INTRODUCTION
Approximately 12% of cancers worldwide can be linked to a viral infection[1]. Oncogenic viruses that have been identified include Epstein-Barr virus (EBV), human herpes virus 8 (HHV8), human papillomavirus (HPV), Simian virus 40 (SV-40), Human T-lymphotropic virus, and human immunodeficiency virus (HIV)[1,2]. Viruses can be categorized into several families and sub-families according to the type of genomic material they contain (DNA or RNA), symmetry of the capsid, presence or absence of an envelope, dimension, and the viral genome replication mechanisms. Tumor viruses belong to a number of families including the DNA virus families: Hepadnaviridae, Herpesviridae, and Papillomaviridae and the RNA virus families: Retroviridae and Flaviviridae[3].
This article focuses on thoracic tumors with viral etiologies, which include post-transplant lymphoproliferative disease, lymphomatoid granulomatosis, Kaposi’s sarcoma (KS), Castleman’s disease, recurrent respiratory papillomatosis (RRP), lung cancer, malignant mesothelioma, leukemia and lymphomas. The participation of the viral infection in the pathogenesis of these tumors as well as their most common imaging findings will be discussed.
EBV
EBV was first discovered in 1964 by Epstein and Barr from a cell line derived from Burkitt lymphoma (BL)[4]. The virus is a DNA double-stranded virus which belongs to the γ herpesvirus subfamily. More than 95% of the healthy population worldwide carries the virus and transmission usually occurs during childhood via saliva. Children under the age of 10 usually have an asymptomatic primary infection. Primary infection occurring over the age of 15 leads to the clinical syndrome of infectious mononucleosis in 30%-40% of cases[5].
EBV infection develops when virions (the infective form of a virus which consists of a DNA or RNA core with a protein shell or capsid) transit across the epithelial cells in the oropharnynx to infect B cells in the mucosa. The virus has two phases of replication, a lytic and a latent cycle. The virus initiates a latent infection by shutting down viral protein expression. It is this latent infectious state that is responsible for malignant transformation[6]. The virus remains in the B cell memory pool, recirculating, and is found predominantly in the blood and pharyngeal lymphoid tissue[3,7].
EBV is known to be associated with several different malignancies in humans, including Hodgkin’s lymphoma (HL), BL, nasopharyngeal carcinoma, and post-transplant proliferative disease (PTLD), in which the malignant cells are characterized by unique viral and cellular phenotypes, as well as unique viral antigen expression[8].
More than 30% of HL cases are EBV-associated, with the clonal virus localized inside the malignant cells. In developing countries this number is higher with 90% of HL being EBV positive. Similarly, the association between EBV and HL is even higher in patients with acquired immunodeficiency syndrome (AIDS) in whom 95% are EBV positive HL. The histology of these tumors also has some distinctive features since HL positive for EBV is more often a mixed cellular type. The clinical presentation also differs from that of the EBV negative counterpart in that this form is more commonly seen in either children younger than 10 years of age or in adults older than 45 years old. Additionally, the prognosis of EBV positive HL in the elderly and immunocompromised is poor, compared with that of patients with EBV negative HL[8].
HL is currently classified by two distinct types: nodular lymphocyte predominant HL and classical HL (CHL). The latter is further categorized into three subgroups: nodular sclerosis, lymphocyte rich, and mixed cellularity (MCCHL)[9]. There is significant overlap in the imaging manifestations of the different types and subtypes of lymphomas, which in the chest may involve the mediastinum, lung, pleura and chest wall, but some differences should be highlighted. First, primary pulmonary lymphoma is rare in Hodgkin’s disease. Pulmonary involvement in HL is more commonly seen either in advanced stages (secondary involvement) or in recurrent disease[10]. Second, even though HL is the most common lymphoma presenting with mediastinal lymphadenopathy, MCCHL, the type most commonly seen in association with EBV involvement, typically spares the thymus gland and mediastinum[9] (Figure 1).
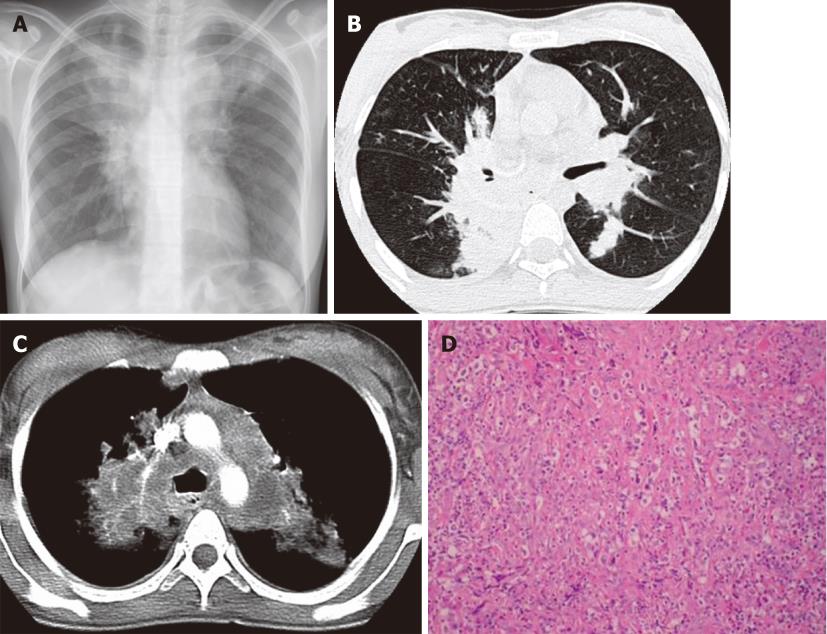
Figure 1 Hodgkin’s lymphoma.
A 23-year-old woman with a four-mo history of dry cough and chest pain. A: Chest X-ray shows mediastinal widening and upper lobe parenchymal opacities; B and C: Contrast-enhanced computed tomography confirms lymphadenopathy involving the mediastinum and infiltrative masses in the bilateral upper lobes; D: Photomicrograph (HE stain). Nodular sclerosing HL lymph node. Fibrous bands divide the lymphoid infiltrate into nodules and contain Hodgkin cells.
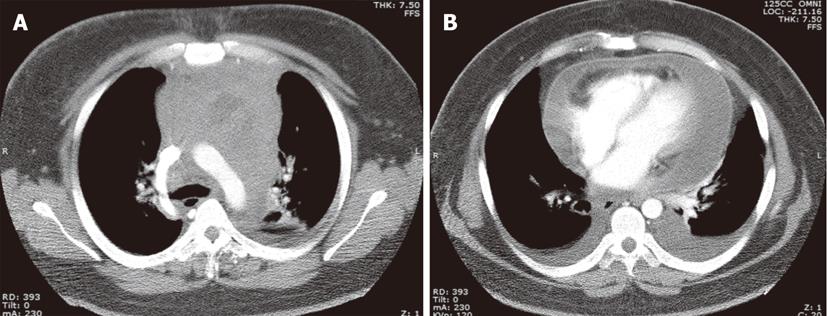
The association between EBV infection and BL varies depending on the subtype of the disease. The endemic form (eBL) is associated with EBV infection in over 95% of cases. Only half of AIDS-related BLs are associated with this viral infection, while the sporadic form of (sBL) is rarely associated with it[11,12]. Thoracic involvement in BL is less common than the more typical facial or abdominal disease, and is more commonly seen in HIV positive (10%) than in HIV negative (2%) patients[13]. The most common thoracic manifestations of BL include mediastinal mass and lymphadenopathy followed by pleural and chest wall involvement. Isolated pleural effusion and pulmonary parenchymal disease are rare[14,15] (Figure 2).
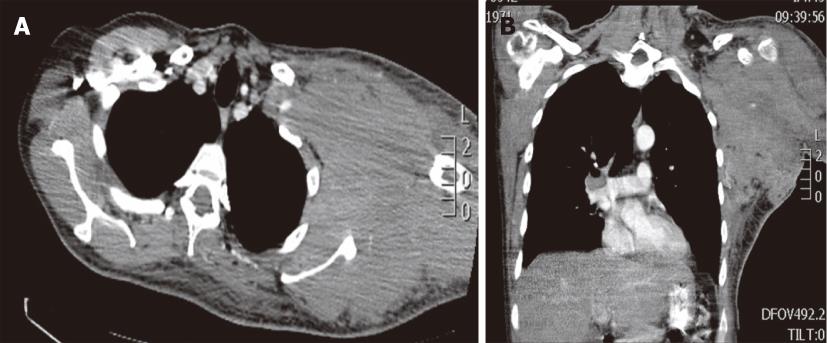
Figure 2 Burkitt lymphoma in a 38-year-old male with a left axilla mass.
Contrast-enhanced computed tomography, axial (A) and coronal (B) images demonstrates a bulky soft tissue mass involving the axilla, subpectoral and subscapular spaces.
Another infectious disease associated with BL is malaria. It is currently thought that the association between malaria and BL arises from a combination of multiple factors including immunosuppression, B-cell activation directly by the malarial parasite Plasmodium falciparum and EBV, since the viral loads for EBV are higher in areas endemic for malaria[11].
EBV and KS virus (KSV) in a complex interaction with the HIV, and the immunosuppression status have also been implicated in the pathophysiology of non-HLs in AIDS, typically B-cell lymphomas. AIDS-related lymphomas remain an important cause of morbidity and mortality in HIV-infected individuals, often occurring in extranodal locations (e.g. bone marrow, brain, viscera) and have an aggressive clinical course[16]. On imaging examinations the extranodal location of these tumors manifest as pleural effusion, interstitial and alveolar lung disease, and pulmonary nodules[17].
PTLD is made up of a heterogeneous group of EBV diseases with malignant lymphoproliferation occurring after hematopoietic or solid organ transplantation secondary to iatrogenic suppression of T-cell function[18]. EBV-infected B cells in the germinal centers, which should be destroyed, persist in the absence of cytotoxic T cells[6].
Approximately 2% of all allograft recipients are afflicted with PTLD, and it is more likely to develop in patients who are seronegative for EBV before transplantation. Onset of the disease can occur at variable intervals with the mean at 2-5 mo after bone marrow, lung or heart-lung transplantation and at a mean of 22-32 mo after kidney, or liver transplantation[19].
PTLD involvement of the lung is more common after heart-lung transplantation than after liver or renal transplant. The most common intrathoracic findings of PTLD include well-circumscribed, often bilateral peripheral pulmonary nodules, patchy air space consolidation and mediastinal and hilar lymphadenopathy[19]. Pleural-based masses, chest wall masses, pleural and pericardial effusion, and thymic enlargement have also been reported[20] (Figures 3 and 4).
Figure 3 Post-transplant proliferative disease in a 50-year-old male post-right lung transplant.
Contrast-enhanced computed tomography shows an infiltrative right hilar mass surrounding the right lower lobe bronchus and involving the subcarinal region. Ipsilateral small pleural effusion is also noted.
Figure 4 Post-right lung transplant post-transplant proliferative disease in a 68-year-old male with pulmonary fibrosis.
Non-contrast computed tomography, axial (A) and sagittal images (B) demonstrates an irregular mass in the right lung involving the middle lobe and lower lobe extending across the major fissure.
Positron Emission Tomography with Fluoro-2-deoxy-D-glucose (FDG-PET) has replaced gallium-67 scintigraphy in the functional and metabolic imaging evaluation of lymphomas. FDG-PET, in particular, is recommended before treatment in typically FDG avid lymphomas like diffuse large B cell lymphoma (DLBCL) and HL[21]. FDG-PET has also proven useful for the diagnosis, staging and therapy monitoring of PTLD both in infants and adult patients, revealing foci of increased uptake, not seen by other imaging techniques[22-24].
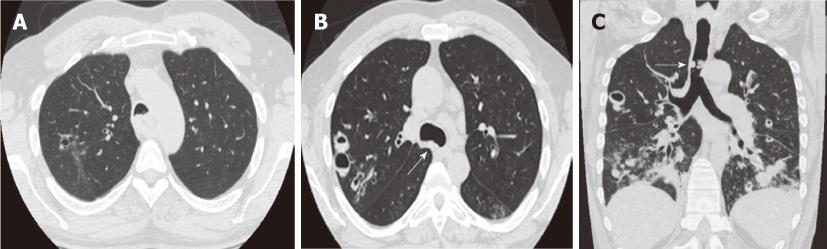
Lymphomatoid Granulomatosis is a B-cell lymphoproliferative disease that is angiocentric and angiodestructive. It is comprised of predominantly reactive T cells and some neoplastic EBV-positive B cells. The pathogenesis of the disease is hypothesized to be similar to that of PTLD, in which infected B cells proliferate in the absence of an adequate number of cytotoxic T cells[25]. Malignant transformation to lymphoma occurs in 12%-47% of patients with a mortality rate between 53% and 63.5%[26].
The most common site of involvement for lymphomatoid granulomatosis is the lung[27]. Imaging findings include poorly marginated pulmonary nodules and lung masses distributed along the bronchovascular bundle and interlobar septa[26]. On computed tomography (CT), the nodular lesions may present with surrounding ground-glass density (halo sign) or central ground-glass surrounded by a denser rim (the reverse halo sign)[28]. Rapid progression, central necrosis and cavitation masquerading as a lung abscess are other imaging features[29,30]. The presence of migratory nodules in the lung parenchyma mimicking the imaging findings of pulmonary vasculitis has also been reported[30] (Figure 5).
Figure 5 Lymphomatoid granulomatosis in a young adult male with acquired immunodeficiency syndrome.
Initial chest computed tomography (CT) (A) demonstrates a round solid mass in the right lower lobe. After surgical resection follow-up CT (B) 4 mo later shows recurrent disease with new multiple nodules and cavitation in the right lower lobe.
HHV8
HHV8 or KS herpes virus was first isolated in a patient with AIDS-associated KS and subsequently isolated in KS patients without HIV[31]. HHV8 is a γ2-herpes virus and similar to EBV has two different life cycles: lytic and latent. Viral proteins produced in both phases of viral replication are responsible for oncogenesis[32].
The virus has been identified in association with KS, primary effusion lymphomas (PEL) and Multicentric Castleman’s Disease[33]. Epidemiologically, the virus is widespread in most of sub-Saharan Africa with 50% of the population having antibodies to HHV8. It is also relatively frequent in the Mediterranean region. In endemic countries, transmission of the virus occurs in childhood from mother to child or among peers. In non-endemic countries, HHV8 is a sexually transmitted disease[34].
PEL is a rare type of AIDS non-HL that predominantly grows in the pleural, pericardial and peritoneal cavities. They are characterized clinically and on imaging examination by the presence of neoplastic effusions in complete absence of a contiguous solid mass, and are considered to develop from HHV8/KSV infection[35].
KS is a low-grade mesenchymal tumor involving blood and lymphatic vessels and is considered one of the major complications of AIDS. Four variants of KS are recognized: classic KS, endemic (African) KS, iatrogenic (organ transplant-related) KS, and AIDS-related KS[36]. In two of the forms of KS (AIDS and iatrogenic), the HHV8 causes a reactive polyclonal angioproliferative response in the presence of host immunosuppression. The polyclonal cells become an oligoclonal cell population which proliferates and undergoes a malignant transformation[37].
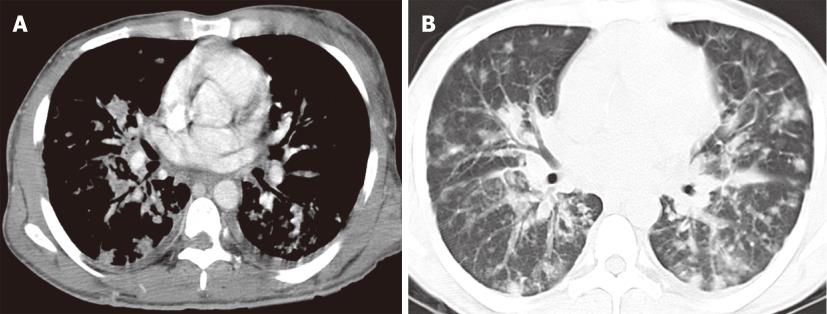
KS with pulmonary involvement is found in approximately 45% of patients with cutaneous AIDS-related KS[37]. Pulmonary involvement in KS is usually secondary to profound immunosuppression. Pulmonary KS was found to be associated with a low CD4 cell count and may be related to late presentation of HIV[38]. Following the introduction of highly active antiretroviral therapy (HAART), thoracic disease has become less frequent[38].
The most common presenting symptoms of pulmonary KS are progressive dyspnea, non-productive cough and fever. Other symptoms reported include pleural effusion with chest pain, hypoxemia, and acute respiratory failure requiring mechanical ventilation[37]. KS may involve the tracheobronchial tree, the lung parenchyma and pleura. Imaging manifestations in the chest include reticular and nodular opacities with a bronchovascular distribution, consolidation and adenopathies (Figure 6). Enlarged lymph nodes in the chest and abdomen may demonstrate significant contrast enhancement. Chest wall involvement with skin lesions and subcutaneous nodules or masses as well as osteolytic bone lesions involving the sternum, ribs and spine are not uncommon[36].
Figure 6 Kaposi sarcoma in a 37-year-old male with acquired immunodeficiency syndrome.
Contrast enhanced computed tomography of the chest, mediastinal window (A) and lung window (B) images demonstrate innumerable peribronchovascular and peripheral pulmonary nodules throughout the bilateral lungs. Enhancing skin lesions and bilateral pleural effusion are also noted.
Castleman’s Disease was identified in a series of 13 cases of localized mediastinal lymph node hyperplasia resembling thymoma described in 1956 by Dr. Benjamin Castleman[39]. Histologically, the disease is characterized by expanded germinal centers with B-cell proliferation and vascular proliferation. Castleman’s disease can be classified histologically as either hyaline-vascular or plasma cell variant, and clinically as either localized or multicentric[33]. Castleman’s disease in the HIV population is usually of the multicentric and plasma cell variant[40].
It is hypothesized that the active viral lytic replication cycle of HHV8 in lymphoid tissue can lead to Multicentric Castleman’s Disease[34]. HIV-related Multicentric Castleman’s disease is associated with Kaposi sarcoma. In fact, in a clinical series, 71% of HIV positive patients with Castleman’s Disease had Kaposi sarcoma[40]. Clinical features include fever, weight loss, respiratory symptoms such as dyspnea and cough, hepatomegaly and splenomegaly. Prognosis is poor with a median survival of 14 mo[40]. There is an increased risk of progression to large B-cell plasmablastic lymphoma and other lymphomas[33]. The most common location for the localized form of Castleman’s disease, typically the hyaline vascular type, is in the chest, where the characteristic imaging manifestation on CT is as a mediastinal or hilar mass, with diffuse homogeneous enhancement after contrast injection[41]. Pleural, pericardial and intrapulmonary forms of Castleman’s disease, which are considered atypical presentations, have also been reported[42]. The multicentric type of Castleman’s disease associated with AIDS, typically the plasma cell type, more commonly manifests as diffuse pulmonary involvement with acute reticular and nodular opacities in patients who also present mediastinal and peripheral lymphadenopathy[43] (Figure 7).
Figure 7 Multicentric Castleman’s disease in a 40-year-old male with acquired immunodeficiency syndrome.
Contrast-enhanced chest computed tomography, axial images at two different levels (A, B) reveal numerous enhancing abnormally enlarged lymph nodes in the axillae and mediastinum.
HPV
HPV is a non-enveloped double-stranded DNA virus with more than 200 types isolated[44]. The virus infects the basal layers of cutaneous and mucosal epithelium and enters the cell via a receptor. E6 and E7 proteins are the portions of the viral genome that encode viral oncoproteins expressed in HPV-positive cancers. E6 interferes with p53, a tumor suppressor protein that regulates the G1/S and G2/M cell cycle checkpoints. The main function of E7 is the binding and degradation of the Rb family of proteins, which are the major regulators of the cell cycle[45].
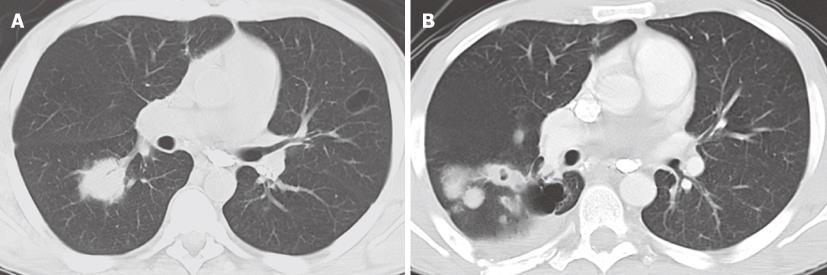
RRP is a benign lesion commonly found in the larynx with rare pulmonary involvement. The disease is difficult to treat because of frequent recurrence and spread throughout the respiratory tract. Ororespiratory exposure during vaginal birth is thought to be the origin of the disease and is often associated with HPV types 6 and 11[46]. HPV 11 is thought to be associated with more aggressive disease than HPV 6[47]. Pulmonary papillomatosis affecting the bronchi and lung parenchyma occurs in 1.8% of patients with RRP. Spread to the lower airways is usually caused by tracheotomy performed in a child with laryngeal papillomatosis. Prognosis of pulmonary papillomatosis is poor with a mortality rate of 57.1%[47]. Malignant transformation in RRP can develop in 10% to 13% of affected patients[48,49].
Radiographic findings of RRP in the lungs include bilateral, multiple, thin-walled cysts and nodular papillomas with predominant lower lobe distribution. Cysts are usually less than 5 cm in size with air-fluid levels. Pulmonary nodules are usually small, but occasionally may be as large as 3 cm in diameter (Figure 8). Chest CT can be more sensitive in detecting small cysts and nodules. Virtual bronchoscopy is a non-invasive technique that can be used to evaluate the tracheobronchial tree and reduces the risk of downward spread of the virus that can occur during an endoscopic exam[46].
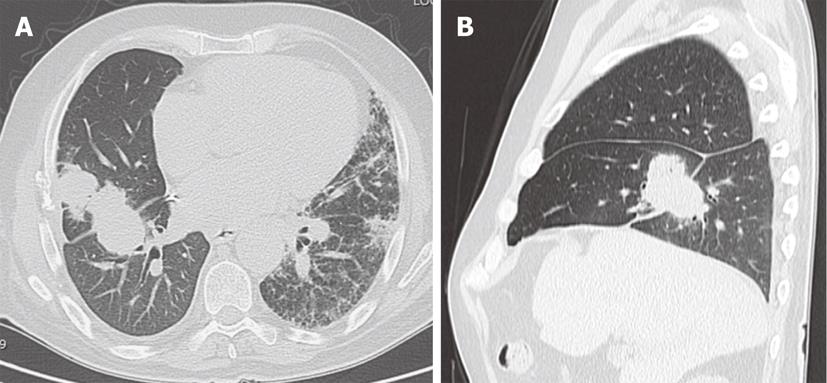
Figure 8 Recurrent respiratory papillomatosis in a 27-year-old patient.
Contrast-enhanced computed tomography, axial (A, B) and coronal (C) images. Numerous nodules and cystic lesion are seen in the bilateral lungs as well as a large lobulated mass partially obstructing the distal trachea (arrows).
The association of lung cancer and HPV was first suggested by Syrjanen in 1979, who described epithelial changes in bronchial carcinomas similar to those of exophytic condylomas of the genital tract seen with HPV[50]. It has been theorized that HPV can infect the lungs hematogenously from other sites such as the cervix[51]. HPV types 16, 18, 31, 33 and 35 have been associated with lung cancer and both adenocarcinoma and squamous cell carcinoma have been seen with HPV infections[44]. HPV DNA has been identified in more than 20% of lung cancers[52].
SV-40
SV-40 is a double-stranded DNA polyoma monkey virus. It is believed that the most likely route of transmission of SV-40 from monkey to human was through contaminated polio vaccines produced between 1955 and 1978[53]. Expression of the oncogenes, large T antigen and small T antigen, causes a high rate of malignant transformation. The large T antigen binds and inhibits p53 and pRB tumor suppressor proteins. Human mesothelial cells are susceptible to SV-40 infection and allow the virus to replicate without lysing leading to malignant transformation[2].
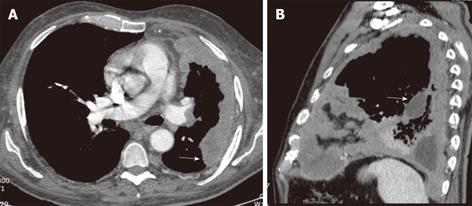
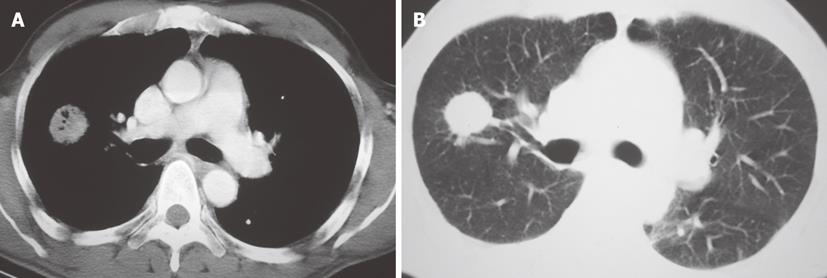
Malignant mesothelioma is an aggressive tumor that arises from mesothelial cells lining the pleura, peritonea and pericardia. SV-40 DNA is found in up to 60% of mesothelioma[54]. There is solid evidence linking SV-40 either alone or with asbestos exposure as a contributing factor in the malignant transformation of mesothelial cells and subsequent development of malignant mesotheliomas[55,56]. Affected patients typically present with pleural effusion associated with chest wall pain. Prognosis is poor with an average survival of just 12 mo after diagnosis[54]. Characteristic imaging findings on CT include unilateral pleural effusion; nodular pleural thickening that may completely encase the affected lung; and pleural thickening extending into the pulmonary fissures (Figure 9). Given the association with asbestos exposure, calcified pleural plaques are seen in approximately in one fifth of affected patients[57].
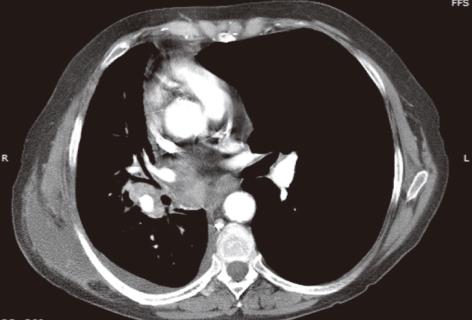
Figure 9 Malignant mesothelioma in a 60-year-old male with progressive chest pain.
Contrast-enhanced computed tomography, axial (A) and sagittal (B) images show an irregular and slightly lobulated soft tissue density mass extensively involving the pleural surface of the left lung including the major fissure (arrows).
HUMAN T-CELL-LYMPHOTROPIC VIRUS 1
Human T-cell-lymphotropic virus 1 (HTLV-1) is an RNA virus belonging to the deltaretrovirus genus. It is the first human retrovirus linked to human malignancy[58]. It was isolated from peripheral blood samples of a patient with cutaneous T-cell lymphoma in the 1980s[59]. HTLV-1 is endemic to Japan, South America, Africa and the Caribbean[3]. Based on a number of studies, it appears that the viral regulatory factors, TAX and HBZ, are the malignant transforming factors. TAX modulates gene expression and induces genomic instability. HBZ stimulates T-lymphocyte proliferation[58,60].
Adult T cell leukemia/lymphoma (ATLL) is a mature T cell neoplasm of post-thymic lymphocytes that has been linked to HTLV-1. Although worldwide, 20 million people are infected with HTLV-1, it is estimated that only 2%-6% will develop ATLL. Patients with ATL have atypical lymphoid cells with multilobulated nuclei[58]. ATL is divided into 4 subcategories based on the diversity of clinical features and prognosis of patients: acute, lymphoma, chronic and smoldering. The acute form is aggressive with poor prognosis due to multidrug-resistant malignant cells. Chronic and smoldering types have an indolent course and do not require chemotherapy[61]. Thoracic involvement in Adult T-cell leukemia and lymphoma is common, with abnormal imaging findings in two thirds of affected patients. The most common abnormalities on CT include ground-glass opacities and thickening of the peribronchovascular interstitium with predominant peripheral distribution. Pleural effusion and enlarged lymph nodes are less common findings[62] (Figure 10).
Figure 10 Adult T cell leukemia in a 24-year-old male.
Contrast-enhanced computed tomography at the level of the aortic arch (A) and mid-ventricular level (B) demonstrates a large mediastinal mass with extensive pericardial involvement and bilateral pleural effusions.
HIV
Patients with HIV have an increased risk of developing cancer compared to the general population. Defined by the Center for Disease Control (CDC, Atlanta GA, USA), some of the AIDS-defining malignancies include KS, non-HL and invasive cervical cancer. Some of the non-AIDS defining malignancies that patients with HIV have an increased risk of developing include: HL, leukemia, lung cancer, invasive anal carcinoma, and multiple myeloma[63]. An increased risk of primary liver cancer has also been reported[64]. The mechanisms for the development of these malignancies are multifactorial and include a complex interaction between the HIV and other oncogenic viruses (e.g. EBV and HPV), immunosuppression and dysregulation of the immune system and environmental factors[65].
While the incidence of KS has decreased with the advent of HAART, HIV patients continue to be at risk for non-HL[66]. The most common AIDS-related lymphomas among patients include: DLBCL and Burkitt’s lymphoma, representing 90% of all lymphomas. The pathogenesis of these lymphomas include: HIV-induced immunosuppression, chronic antigenic stimulation, genetic abnormalities, cytokine release and dysregulation, dendritic cell impairment, and the role of EBV and HHV8[63].
Compared to the general population with NHL, AIDS patients with NHL tend to have advanced disease and present with B symptoms. The AIDS patients also have extranodal disease including bone marrow involvement and have disease in unusual locations such as body cavities, jaw, rectum, and soft tissues. In addition, patients have frequent plasmacellular differentiation and the disease is commonly associated with EBV and HHV8[63]. The respiratory system is the most common extranodal site (70%) involved in individuals with AIDS-related NHL. Pleural effusion and mediastinal lymphadenopathy are the most common findings on CT (60%), followed by pulmonary nodules in half of them, and lobar opacities or consolidation in one fourth[67]. Cavitation of a lung or mediastinal mass is not an uncommon imaging finding[68,69] (Figure 11).
Figure 11 Non-Hodgkin lymphoma in two different patients with acquired immunodeficiency syndrome.
A: Contrast-enhanced computed tomography (CT) at the level of the AP window demonstrates a large mediastinal mass with areas of cavitation with air-fluid levels (arrows); B: Contrast-enhanced CT reveals a large mediastinal mass with low-density foci consistent with tumoral necrosis. There is small left sided pleural effusion in both cases.
Patients with HIV/AIDS also have an elevated risk for developing lung cancer. The incidence of HIV-related lung cancer has significantly increased, independent of smoking history, after the introduction HAART from 0.8% (pre-HAART) to 6.7% (post-HAART). The majority of these cancers (94%) are non-small cell lung cancers with adenocarcinoma as the most common subtype (34%). Mortality is high (97%) with a median survival of 3 mo[70] (Figure 12).
Figure 12 Contrast enhanced chest CT.
A: Mediastinum window, axial image at the level of the pulmonary arteries demonstrates a soft tissue density mass with air-bronchogram in the right lung. B: Lung window image at the same level shows the spiculated contour of the lung mass.
Marginal zone lymphomas, a subtype of B-cell non-Hodgkin lymphoma has also been associated with bacterial infection (e.g. Helicobacter pylori, Campylobacter jejuni, Borrelia burdoferi, and Chlamydia psittaci) and viral infection (HIV, EBV and hepatitis C virus)[71].
CONCLUSION
A significant number of malignancies, many of which originate or manifest in the thoracic region, are known to be associated with viral infections. The pathophysiology for the development of these neoplastic processes are extremely complex and in some cases not yet completely understood. Multiple variables in addition to the initial viral infection including immunosuppression and dysregulation of the immune system, co-infection with another virus, as well as genetic and environmental factors come into play and interact. CT, with its capability for imaging the lung, mediastinum, pleural and chest wall, remains the most common imaging modality used for the diagnosis, staging and follow-up of these lesions.
Peer reviewer: Patrick K Ha, MD, Assistant Professor, Johns Hopkins Department of Otolaryngology, Johns Hopkins Head and Neck Surgery at GBMC, 1550 Orleans Street, David H Koch Cancer Research Building, Room 5M06, Baltimore, MD 21231, United States
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM