INTRODUCTION
Radiation therapy still plays a major role in the management of hematological malignancies. Its place and modalities for treatment of lymphoma have evolved over recent decades. First, randomized studies supported reduction of field size and dose radiation in treatment programs for Hodgkin disease[1]. These developments were encouraged by reports that mediastinal radiotherapy was associated with cardiac toxicity and second malignancies, particularly when chemotherapy agents were used concomitantly or sequentially. Second, sophisticated imaging technologies and new radiation delivery techniques have become available[2]. With the recent advances in irradiation devices, new intensity modulated irradiation modalities have emerged. Those offer both increased target dose conformality and improved normal tissue avoidance. Helical tomotherapy combines inversely planned intensity modulated radiotherapy (IMRT) with on-board megavoltage imaging devices[3]. In this way, it has become possible to tailor very sharp dose distributions around the target volumes, close to critical organs[4]. It has emerged as one of the most promising techniques for IMRT delivery.
Here we summarize some of the most promising applications of helical tomotherapy in patients with hematological malignancies.
CLINICAL APPLICATIONS
For lymphoma irradiation, it is now the standard of care to use involved-field radiotherapy rather than the extended radiation fields of the past[5]. In this setting of volume reduction, implementation of new strategies aimed at further improving target coverage is promising. Helical tomotherapy combines inversely planned IMRT with on-board megavoltage imaging devices[3]. In this way, it has become possible to tailor very sharp dose distributions around the target volumes, close to critical organs. Improving dose conformality around the volumes has become an important end-point for radiation oncologists. Dosimetric results from planning studies of helical tomotherapy have demonstrated its ability in better sparing critical organs from irradiation, in comparison with more conventional irradiation modalities. Helical tomotherapy was shown to provide similar target coverage, and to improve both dose conformality and dose homogeneity within the target volume. This modern irradiation device allows accurate repositioning and critical organs visualization. Tomita et al[6] compared radiation treatment plans that used IMRT with helical tomotherapy or three-dimensional conformal radiation therapy for nasal natural killer/T-cell lymphoma. Authors found that IMRT achieved significantly better coverage of the planning target volume (PTV), with more than 99% of the PTV receiving 90% of the prescribed dose, whereas 3D-CRT could not provide adequate coverage of the PTV, with only 90.0% receiving 90% (P < 0.0001). These results and others demonstrated that helical tomotherapy could significantly improve target coverage when the PTV was close to critical organs.
Prospective data with long-term follow-up evidenced that heart dose exposure may cause cardiac disease and adversely affect quality of life, particularly in young patients with mediastinal radiotherapy for Hodgkin lymphoma. Hudson et al[7] assessed the impact of treatment toxicity on long-term survival in pediatric Hodgkin’s disease, and reported an excess mortality from cardiac disease in survivors of pediatric Hodgkin’s disease (22, 95% CI: 8-48), compared with age- and sex-matched control populations. Cardiac irradiation contributes to this excess of risk[8]. Recent data reported that helical tomotherapy could decrease radiation dose exposure for breasts, lung, heart and thyroid gland in patients treated for advanced Hodgkin’s disease[9].
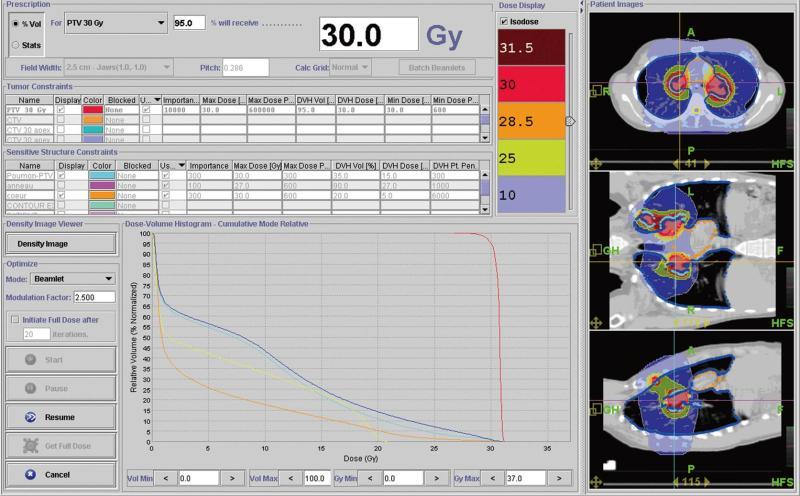
Since radiation-induced cardiovascular pathology is a major concern in patients undergoing therapeutic chest irradiation, helical tomotherapy has been logically investigated for improving heart avoidance. The physiopathology and manifestations of radiation-induced heart disease may considerably vary according to the dose, volume and technique of irradiation, and every effort should be made to avoid irradiating cardiac structures[10]. In this way, it will be possible to substantially decrease the risk of death from ischemic heart disease associated with radiation, which is particularly significant in patients receiving other cardiotoxic agents, such as anthracyclines. Actually, helical tomotherapy also allows treatments that would be difficult for conventional radiotherapy machines to deliver, such as treating mediastinal lymph nodes[11]. Figure 1 shows the distribution dose during radiotherapy of a patient who was diagnosed with multiple pleural and mediastinal locations from lymphoma. Dose-volumes histograms evidence accurate sparing of some organs at risk, with the possibility to treat multiple targets simultaneously.
Figure 1 This figure shows the dose distribution during radiotherapy for the first patient who was diagnosed with multiple pleural and mediastinal tumors and also the dose-volumes histograms showing the sparing of some organs at risk (for example heart in orange with 5 Gy received by 20% of heart volume, in red planning target volume (planning treatment volume with homogeneous dose distribution and adequate coverage).
Since irradiation to the bilateral hilum increases the risk of radiation pneumonitis, every effort was made to decrease the doses to the lung. Helical tomotherapy could be particularly useful in this setting. In this example, no more than 10% of the volume defined as the [lung - PTV] received 20 Gy.
Several other promising applications for helical tomotherapy have emerged. These strategies include treatment of patients who are at high risk of radiation-induced toxicity because of individual susceptibility, such as patients with acquired immunodeficiency[12]. Helical tomotherapy could also be used for decreasing the doses to critical structures in patients treated with concurrent targeted agents, which might potentially increase the risk of side effects[13]. Moreover, it permits re-irradiation of relapsed disease, a setting that considerably increases the risk of consequent delayed toxicity. Introducing helical tomotherapy to the field of lymphoma may also provide safer and more accurate radiotherapy to selected patients with bulky residual disease[1]. We previously reported the feasibility of helical tomotherapy to decrease the acute toxicity of debulking irradiation before allograft in patients with refractory lymphoma. In other malignancies, our retrospective data in patients with solitary plasmocytoma demonstrated that doses to critical organs, including the heart, lungs, or kidneys could be decreased[14]. This may be clinically relevant in heavily pretreated patients who are at risk for subsequent treatment-related cardiac toxicity. High response rates were also reported and encouraged further prospective assessment, and most patients experienced a complete response prior to stem cell allograft.
An increased risk of secondary malignancies has been reported after radiotherapy for lymphoma. In particular, young patients have a high risk of developing breast cancer in their life after mediastinal radiotherapy for a lymphoma[15]. The improved outcome among patients with Hodgkin’s lymphoma has been associated with increased incidence of second malignancies. This risk becomes significantly elevated 5 to 10 years after irradiation for Hodgkin lymphoma[16,17] and the incidence of breast cancer has been reported to increase by a factor of 4.3 (95% CI: 2.0-8.4) for patients treated with mantle irradiation[18]. Koh et al[19] quantified the reduction in radiation dose to normal tissues and modeled the reduction in secondary breast cancer risk, and suggested significant relative risk reduction for second cancers with involved field radiotherapy. While the dose response for radiation dose above 10 Gy remains uncertain, carcinogenesis after radiation is exacerbated by the large dose gradient across the breast and treatment field position[20]. Although helical tomotherapy might significantly decrease high doses delivered to the breast, it increases the volume that receives lower doses, which has also been implicated in the carcinogenesis process. For that reason, intensity-modulated irradiation should not be delivered in children outside of a clinical trial.
Finally, preliminary results suggested that helical tomotherapy could be employed for total lymphoid irradiation in the preparative regimens for allogeneic bone marrow and chronic graft-versus-host disease. When using conventional irradiation devices, extended source-to-skin patient setup and/or field matching are required, and all critical organs are within the beam coverage. Treatment planning with helical tomotherapy for total lymphoid irradiation in adults demonstrated that the dose to the spinal cord, kidneys, intestinal compartment, and lungs could be decreased[21,22].
ALTERNATIVE IRRADIATION MODALITIES
We have pointed out the potential of helical tomotherapy in the light of our institutional experience. Actually, helical tomotherapy is not the only solution to improve both dose conformality and dose homogeneity within the target volume, and its availability remains rather limited (low number of helical tomotherapy devices). Other IMRT techniques could also be applied for delivering highly conformal irradiation. In 2005, Goodman et al[23] assessed the feasibility and potential advantages of linear accelerator based IMRT in the treatment of lymphoma involving large mediastinal disease volumes or requiring reirradiation. Compared to conventional parallel-opposed plans and conformal radiotherapy plans, IMRT could decrease the dose delivered to the lung by 12% and 14%, respectively. The PTV coverage was also improved, compared with conventional RT[23]. Recent dosimetric data demonstrated that the forward planned IMRT technique could be easily used for improving PTV conformity while sparing normal tissue in Hodgkin’s lymphoma[24].
Volumetric modulated arc therapy (VMAT) has also demonstrated its ability in tailoring accurate dose distributions around the target volumes. Weber et al[25] compared VMAT to conventional fixed beam IMRT in ten patients with early Hodgkin disease. They found no difference in levels of dose homogeneity. However, for involved node radiotherapy, doses to the PTV and OAR were higher and lower with VMAT when compared to IMRT, respectively.
Finally, the dosimetric advantages of proton therapy could also be used for reducing the risk of late radiation-induced toxicity related to low-to-moderate doses in critical organs. Chera et al[26] compared the dose distribution in Hodgkin’s lymphoma patients using conventional radiotherapy, IMRT, and 3D proton therapy in Hodgkin’s lymphoma patients with stage II disease. Authors found that 3D proton therapy could reduce the dose to the breast, lung, and total body. However, the availability of proton therapy is very low and only a few patients could benefit from this highly conformal irradiation modality.