Revised: January 10, 2011
Accepted: January 17, 2011
Published online: March 28, 2011
We describe a case of worsening paraparesis induced by spinal cord compression at T6-T7 levels associated with compensatory extramedullary haematopoiesis from a compound heterozygote for haemoglobin E and for β-thalassemia. An emergency T3-T9 laminectomy was performed with excision of the masses and complete rehabilitation of the patient.
- Citation: Savini P, Lanzi A, Marano G, Moretti CC, Poletti G, Musardo G, Foschi FG, Stefanini GF. Paraparesis induced by extramedullary haematopoiesis. World J Radiol 2011; 3(3): 82-84
- URL: https://www.wjgnet.com/1949-8470/full/v3/i3/82.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i3.82
Extramedullary haematopoiesis (EMH) is a physiologic response to chronic anemia, observed in various hematologic disorders such as thalassemias[1], myelofibrosis and polycythemia. Since the first description by Gatto et al[2], several cases of EMH at different sites have been reported.
The EMH, as a tumor-like mass, is commonly seen in the liver, spleen and lymph nodes. Involvement of the epidural space causing spinal cord compression has rarely been reported[3].
Because of its rarity, there are no evidence-based guidelines for the treatment of paraspinal pseudotumors caused by EMH. Management options include hypertransfusion, radiotherapy, surgery or a combination of these modalities. Recently, hydroxyurea or erythropoietin in combination with radiotherapy as treatment was described[4-6], but management still remains controversial.
Decompressive laminectomy, as in our case, has been used in patients with rapid neurological deterioration[7].
We hereby present a case of EMH in a 21-year-old man with thalassemia presenting with paraplegia due to a spinal cord compression that was treated successfully with surgery.
A 21-year-old man with a not well defined history of transfusion-dependent microcytic anemia in his native country, Bangladesh, presented to the emergency department with worsening paraparesis.
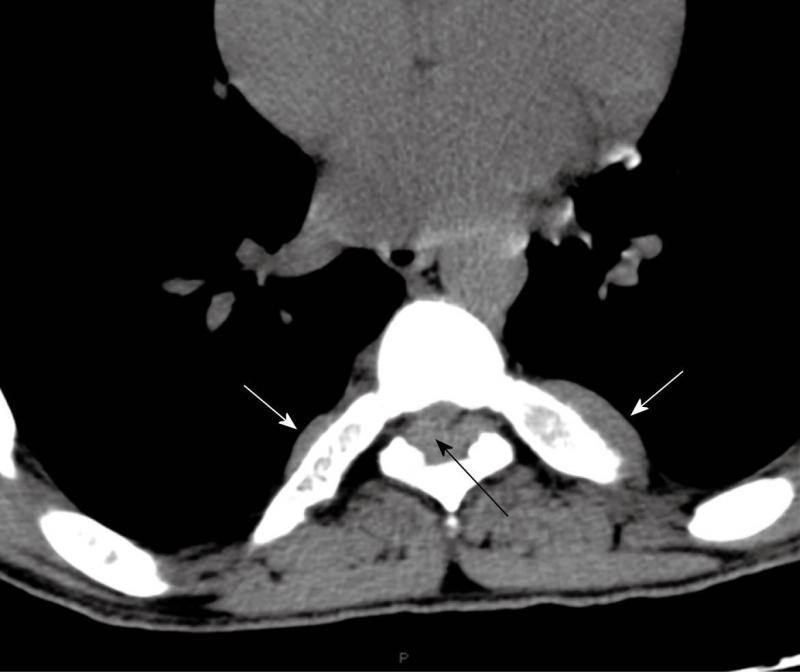
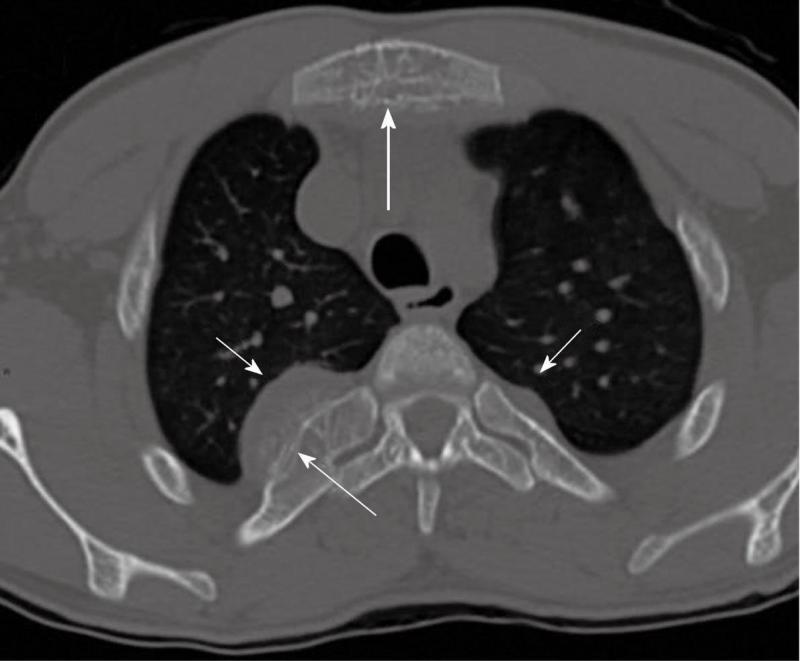
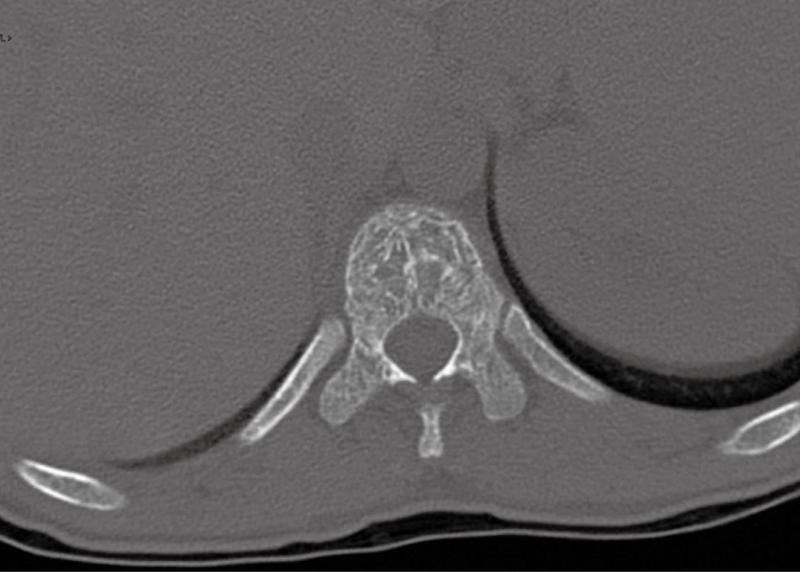
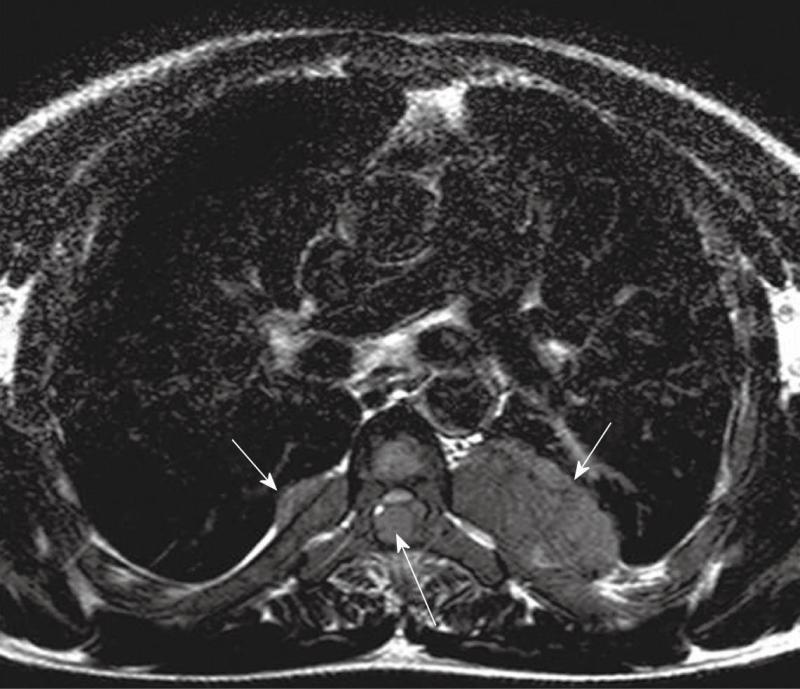
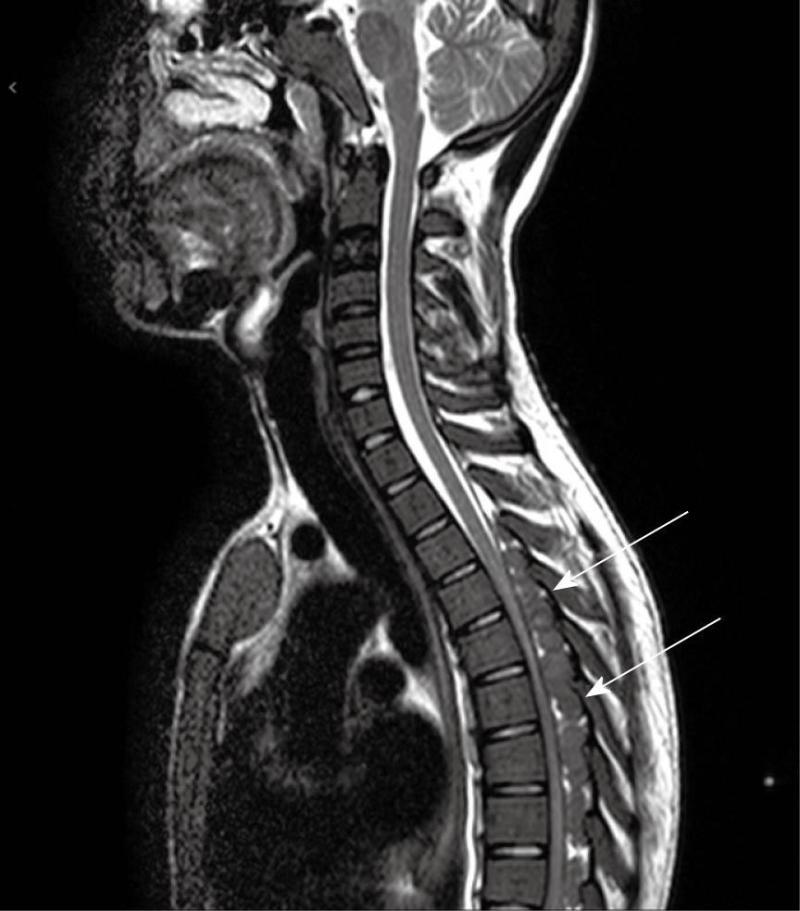
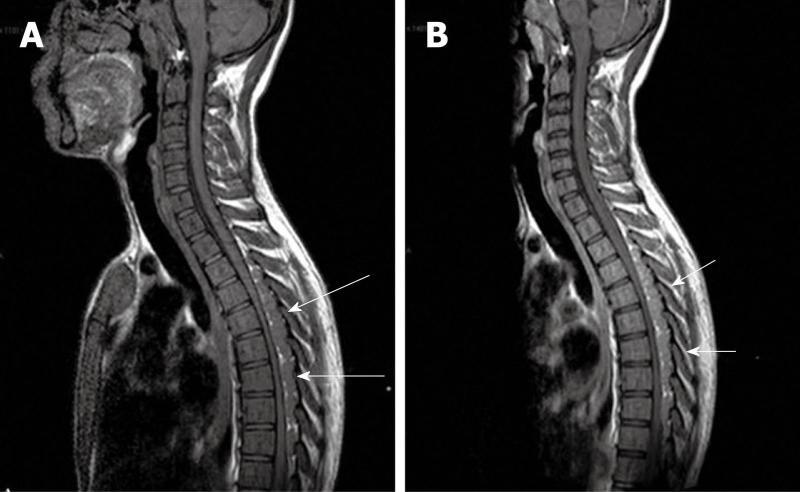
Computed tomography (Figures 1-3) and magnetic resonance imaging (MRI, Figures 4-6) revealed bilateral paravertebral soft-tissue masses at T4-L1 levels and a mass with the same features was seen inside the vertebral canal inducing spinal cord compression at T4-T9 levels. Furthermore, a marked medullary expansion of the bony structures was present. Laboratory analyses showed a haemoglobin (Hb) level of 8.5 g/dL and mean corpuscular volume (MCV) of 68 fL. Hb electrophoresis revealed HbE at 45%, HbF at 30% and HbA2 at 20%.
Molecular analysis showed the patient was a compound heterozygote for HbE (β-26 glutamine → lysine) and for β-thalassemia (β+IVS1-nt5). All these findings appeared to be associated with compensatory extramedullary haematopoiesis.
An emergency T3-T9 laminectomy was performed with a complete excision of the masses. A morphological and immunohistochemical analysis was then carried out. The masses were composed of a heterogeneous cellular population resembling physiological haematopoiesis in all its components.
The patient received a rehabilitation cycle and five months after the surgery, with continuing red blood cell transfusions, remains free of symptoms.
HbE (β26Glu → Lys) is the most common Hb variant in Southeast Asia and the second most prevalent worldwide.
HbE, when associated with β thalassemia (HbE/β-thalassemia) in a compound heterozygous state, results in a clinically severe condition. HbE activates a cryptic splice site that produces non-functional mRNAs. Hb IVS1-1 is a Mediterranean mutation that affects mRNA processing giving rise to β (o)-thalassemia. HbE/β thalassemia has a very variable clinical phenotype. HbE/β-thalassemia generally manifests with severe anemia where individuals exhibit β-thalassemia major with regular blood transfusions or β-thalassemia intermedia with periodic blood transfusions. Extramedullary haematopoiesis is a consequence of insufficient bone marrow function that is unable to meet circulatory demands. Thalassemia major or intermedia, congenital spherocytosis, congenital haemolytic anemia and sickle cell anemia account for most cases of EMH. It is rarely seen in myelofibrosis and Gaucher’s disease.
Intrathoracic EMH is most often visualized on the chest roentgenogram or the chest computed tomography (CT) scan as single or multiple paravertebral mass lesions. While a paravertebral mass may represent EMH, other disorders of the posterior mediastinum, such as neurogenic tumor, lymphoma, primary and metastatic malignancy, paravertebral abscess, lateral meningocele, and extrapleural cyst, must be considered.
The characteristic features observed in the chest roentgenogram and the chest CT scan were helpful in recognizing intrathoracic EMH. These included the following8]: (1) Widening of the ribs by expansion of the medullary cavity or by periosteal elevation, without bony erosion; (2) The presence of a unilateral or bilateral well-circumscribed lobulated, paravertebral mass lesion usually located caudal to the sixth thoracic vertebrae; and (3) The absence of calcification and the presence of adipose tissue within the mass.
MRI is currently the technique of choice in evaluating spinal EMH. On MRI, EMH is usually characterized by lobular masses with increased signal intensity compared to that of the red marrow in the adjacent vertebral bodies[7]. The lack of gadolinium enhancement allows its differentiation from other epidural masses such as abscesses or metastases.
Peer reviewers: Yi-Xiang Wang, MMed, PhD, Associate Professor, Department of Diagnostic Radiology and Organ Imaging, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, NT, Hong Kong, China; Kennith F Layton, MD, FAHA, Division of Neuroradiology and Director of Interventional Neuroradiology, Department of Radiology, Baylor University Medical Center, 3500 Gaston Avenue, Dallas, TX 75246, United States
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM
| 1. | Cappellini MD, Musallam KM, Taher AT. Insight onto the pathophysiology and clinical complications of thalassemia intermedia. Hemoglobin. 2009;33 Suppl 1:S145-S159. |
| 2. | Gatto I, Terrana V, Biondi L. [Compression of the spinal cord due to proliferation of bone marrow in epidural space in a splenectomized person with Cooley's disease]. Haematologica. 1954;38:61-76. |
| 3. | Logothetis J, Constantoulakis M, Economidou J, Stefanis C, Hakas P, Augoustaki O, Sofroniadou K, Loewenson R, Bilek M. Thalassemia major (homozygous beta-thalassemia). A survey of 138 cases with emphasis on neurologic and muscular aspects. Neurology. 1972;22:294-304. |
| 4. | Cario H, Wegener M, Debatin KM, Kohne E. Treatment with hydroxyurea in thalassemia intermedia with paravertebral pseudotumors of extramedullary hematopoiesis. Ann Hematol. 2002;81:478-482. |
| 5. | Cianciulli P, Sorrentino F, Morino L, Massa A, Sergiacomi GL, Donato V, Amadori S. Radiotherapy combined with erythropoietin for the treatment of extramedullary hematopoiesis in an alloimmunized patient with thalassemia intermedia. Ann Hematol. 1996;72:379-381. |
| 6. | Malik M, Pillai LS, Gogia N, Puri T, Mahapatra M, Sharma DN, Kumar R. Paraplegia due to extramedullary hematopoiesis in thalassemia treated successfully with radiation therapy. Haematologica. 2007;92:e28-e30. |
| 7. | Dibbern DA Jr, Loevner LA, Lieberman AP, Salhany KE, Freese A, Marcotte PJ. MR of thoracic cord compression caused by epidural extramedullary hematopoiesis in myelodysplastic syndrome. AJNR Am J Neuroradiol. 1997;18:363-366. |