normal triphasic waveform of patent hepatic vein; B: Monophasic waveform in hepatic venous occlusion.
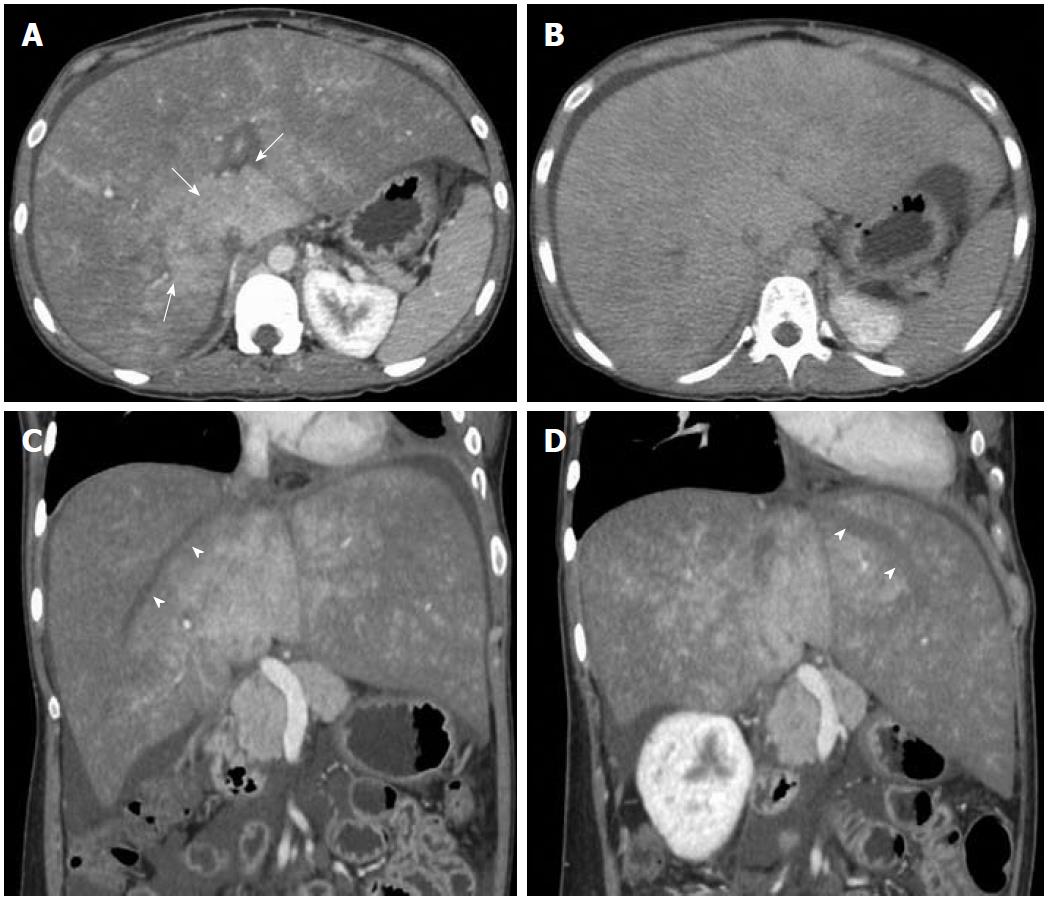
Imaging findings on CT consist of hepatomegaly with low attenuation in acute disease, whereas in the chronic form of disease there is patchy and heterogeneous enhancement with reduced enhancement in the periphery of the liver and strong enhancement in the center. Arterial phase images in chronic forms of the disease either show strongly enhancing nodular lesions or may show patchy enhancing areas due to arterio-portal shunting and opacification of portal vein (Figure 4). The portal phase images show a patchy and mottled type of heterogeneous enhancement pattern having strong enhancement in the central part of the liver adjacent to the IVC and relatively poor or no enhancement in the periphery (Figure 5). Peripherally wedge-shaped hypoattenuating areas may also be seen. In the delayed phase, the enhancement pattern becomes more homogeneous; however, the HV remain unopacified in all the phases (Figure 5). This is mainly related to portal and sinusoidal stasis secondary to hepatic venous outflow obstruction. Also, the HV are occluded with or without multiple tortuous intrahepatic collaterals. In the chronic form of the disease, associated morphologic changes in the liver are also seen. Sometimes nodular regeneration may be seen, referred to as NRH, characterized by nodules which are multiple in number with size ranging from 0.5 to 4.0 cm (Figure 4). These changes are considered to occur due to arterialization of focal areas having preserved hepatic venous outflow with loss of portal perfusion[28,29]. Hence, these lesions markedly enhance on arterial phase images and are generally hyperattenuating on portal phase images. Hepatocellular carcinoma (HCC) is a strong differential diagnosis for these lesions. Features which may help NRH to be differentiated from HCC are: NRH is either hyper- or isoattenuating on unenhanced scans as compared to HCC which is mostly hypoattenuating; moreover, HCC shows contrast wash-out in portal phase images.
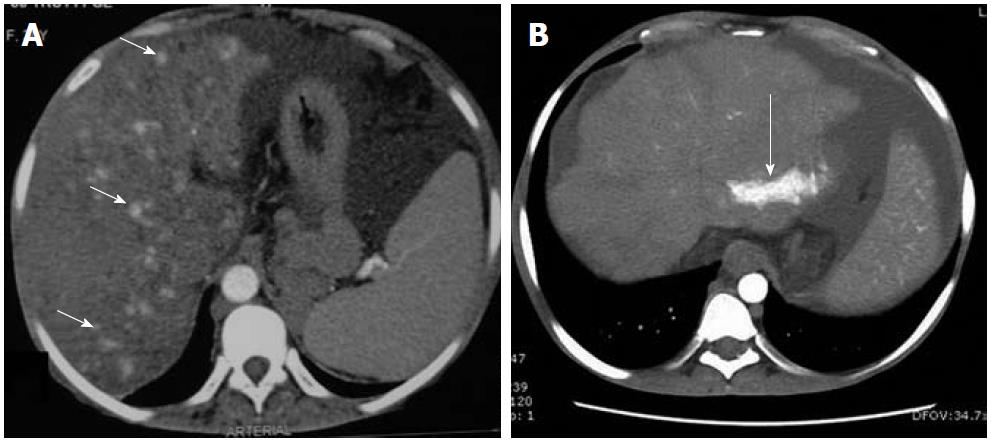
Figure 4 Contrast-enhanced arterial phase computed tomography images.
A: Nodular arterial enhancing lesions (short arrows) in a background of Budd-Chiari syndrome representing nodular regenerative hyperplasia; B: Another case of chronic Budd-Chiari syndrome. Focal enhancing area (long arrow) due to arterio-portal shunting.
Figure 5 Axial computed tomography images.
Portal venous phase image (A) shows heterogeneous and mottled enhancement pattern with central prominence (arrows) which becomes more homogeneous in delayed phase (B). Oblique reformatted images (C, D) along the axis of the right and left hepatic veins show unopacified hepatic veins (arrowheads).
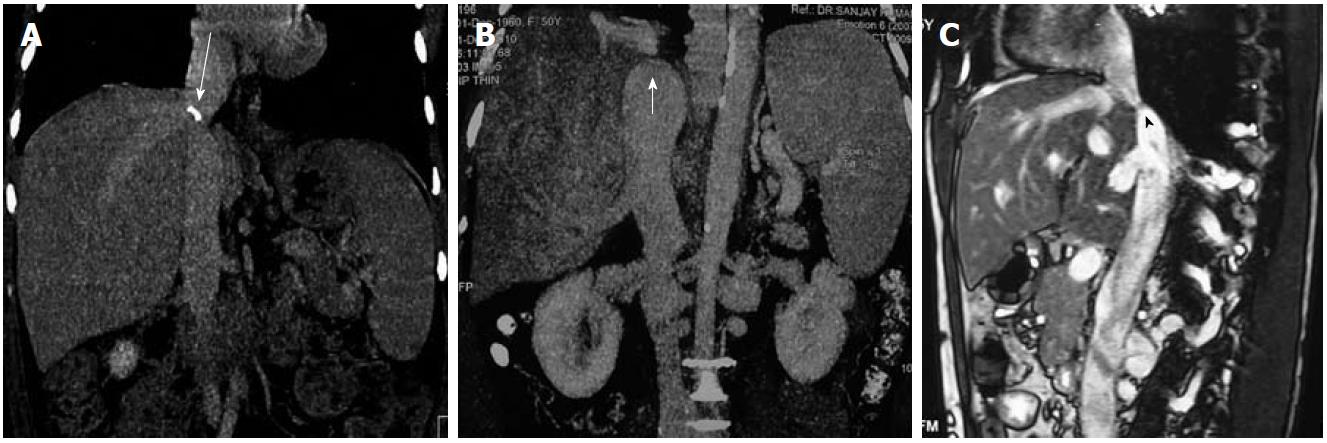
Similarly, in the acute phase, MRI shows enlarged liver having low signal intensity on T1 weighted images and heterogeneously increased signal on T2 weighted images. These findings are more prominent in the periphery. In the chronic form of the disease, the liver is shrunken and fibrosed with variable signal intensity. T2 weighted gradient echo sequence or multiphasic contrast-enhanced images are required to look for vessels and collaterals. Similar to CT, apparent on contrast-enhanced MRI, there is reduced enhancement in the acute phase and variable enhancement in chronic disease. NRH are hyperintense on T1 weighted images and iso- to hypointense relative to liver on T2 weighted images. They enhance intensely on administration of contrast. With the availability of liver-specific contrast agents such as gadobenate dimeglumine (Gd-BOPTA) and gadoxetic acid (Gd-EOB-DTPA), the differentiation between benign lesions and HCC may be determined on hepato-biliary phase images (delayed phase). On delayed phase, benign lesions show homogeneous contrast uptake, whereas HCC appears hypointense relative to the normal liver parenchyma. Moreover, there are a few reports which suggest that liver-specific agents such as gadoxetic acid may help in better visualization of hepatic congestion[30]. Therefore, MRI may sometimes help in differentiating HCC from NRH as HCC are hypointense relative to liver on T1 weighted images and hyperintense on T2 weighted images, also contrast-enhanced scans using liver-specific agents may further help. Also, CT and MRI may be helpful in differentiating IVC compression due to caudate hypertrophy and IVC occlusion in cases where US is equivocal (Figure 6).
Figure 6 Inferior vena cava.
A, B: Computed tomography images (coronal reformatted) show calcified (long arrow) and non-calcified inferior vena cava (IVC) web (short arrow); C: Sagittal T2 weighted magnetic resonance imaging shows membranous occlusion of IVC (arrowhead).
Of late, various studies have shown the effectiveness as well as good outcome of various radiological interventions in patients with BCS, and now there has been a consensus over the management consisting of radiological interventions over shunt surgeries[20-24]. If technically feasible, restoration of hepatic venous outflow along with opening of occluded IVC is the preferred treatment for BCS. If opening of the hepatic vein is not possible due to complete occlusion of the HV then alternative treatment consists of creation of TIPS, and surgical options are reserved for patients with failed radiological interventions or having fulminant disease with liver failure requiring liver transplantation.
Patients having BCS may be categorized into two subsets for the planning of interventions. Patients having membranous or short segment occlusion of HV/IVC are considered for restoration of normal physiological venous outflow by opening of the occluded segment. The other group of patients in whom all the native HV are totally replaced by small intrahepatic collaterals are not suitable for interventions aimed at restoring normal physiological hepatic venous outflow, hence they require creation of non-surgical portocaval shunt (TIPS) or in the case of fulminant liver failure, liver transplantation remains the ultimate treatment. All these procedures should be supplemented with hematological management, because if these underlying disorders are not corrected then there is a very high likelihood of treatment failure.
VARIOUS INTERVENTIONS IN BCS
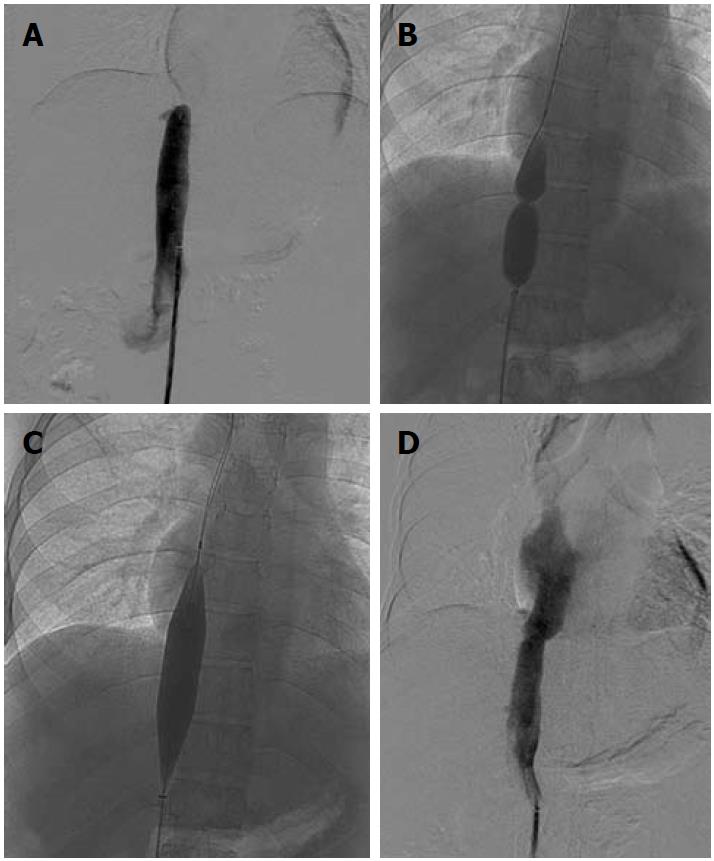
A subset of patients having membranous/short segment occlusion of IVC and/or HV require opening of the occluded segment followed by balloon dilatation and stenting if required. Dilatation may be attempted via the transjugular or transfemoral route; however, in a few selected cases combined jugular and femoral approach is required. IVC interventions may be performed using either the jugular or femoral route. Mostly, the femoral route is preferred as negotiation is easier. Initially the occluded segment/web is crossed using a guidewire and thereafter serial dilatation of the stenosed segment is done using a balloon catheter (Figure 7). Generally, balloon dilatation of the occluded segment is sufficient for opening of the stricture and stenting is rarely required. This is due to the fairly large lumen of the IVC. If there is persistent stricture after dilatation with a balloon having equal size to the lumen of an adjacent normal segment of IVC, then stenting is required[31,32]. IVC dilatation has a good midterm outcome and patients have immediate relief from symptoms such as pedal edema, dilated veins over the trunk and varicose veins.
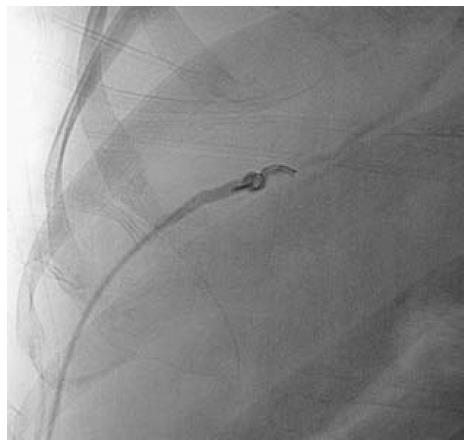
Figure 7 Inferior vena cavogram image.
A: Occlusion of suprahepatic inferior vena cava, which was crossed and balloon dilated; B, C: Dilatation of stenosed segment; D: Post dilatation venogram.
In patients with short segment occlusion of HV, the best hepatic vein is selected for dilatation. A hepatic vein having a straight course, echofree lumen and good caliber (7-8 mm in adults) is considered suitable for interventions. Balloon dilatation of hepatic venous obstruction is mostly attempted via the transjugular route but sometimes crossing of the stricture may not be easy due to longstanding fibrosis; such cases may require other approaches such as the percutaneous transhepatic or rarely transfemoral route (Figure 8). The percutaneous approach is relatively simple to perform due to the small and linear course of the hepatic vein providing easy manipulation of catheters and guidewire. After crossing the stricture the wire is snared out through the access sheath placed in the internal jugular vein and then balloon dilatation may be performed via the jugular route. Alternatively, dilatation may be done via the percutaneous track but it carries a risk of post-procedure hemoperitoneum secondary to the large rent created due to placement of an access sheath in the track. This complication can be minimized by embolizing the percutaneous track using embolization coils and Gelfoam pledgets (Figure 9). This is frequently practiced in our institute and in our experience we have not encountered any complication secondary to percutaneous balloon dilatation/stenting of the stricture. If needed, a stent may be placed via either of the routes available.
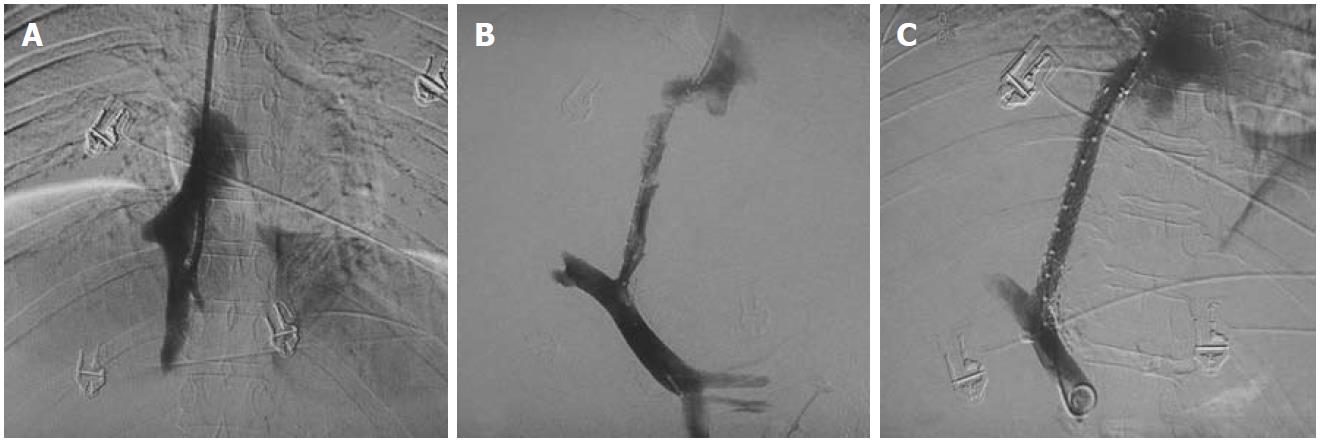
Figure 8 Hepatic venogram after percutaneous puncture.
A: Occlusion of hepatic veins with intrahepatic collaterals (short arrows); B: Balloon dilatation of the stricture with waist formation at the mid segment of balloon (arrowhead), The wire is placed percutaneously with distal end of wire in the left subclavian vein; C: Disappearance of waist; D: Residual stricture (long arrow) post dilatation hence leading to stent placement; E, F: Stent across the stricture with obliterated collaterals on post stenting venogram.
Figure 9 Fluoroscopic spot image showing closure of percutaneous track using embolization coil and Gelfoam slurry.
Patients with IVC as well as HV occlusion require opening of both the segments. These patients may sometimes be difficult to treat due to tight fibrosis of the hepatic venous ostium or the IVC. These cases may be challenging as negotiation of the wire across the strictured segment is not easy and several multiple approaches (transjugular, transfemoral and percutaneous) may be required. In selected few cases use of long needles may be required to cross the stricture. However, this should be attempted with great caution and care as the smallest of mistakes may be fatal.
The role of radiological intervention in acute BCS is to thrombolyse the involved HV. This may be done either by using local/direct pharmacological agents or it may require mechanical thrombolysis to restore deteriorating liver function. The decision regarding the use of thrombolysis technique depends upon the age of the thrombus; hyperacute thrombus may be lysed by using pharmacological agents only but an older thrombus may require mechanical thrombolysis. There are a few case reports in the literature describing the benefits of systemic thrombolysis[33], whereas few investigators have used combined local and systemic thrombolysis[34]. However, patients with fulminant disease not responding to systemic thrombolysis may benefit from local direct pharmacological as well as mechanical thrombolysis.
TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT
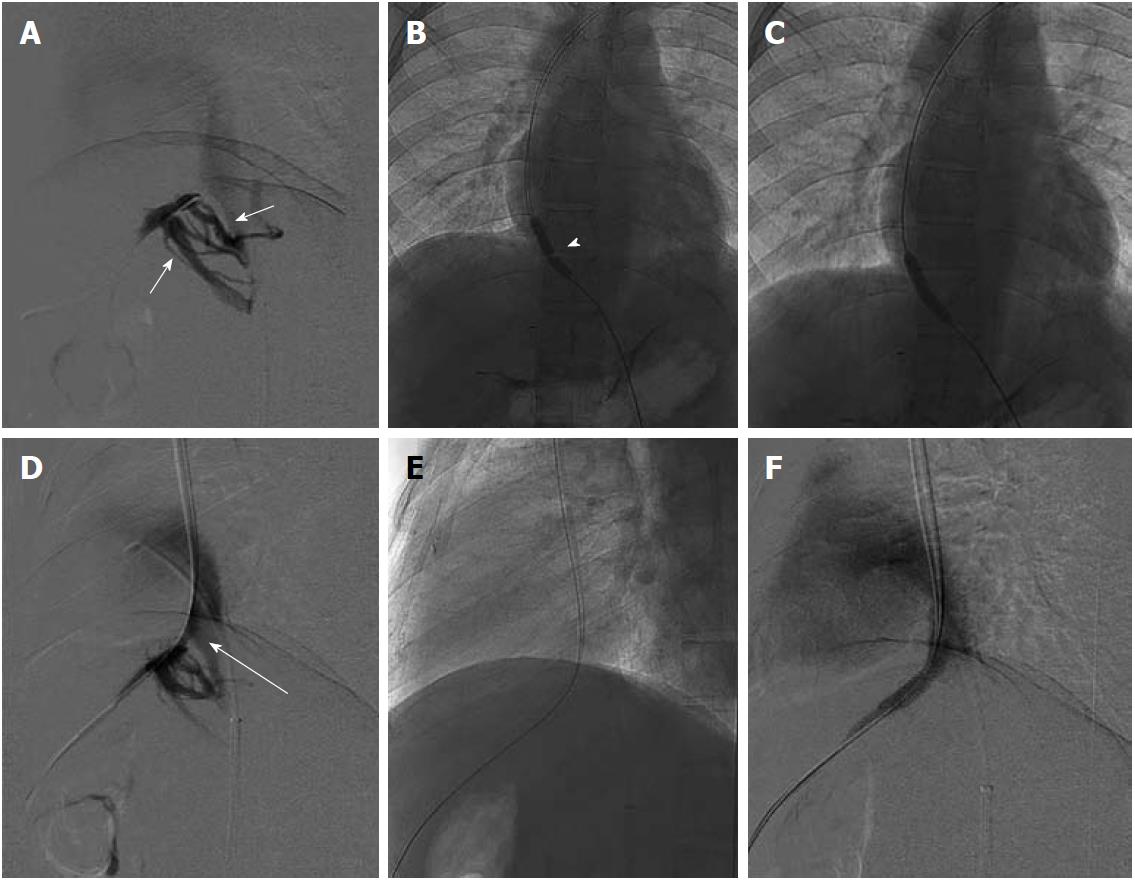
Chronic BCS patients having all the native HV replaced by intrahepatic collaterals form the other subgroup of patients where restoration of normal hepatic venous outflow is not possible. A possible radiological intervention in these patients consists of creating a shunt across the portal vein and IVC. With a relatively good midterm outcome, currently TIPS is the preferred intervention for patients having chronic BCS[23-25,35-41]. Creating TIPS in patients having chronic BCS with no available hepatic vein may be technically challenging and difficult and in some cases there may not even be a small notch in the IVC to suggest the site of confluence of hepatic vein and IVC. Such cases require a direct porto-caval shunt. Mostly these shunts are created using a transjugular approach but in difficult situations a combined percutaneous transhepatic approach may also be needed (Figure 10). A few authors have performed and described the technique for percutaneous transhepatic TIPS under US guidance[42]. After successful creation of this shunt the liver congestion is relieved, and thus there is improvement in the clinical symptoms and liver function.
Figure 10 DSA images of chronic Budd-Chiari syndrome with complete occlusion of all hepatic veins, cavogram.
A: Small stump of right hepatic vein; B, C: Creation of porto-caval shunt and placement of transjugular intrahepatic portosystemic shunt stent.
ROLE OF SURGERY
With advances in various non-invasive radiological interventions and techniques, and a fairly good midterm outcome, shunt surgery no longer remains the standard of care for BCS. Surgery consisting of liver transplantation remains the definitive treatment for patients having end stage liver disease or with fulminant disease and is the ultimate treatment and salvage procedure.
FOLLOW UP AND POST-INTERVENTION MANAGEMENT
As the majority of chronic BCS patients have thrombotic tendency with underlying thrombotic disorder(s), therefore identification and correction of these disorders is of utmost importance for long term success of the procedure, or else restenosis/thrombosis of the recanalized segment may occur leading to early failure of the procedure. Hence, it is imperative to maintain an INR (international normalized ratio) around 2.5. Regular follow up (clinical as well as radiological) is an important part of the management of these patients. The patient is reviewed three monthly initially for the first year; later he may be kept on annual follow up. Follow up radiological assessment comprises of US and color Doppler evaluation of the recanalized segment of HV and/or IVC and liver (Figures 11 and 12). Wherever there is suspicion regarding the patency of the recanalized segment, a hepatic venogram and inferior vena cavogram should be performed. If there is any suggestion of restenosis, balloon dilatation should be undertaken and if deemed necessary restenting may be done.
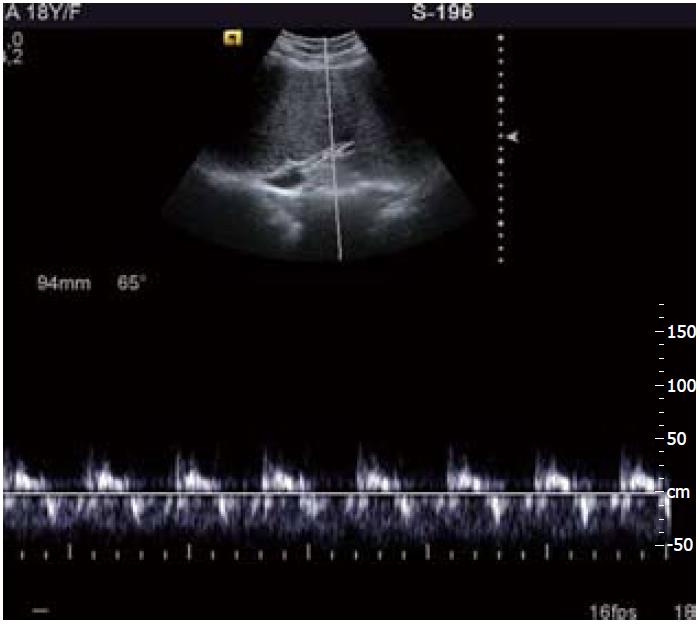
Figure 11 Follow up ultrasound Doppler image shows patent recanalized hepatic vein with normal triphasic waveform.
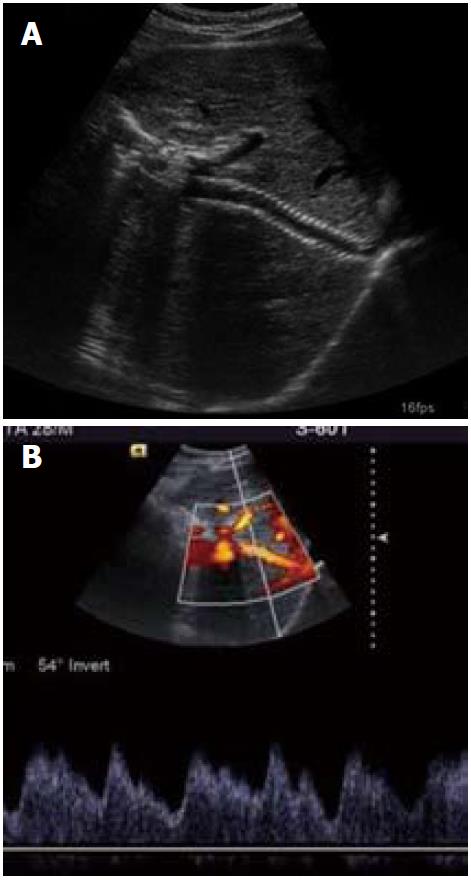
Figure 12 Follow up ultrasound and Doppler images (A, B) showing patent transjugular intrahepatic portosystemic shunt stent with normal spectral waveform.
CONCLUSION
BCS refers to a collection of disorders having hepatic venous outflow obstruction leading to raised hepatic sinusoidal pressure, hepatic fibrosis and portal hypertension. Due to advances in various interventional techniques and hardware, radiological intervention is currently considered the primary treatment for this disorder. These interventional procedures consist of recanalization, balloon dilatation, stent placement and creation of a porto-systemic shunt. With recent research showing good midterm outcome in BCS patients undergoing interventional procedures, shunt surgery no longer remains the primary treatment modality. However, liver transplant remains the ultimate treatment in fulminant disease and end stage liver failure.
Peer reviewer: Joon Koo Han, MD, PhD, Professor, Department of Radiology, Seoul National University College of Medicine, 103, Daehangno, Jongno-gu, Seoul, 110-744, South Korea
S- Editor Cheng JX L- Editor Logan S E- Editor Zheng XM