INTRODUCTION
The term atherosclerosis is derived from the Greek words “atheroma” and “sclerosis” which mean “mush” and “hardening”, respectively. These two words indicate the hardening of the vascular wall and lumen reduction of the same vessel. Atherosclerosis remains the commonest cause of death in the Western world. A degree of carotid artery narrowing has been reported in up to 75% of men and 62% of women aged 65 years[1]. The first study to identify an association between carotid artery lesions and the incidence of stroke is attributed to Savory, who in 1856[2] reported the case of a woman with cerebrovascular symptoms and where the autopsy examination revealed an occlusion of the distal tract of the left internal carotid artery associated with a bilateral obstruction of the subclavian artery. Since this first report many others have been described[3-5] providing evidence of a strong correlation between carotid pathology and cerebral symptoms. Currently, this pathology is the third leading cause of severe disability in the Western world causing 4.5 million deaths[6,7]. It has been demonstrated that the majority of cerebrovascular events appear be a consequence of emboli from an atherosclerotic plaque or, acute occlusion of the carotid artery, with distal propagation of the thrombus. Therefore, the identification and characterization of atherosclerotic disease affecting the carotid arteries is fundamental.
DIGITAL SUBTRACTION ANGIOGRAPHY OF CAROTID ARTERIES
The history of intra-arterial angiography is glorious and from the first study published in 1924 by Brooks[8], techniques have continuously improved and ultrasoft catheters, high-resolution biplane angiography equipment and Digital Subtraction Angiography (DSA) are currently used. DSA is an imaging technique used in interventional and diagnostic radiology that requires femoral artery puncture and intra-arterial injection of contrast medium, usually into the aortic arch or the common carotid artery. It is possible to obtain images of the carotid stenotic lumen with high spatial resolution and an estimation of low dynamics, such as slow and delayed blood flow.
DSA was previously considered the gold standard for the study of carotid artery pathology, because of its high spatial (50 μm) and temporal resolution (10 ms). This method allows us to obtain optimal definition of the opacified lumen, as well as plaque characteristics such as lumen irregularity or plaque ulcerations[9-11]. However, DSA has some limitations, it is invasive, expensive (compared to Computed Tomography and Magnetic Resonance), time expensive and requires a period of bed rest. It is important to underline that with current technological improvements (use of small catheter sizes) DSA can now be considered a minimally-invasive procedure and that the period of bed rest, with the use of a closure device, is limited. The main concern with DSA is the risk of neurological complications, however, this point is debated: in 1990 the risk of permanent neurological complications reached approximately 0.9% and transient neurological complications approximately 2%[12]. However, in the last few years the risk of neurological complications after DSA is low; Thiex et al[13] in a paper published in the American Journal of Neuroradiology in 2010, evaluated the safety of DSA provided by a dedicated neurointerventional team in a high-volume university hospital. They analyzed a cohort of 1715 patients from 2000 to 2008 and observed that no strokes or permanent neurologic deficits were seen in any of the 1715 patients undergoing diagnostic neuroangiography and only one patient experienced a transitory ischemic attack (TIA). This study demonstrated that within a high-volume neurointerventional practice, the risk of neurologic complications related to DSA can approach zero.
For about 50 years, angiography has represented the only imaging method for studying carotid arteries in order to detect the presence of pathological stenosis due to atherosclerotic plaque[14,15]. Conventional angiography was considered the most accurate technique for the diagnosis of carotid bifurcation stenosis and remains the standard against which other methods are compared.
Carotid endarterectomy (CEA) trials were undertaken during the 1980s up to the mid-1990s[16], and the benefit of CEA according to the degree of stenosis measured by angiography was graded. Nowadays, conventional angiography, always considered the gold standard imaging technique, plays a role only when there is no agreement between different imaging modalities or when endovascular treatment (carotid artery stenting) is needed. For these reasons, non-invasive and less risky carotid imaging techniques have gradually replaced intra-arterial angiography in the quantification of the degree of carotid stenosis.
STENOSIS DEGREE QUANTIFICATION
Three multi-centric randomized studies, North American Symptomatic Carotid Endarterectomy Trial (NASCET), European Carotid Surgery Trial (ECST) and Asymptomatic Carotid AtheroSclerosis Group (ACAS), provided cut-off values for stenosis degree indicating the possible benefits of CEA[17-19]. In particular, for those patients with symptomatic high-grade stenosis (70%-99%) after 5 years of follow-up, the pooled analysis of these three trials proved the benefits (70%-99% NASCET stenosis with a risk reduction of 16%, P < 0.001) of undergoing endarterectomy.
NASCET, ECST and ACAS proposed that when the only imaging technique available was DSA, the degree of stenosis was evaluated as the percentage reduction in the luminal diameter of the artery. Differences existed in the evaluation of stenosis degree between NASCET, ECST and CSI. With the NASCET criteria, the ratio between the lumen diameter at the stenosis and distal normal lumen diameter with no stenosis was calculated. With the ECST criteria, the ratio between the lumen diameter at the stenosis and the total carotid diameter (including the plaque) was calculated, whereas with the CSI-index criteria, the ratio between the lumen diameter at the stenosis and normal lumen of the proximal common carotid artery first multiplied by 1.2 was calculated. It was demonstrated by Saba et al[20] that the NASCET and ECST methods showed a strong correlation and that inter-observer and intra-observer agreement values were high for both NASCET and ECST.
The methodology for carotid stenosis quantification is widely debated because the NACET, ECTS and CSI-index are indirect ratio-percent methods. These trials imaged carotid arteries using conventional angiography, and methods of deriving ratio-percent were adopted because standardized stenosis measurements were not consistent with film (in conventional angiography) and because when used, digital angiography demonstrated different degrees of magnification and a lack of millimetre (mm) calibration.
With the introduction of MDCTA, thanks to its high spatial resolution (with an isotropic voxel of 0.5 mm), Bartlett et al[21-23] proposed a new direct mm-method in order to overcome the limitations of the classical percent-methods. It was demonstrated that the simple mm measurement of stenosis can reliably predict NASCET-type, ECST-type and CSI-type percent stenosis[24].
In an editorial by Forsting et al[25] published in Stroke 2003, the Editors suggested that, from a clinical point of view, DSA could be replaced by non-invasive methods for the quantification of carotid artery stenosis degree. However, in a meta-analysis published by the Lancet in 2006, Wardlaw et al[15] suggested caution because, even if non-invasive tests could probably replace DSA for 70%-99% of stenoses, insufficient data to determine their accuracy were provided, mostly in the presence of 50%-69% stenosis. These data have been confirmed in a paper recently published in Radiology by the same research group[26], where the authors stated that “Combinations of non-invasive tests do not improve both sensitivity and specificity but rather improve one at the expense of another”.
On the basis of these data it is reasonable to assume that the measurement of carotid stenosis can be adequately performed with non-invasive techniques, bearing in mind that the diagnostic accuracy of DSA is still unsurpassed and represents the gold standard. Nevertheless, the relevance of and interest in imaging tests for carotid artery occlusive disease remains high. On the basis of data from randomized clinical trials, we know that accurate diagnosis of stenosis severity is critical for clinicians to sensibly recommend patients for subsequent intervention.
THE CAROTID ARTERY VULNERABLE PLAQUE
In the past few years, the degree of luminal stenosis has been used as a measure of atherosclerotic disease severity. Recent studies demonstrated that moderate carotid artery stenosis may lead to acute cerebral infarction and subsequent histopathology results showed that plaque erosion and disruption were common morphologic features found in symptomatic lesions indicating that luminal narrowing was not the sole predictor of cerebrovascular events[27-29].
These studies have enabled the introduction of new concepts: (1) the degree of carotid stenosis is approximate to the volume and extension of carotid plaque[30,31]; and (2) a set of parameters, easily identifiable by CTA, MRA and US-ECD, are closely linked to the development of ischemic symptoms and can significantly increase the risk of stroke regardless of the degree of stenosis[32,33].
At the same time, a greater understanding of the pathophysiology of cerebrovascular events has been reached and it is understood that stroke/TIA originating from the carotid arteries can be determined by the presence of elements of the plaque’s embolization[34,35]. Another important parameter recently underlined in the literature is plaque volume. In fact, accumulation of atherosclerotic plaque in the carotid artery may lead to positive remodelling in which the artery enlarges to preserve the luminal area. In addition, a certain amount of atherosclerosis needs to be present in the carotid bulb before it causes stenosis. Some researchers have proposed that plaque volume is a better descriptor of the severity of atherosclerotic disease than the degree of stenosis[30]. Another interesting observation is that plaque composition changes with increasing plaque volume. According to the AHA criteria, which describe advanced atherosclerotic lesions in the carotid artery as containing more lipid and more calcium, an increase in the proportions of lipid and calcification were found with increasing plaque volume. Obviously, DSA does not provide information on plaque volume, because it is a purely “luminal” technique and for this type of analysis non-invasive techniques such as CTA, MRA or US are necessary.
CAROTID DIAGNOSTIC FLOW-CHART
The choice of a specific imaging modality to assess the carotid artery depends on several parameters and depends largely on the clinical indication for imaging and the skills available in individual centres. Recently, Jaff and colleagues[36] proposed a diagnostic algorithm for the correct use of imaging modalities according to the different clinical indication of the patients. In this paper published in 2008, it was proposed that for patients with a high likelihood of vascular disease, CTA may represent an appropriate first exam. On the other hand, for the screening of patients with a lower likelihood of neurovascular pathology, US-ECD should be selected. If significant stenotic disease of the ICA is detected, CTA as well as MRA (Figure 1) can be used to confirm the diagnosis and to accurately determine the precise degree of stenosis. DSA is necessary infrequently and only in cases of severe multiple vessel disease, for which assessment of flow direction and collateral patterns may be important or when the image quality of a non-invasive procedure is of limited value.
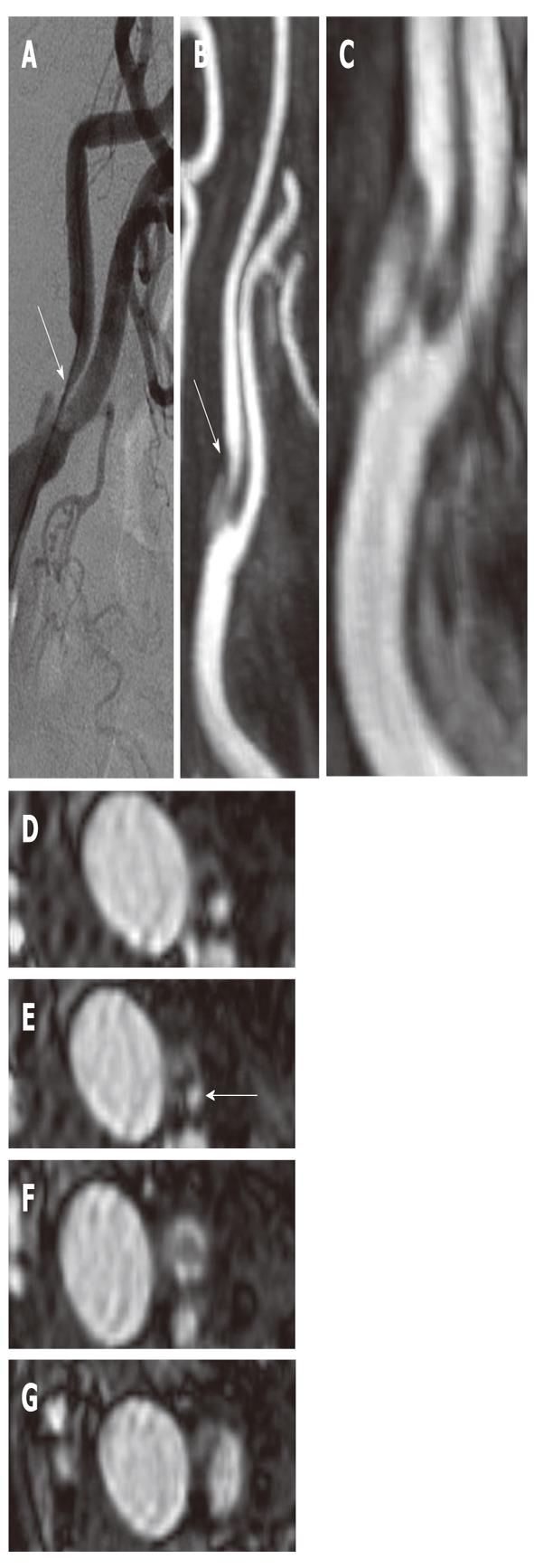
Figure 1 Digital Subtraction Angiography (A), CTA (B) and MRA (C-F) of the right carotid artery of a 74-year-old patient with right transitory ischemic attack.
The blue arrows indicate the point of maximum stenosis degree
CONCLUSION
Gradually, we have evolved from the concept of risk arising from the degree of stenosis to the concept of vulnerable plaque[37]. Identification of high risk atherosclerotic plaque and quantification of biological markers, especially inflammation, have considerably boosted the prediction potential of vascular risk. As a result, we can assume that stenosis may be considered only one among several indirect signs of a plaque showing embolic risk.
In light of these findings, vulnerable plaques should be identified early, and the role of DSA which is a purely technical luminal technique should be determined.
DSA should not be regarded as a reference method in the assessment and risk stratification of the carotid artery. In fact, the degree of stenosis can be adequately assessed with non-invasive techniques allowing evaluation of all structural parameters of the plaque, which cannot be obtained with angiography.
New trials are now required, in order to incorporate the potential of new imaging techniques to determine which patients require treatment, whether medical, surgical or interventional. Moreover, non-invasive imaging techniques should help to gain further insight into the natural history of atherosclerotic carotid plaques[38].
Peer reviewers: Patrick M Colletti, MD, Professor of Radiology and Medicine, Director Nuclear Medicine Fellowship, USC Keck School of Medicine; Professor of Biokinesiology; Professor of Pharmacology and Pharmaceutical Sciences, Chief of MRI, LAC+USC Imaging Science Center, University of Southern California, 1200 N State Street Room 3566 ,Los Angeles, CA 90033, United States;Poul Erik Andersen, MD, PhD, Associate Professor, Department of Radiology, Odense University Hospital, Sdr. Boulevard, DK-5000 Odense C, Denmark
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM