Published online Nov 28, 2011. doi: 10.4329/wjr.v3.i11.266
Revised: September 8, 2011
Accepted: September 15, 2011
Published online: November 28, 2011
AIM: To investigate changes on magnetic resonance imaging (MRI) which occur with intracavitary Gliadel wafer placement in patients with glioblastoma multiforme (GBM).
METHODS: This retrospective Health Insurance Portability and Accountability Act-compliant study was approved by the institutional review board, with a waiver of informed consent. A total of eight patients aged 29-67 years with GBM underwent Gliadel wafer placement. T2-weighted/FLAIR images and post-contrast T1-weighted images both before and after wafer placement were retrospectively reviewed in consensus to determine changes in the following parameters: appearance of the pericavitary tissue, pattern of tumor recurrence or progression and appearance of the Gliadel wafer itself.
RESULTS: Five out of the eight patients had a progressive increase in enhancement and pericavitary T2/ FLAIR hyperintensity within the first 2 mo and a subsequent decrease in these MRI findings. None of these patients had tumor recurrence within the first 6 mo. Three out of the eight patients demonstrated a progressive increase in enhancement and pericavitary T2 hyperintensity, which continued after the first 6 mo, and were subsequently diagnosed with true tumor progression. There was no increase in distant/nonlocal tumor recurrence. The Gliadel wafer appearance changed over time.
CONCLUSION: Pseudoprogression is common after intracavitary Gliadel wafer placement and thus care should be taken before diagnosing tumor progression or recurrence within the first 2 mo.
- Citation: Colen RR, Zinn PO, Hazany S, Do-Dai D, Wu JK, Yao K, Zhu JJ. Magnetic resonance imaging appearance and changes on intracavitary Gliadel wafer placement: A pilot study. World J Radiol 2011; 3(11): 266-272
- URL: https://www.wjgnet.com/1949-8470/full/v3/i11/266.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i11.266
Despite vast efforts in drug development, only limited advancements in malignant glioma therapy have been achieved[1]. Various new chemotherapeutic agents for glioblastoma multiforme (GBM) treatment, including but not limited to alkylating agents, tyrosine kinase inhibitors, and angiogenesis inhibitors, have yielded only minor benefits in patient survival[1] and the standard of care remains surgery, radiation, and concurrent chemotherapy with the alkylating agent temozolomide (TMZ)[2]. For most patients, the prognosis remains dismal with a median overall survival of 14.6 mo[2].
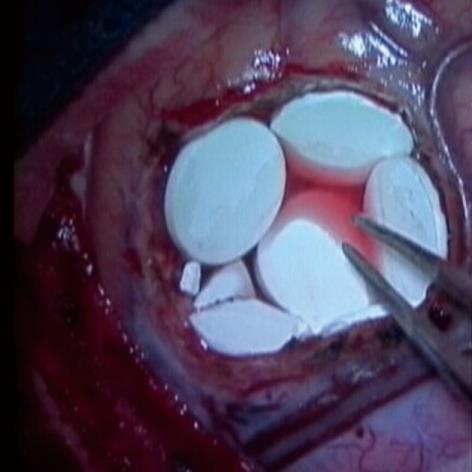
Thus, in addition to standard oral and intravenous chemotherapy, other routes of drug delivery are being explored. One example is the placement of chemotherapeutic wafers in the tumor resection cavity, called Gliadel wafers[3] (Eisai Co. Ltd., Tokyo, Japan). Gliadel wafers are composed of a biodegradable polymer wafer impregnated with 3.85% carmustine [1,3-Bis(2-Chloroethyl)-1-Nitroso-urea, BCNU]. BCNU is a chemotherapeutic agent which alkylates reactive sites on nucleoproteins and interferes with DNA synthesis and repair[4]. In 1996, Gliadel wafers became the first new treatment to be FDA-approved for malignant glioma, specifically recurrent GBM, since the introduction of systemic chemotherapy. It was the first and currently remains the only interstitial chemotherapeutic drug approved for malignant glioma[5]. Subsequently, in 2003, it was approved for primary therapy for GBM as well. During the surgical procedure, right after tumor resection or debulking, up to 8 wafers are deposited along the wall of the resection cavity (Figure 1). The implanted wafers are left in situ, providing a controlled release of BCNU over a period of 2 to 3 wk[6].
However, the introduction of different chemotherapies invariably results in new and different imaging findings both in the peritumoral and pericavitary regions. These pericavitary parenchymal changes can mimic tumor recurrence and progression, referred to as pseudoprogression. Therefore, knowledge of these changes on MR imaging is of utmost importance for proper identification and accurate interpretation. The radiologist (1) must be aware of appearance of the wafers on imaging and, once correctly identified, be able to (2) contextually analyze the associated pericavitary parenchymal changes on the images and provide the appropriate interpretation of those changes.
Despite multiple randomized clinical trials demonstrating a clinical efficacy in both primary[7,8] and recurrent[9] GBM, little has been published regarding the radiological findings of these wafers. Although multiple non-radiological studies have briefly touched on the parenchymal changes and complications seen after intracavitary placement of Gliadel wafers[9-11], to date, no published radiological study exists describing the peritumoral or pericavitary imaging findings. Only two dedicated radiology articles have been published describing the imaging appearance of carmustine wafers itself[12] and a second one additionally describing the pattern of recurrence and evolutionary changes in the surgical bed without particular focus on the pericavitary region[13]. The fact that Gliadel wafers can cause pseudoprogression, mimicking true tumor recurrence or progression, is clinically significant. The phenomenon of pseudoprogression is a well-known treatment-related response known to occur most commonly with concurrent radiation and TMZ administration[14]. However, this has not been described to occur with Gliadel wafers.
Therefore, the purpose of this article is to describe the parenchymal changes seen post- intracavitary Gliadel wafer placement and to provide potential ways to help differentiate Gliadel wafer pseudoprogression changes from true tumor progression. We will also illustrate the appearance of the Gliadel wafer itself on imaging modalities and describe patterns of tumor recurrence and progression.
This retrospective Health Insurance Portability and Accountability Act-compliant study was approved by the institutional review board, with a waiver of informed consent.
We retrospectively identified eight patients (6 males, 2 females; age range: 29-76 years; median age: 56 years) between 2004-2009 whom had undergone intracavitary placement of Gliadel wafers, had histopathologically confirmed GBM, and had undergone pre and post T1-weighted image (T1WI) and T2-weighted image (T2WI) or FLAIR MR imaging. A ninth patient who had undergone Gliadel wafer placement and subsequent removal of the wafer a day later due to immediate post- operative wound infection was excluded.
All patients underwent conventional MR imaging in a 1.5T magnet (GE Healthcare, Milwaukee, Wisconsin) using a standard head coil. Axial spin-echo T1-weighted sequences (TR, 509 ms; TE, 14 ms), before and after intravenous administration of gadopentetate dimeglumine, and axial FLAIR sequences (TR, 11 000 ms; TE, 140 ms; TI, 2600 ms) were obtained in the standard fashion. Of note, it has been shown that both 1.5T and 3T magnetic resonance imaging (MRI) are unable to differentiate between true and pseudoprogression[15]. T2-weighted images were obtained with the following parameters (TR/TE 3500/90).The section thickness for all imaging sequences was 5 mm, with an intersection gap of 0.5 mm, with matrix 512 × 512, FOV 230 × 230. Two patients underwent perfusion imaging. Perfusion-weighted imaging was obtained using T2*-weighted echo-planar imaging sequence with the following parameters: repetition time, 2020 ms; echo time, 40 ms and flip angle 90; field of view, 230 mm × 230 mm; section thickness, 4 mm, gap 0; data matrix, 256 × 256 matrix; and in-plane voxel size, 2.33 mm × 2.33 mm was acquired in 40 dynamic scans every 2 s during the first pass of the contrast agent. Gadopentetate dimeglumine (0.1 mmoL/kg) was injected with a Medrad MR-compatible power injector (Medrad, Pittsburgh, PA) at a rate of 5 mL/s through an 18-gauge intravenous catheter followed immediately by a 20 mL continuous saline flush at 5 mL/s).
Time points for the scans were as follows in all patients as per clinical protocol: preoperative scan, immediate (within 72 h) post-operative scan, second (6 wk) post-operative scan, and subsequent scans every 1-2 mo for up to 1-2 years/until death. Some patients had additional scans between these standard time points.
The T2WI/FLAIR and post-contrast T1WI images were retrospectively reviewed by two neuroradiologists, with 6 (RRC) and 18 (DDD) years of experience, working in consensus. Both were blinded to the histopathological diagnosis of whether there was recurrence or no recurrence, patient’s survival and clinical status, and imaging interpretation reports. The MRI studies before and after Gliadel wafer placement were placed side by side on the same plane on a standard digital workstation and were analyzed accordingly. The first comparison was done between the MRI prior to Gliadel wafer deposition and the baseline post-operative study. All studies subsequent to the baseline MRI post-Gliadel wafer placement were also evaluated and compared to the prior. The following parameters were analyzed and recorded by the neuroradiologists: (1) the appearance of the pericavitary tissue relative to the baseline MRI and subsequently the immediate prior MRI; (2) the pattern of tumor recurrence and progression whether local or non-local; and (3) the appearance of the Gliadel wafer itself.
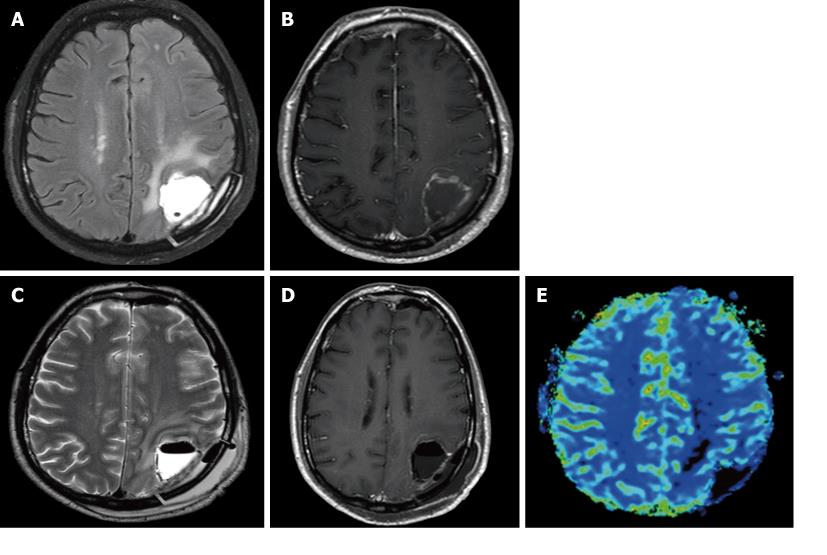
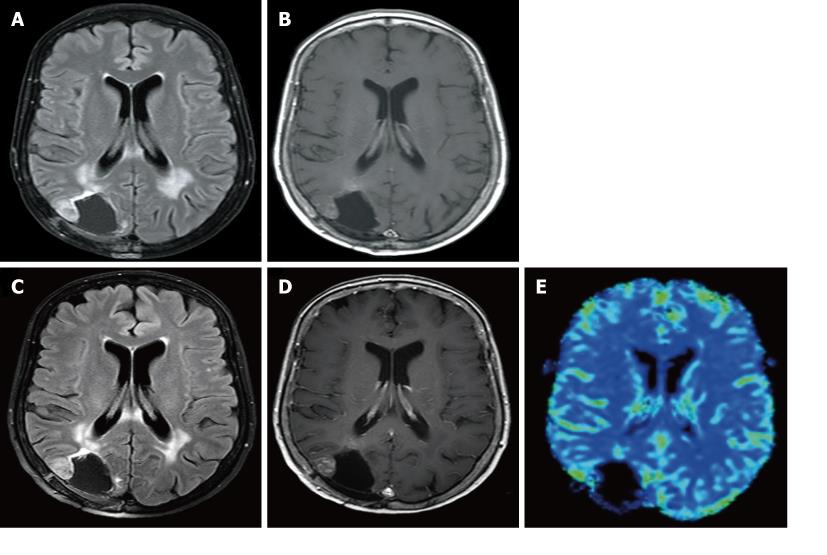
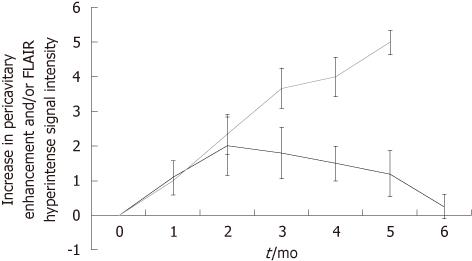
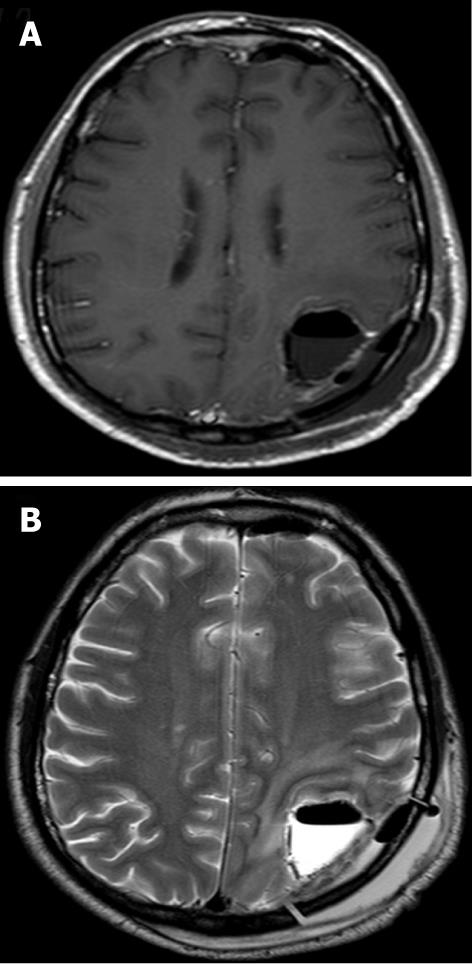
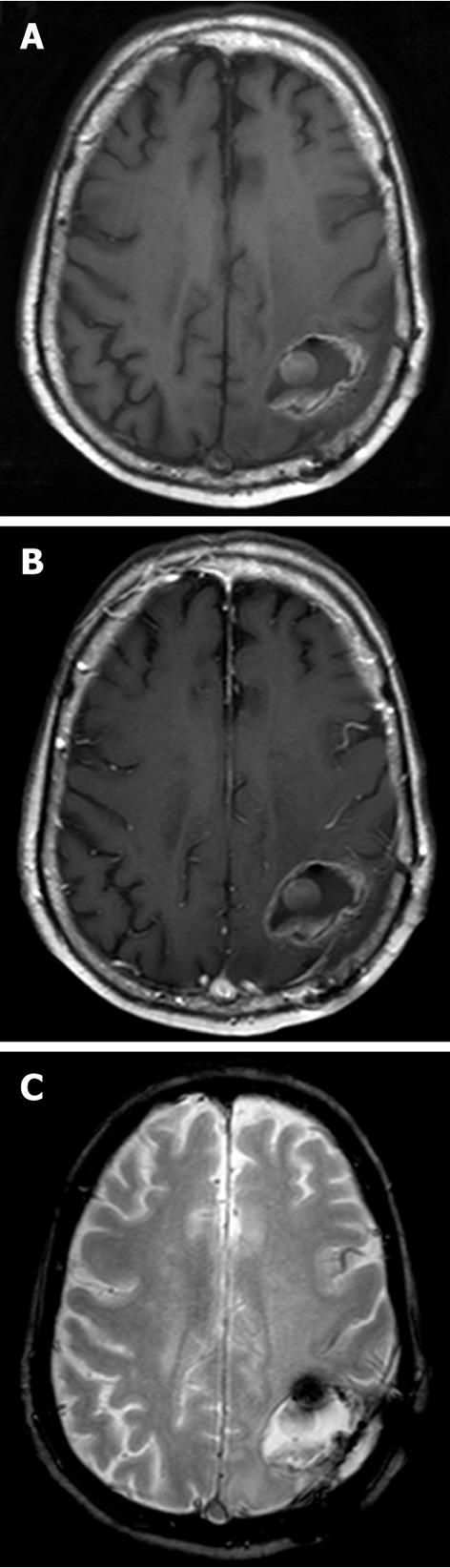
The MR appearance and changes of the pericavitary tissue was assessed and evaluated during the first 6 mo after intracavitary placement of Gliadel wafers. Five out of the eight patients demonstrated a progressive increase in enhancement on post-contrast T1WI and pericavitary hyperintense T2WI/FLAIR signal within the first 2 mo with a subsequent decrease in enhancement and FLAIR hyperintensity at 2 mo time point without a change of therapy (Figure 2). This increase in enhancement was relative to the baseline MRI and subsequently immediate prior comparison MRI examination. Three out of the eight patients demonstrated a non-abating progressive increase in enhancement and pericavitary FLAIR hyperintensity in each subsequent scan when compared to the baseline and immediate prior MRI which continued after the first 2 mo and even after 6 mo (Figure 3). The latter three patients (recurrent group) were subsequently diagnosed with true tumor progression, confirmed by both continued radiographic progression and histopathological analysis subsequent to re-resection. All former 5 patients (non-recurrent group) who showed a decrease in enhancement and FLAIR hyperintensity at 2 mo did not demonstrate true tumor progression or recurrence within the first 6 mo. MR perfusion imaging was performed within the first 6 mo in two patients, from each respective group. MR perfusion, specifically relative cerebral blood volume maps, accurately demonstrated concordance with the subsequent histopathological diagnosis (Figures 2 and 3). Of note, there was no post-operative ischemia seen on the diffusion weighted images (not shown). No patients in the non-recurrent group demonstrated increase in enhancement after 2 mo (Figure 4). All patients in the recurrent group demonstrated a continued increase in enhancement after the 2 mo mark.
Two patterns of tumor recurrence were identified: nonlocal/distant and local, using standard brain tumor criteria for patterns of recurrence[16]. The nonlocal/distant pattern was defined as recurrent tumor at a distance of 1.5 cm or more from the resection cavity site. Local recurrence was defined as tumor occurring within 1.5 cm from the resection cavity site. To assess for pattern of recurrence, the entire spectrum of MRIs for each patient were reviewed. When following the patients over a period of time, for the most part over more than 1.5 years, seven out of the eight patients had recurrence. Of these, two out of seven patients had a nonlocal pattern of recurrence. Six out of seven patients had local recurrence. One patient has not shown any sign of recurrence after more than 5.5 years after initial diagnosis.
When evaluating the appearance of the wafers themselves, three types of phases were defined similar to Prager et al[12]. In the acute phase (less than 7 d post-wafer placement), all wafers were seen as linear hypointense structures on T1WI and T2/FLAIR images relative to the fluid within the resection cavity (Figure 5). In the subacute phase (from 1 wk to 3 mo post-wafer placement), the wafers progressively appeared as a linear T1-hyperintense and T2-hypointense structures (Figure 6). In the chronic phase (after 3 mo post-wafer placement), the wafers were not detectable on imaging due to dissolution. No wafer enhancement was seen in any of the phases.
Our results demonstrate that the placement of Gliadel wafers into the resection cavity may result in a tissue reaction that may mimic tumor progression on MRI, a phenomenon referred to as pseudoprogression. This is evidenced radiologically as an increase in pericavitary enhancement and T2WI/FLAIR hyperintensity within the first 2 mo after wafer deposition and subsequent decrease of these MRI findings. Previous studies have briefly described parenchymal changes on Gliadel wafer placement[9,10]; however, these studies have not established a time point when these changes are most common or expected and when pseudoprogression is unlikely based on timing and ancillary advanced MR techniques such as MR perfusion imaging. Our results demonstrate that continued increase in enhancement after the 2 mo period should be considered as probable tumor progression rather than post Gliadel wafer changes; and, MR perfusion imaging can be helpful to further characterize and differentiate between these entities. It is essential in both clinical practice and clinical trials that radiographic time-to-progression be interpreted cautiously in this setting. The timing of pseudoprogression with Gliadel wafers is concordant with the new Response Assessment in Neurooncology (RANO) criteria which states the progression cannot be diagnosed within the first 3 mo after concurrent chemoradiation therapy unless it is outside the radiation field or there is pathological confirmation of progressive disease[17]. Our results are concordant with the latter definition of pseudoprogression standardized in the RANO criteria for brain tumors. Intracavitary Gliadel wafers can cause pseudoprogression; therefore, recognition is paramount to avoid misdiagnosis of recurrent tumor resulting in an unnecessary second surgery or change in the patient’s chemotherapeutic regimen based on the assumption that this reflects treatment failure. On the other hand, it is important to recognize that when these MRI changes increase or persist beyond 2 mo, these imaging findings are unlikely to represent post-Gliadel wafer changes; and advanced MR techniques can be employed to further characterize and help differentiate recurrent tumor from post-treatment changes.
This study also confirms that there is no increase in nonlocal tumor recurrence. Presumably, given that Gliadel wafers work locally on the pericavitary infiltrated tissue, one can hypothesize that the consequence of local control of disease might be an increase in distant/nonlocal (a distance of 1.5 cm or more from the primary tumor) recurrence of tumor[16]. Our results showed that 29% of Gliadel wafer patients had nonlocal tumor recurrence. Similar findings also resulted from the Giese et al[16] study which demonstrated no statistical difference in the pattern of recurrence between the Gliadel and placebo group; in this study, nonlocal recurrence occurred in 30% of patients in the Gliadel group vs 28% in the placebo group[16].
In our study, the differing patterns of Gliadel wafer appearance depend on their in situ time and are concordant with the literature[12]. In the acute phase (less than 7 d), the wafers appear hypointense on both T1WI and T2WI images. This appearance reflects their hydrophobic composition[12]. In the subacute phase (1 wk to 3 mo), the wafers become more proteinaceous and thus demonstrate hyperintensity on T1WI and hypointensity on T2WI[12]. After 3 mo, the wafers for the most part have completely degraded and thus cannot be seen on imaging. Awareness in local chemotherapy options such as Gliadel wafer placement available for GBM treatment as well as the ability to, independently from medical records, identify these on MRI can help a radiologist to entertain the possibility that an increase in enhancement and T2WI/FLAIR hyperintensity within the first 2 mo post Gliadel wafer deposition might be due to pseudoprogression rather than true tumor recurrence or progression.
This pilot study is the first study dedicated to fully describing the phenomenon of pseudoprogression and the post-treatment changes that occur with Gliadel wafer deposition. This increases the awareness of the radiologist not to potentially misdiagnose these post-treatment changes as true tumor recurrence or progression. However, a prospective study with a larger patient population is needed to confirm our preliminary results and to more precisely determine a time point where post-treatment changes vs tumor progression unmistakably diverge. Furthermore, unlike a retrospective study which might have potential biases regarding imaging times and particular sequences, a prospective study in which standardized imaging protocols and sequences are done can address these limitations and will be helpful to evaluate the role of the MR perfusion and diffusion in diagnosing pseudoprogression. This is expected to lead to a more accurate diagnosis early on during treatment.
In conclusion, pseudoprogression is common after intracavitary placement of Gliadel wafers and should not be automatically interpreted as tumor progression or recurrence within the first 2 mo.
Despite vast efforts in drug development, only limited advancements in malignant glioma therapy have been achieved. Placement of chemotherapeutic wafers in the tumor resection cavity, called Gliadel wafers, have been approved as a therapeutic option for primary and recurrent malignant glioma.
Knowledge of MR imaging of Gliadel wafers is essential for proper identification and accurate interpretation of their treatment effect and resources on this subject are scarces in the literature. The purpose of this article is to describe the parenchymal changes seen post- intracavitary Gliadel wafer placement and to provide potential ways to help differentiate Gliadel wafer pseudoprogression changes from true tumor progression.
Despite multiple randomized clinical trials demonstrating clinical efficacy in both primary and recurrent GBM, little has been published regarding the radiological findings of these wafers. And, although multiple non-radiological studies have briefly touched on the parenchymal changes and complications seen after intracavitary placement of Gliadel wafers, to date, no published radiological study exists describing the peritumoral or pericavitary imaging findings. Only two dedicated radiology articles have been published describing the imaging appearance of carmustine wafers and a second one additionally describes the pattern of recurrence and evolutionary changes in the surgical bed without particular focus on the pericavitary region.
The fact that Gliadel wafers can cause pseudoprogression, mimicking true tumor recurrence or progression, is clinically significant and has not been described to occur with Gliadel wafers. We hope that illustration of the appearance of the Gliadel wafer on imaging modalities and patterns of tumor recurrence and progression, helps radiologists and clinicians make appropriate imaging interpretation and clinical decision.
“Pseudoprogression” is a well-known treatment-related response known to occur most commonly with concurrent radiation and temozolomide administration for treatment of high-grade glioma. Shortly after completion of treatment, there can be an increase in contrast-enhancing lesion size, followed by subsequent improvement or stabilization without any further treatment. This phenomenon, which mimics tumor progression, is termed “pseudoprogression”; a subacute treatment-related reaction commonly without clinical deterioration.
This study appears to be a clinically-relevant exploration of radiological appearance of Gliadel wafers and pericavitary tissue on contrast-enhanced T1, T2-weighted, and perfusion MRI. This report confirms previously described appearance of the wafers and adds pericavitary tissue findings as a novel contribution.
Peer reviewers: Owen Carmichael, PhD, Assistant Professor, Department of Neurology, University of California, Davis, 1544 Newton Court, Davis, CA 95618-4859, United States; Ulf Jensen, MD, Institute of Neuroradiology, University of Schleswig-Holstein, Schittenhelmstr. 10, 24105 Kiel, Germany
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM
| 1. | Quant EC, Wen PY. Novel medical therapeutics in glioblastomas, including targeted molecular therapies, current and future clinical trials. Neuroimaging Clin N Am. 2010;20:425-448. [PubMed] |
| 2. | Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996. [PubMed] |
| 3. | Gliadel wafers for treatment of brain tumors. Med Lett Drugs Ther. 1998;40:92. [PubMed] |
| 4. | Gerosa MA, Rosenblum ML, Stevanoni G, Licata C, Della Corte V, Marcon C, Bricolo A, Tridente G. In vitro analysis of BCNU-sensitivity in human malignant gliomas. II. Cross-resistance studies with cisplatinum and nitrosoureas. Acta Neurol Scand. 1986;73:66-70. [PubMed] |
| 5. | Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien). 2006;148:269-275; discussion 275. [PubMed] |
| 6. | Bota DA, Desjardins A, Quinn JA, Affronti ML, Friedman HS. Interstitial chemotherapy with biodegradable BCNU (Gliadel) wafers in the treatment of malignant gliomas. Ther Clin Risk Manag. 2007;3:707-715. [PubMed] |
| 7. | Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41:44-48; discussion 48-49. [PubMed] |
| 8. | Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jääskeläinen J, Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79-88. [PubMed] |
| 9. | Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008-1012. [PubMed] |
| 10. | Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15:2887-2893. [PubMed] |
| 11. | Weber EL, Goebel EA. Cerebral edema associated with Gliadel wafers: two case studies. Neuro Oncol. 2005;7:84-89. [PubMed] |
| 12. | Prager JM, Grenier Y, Cozzens JW, Chiowanich P, Gorey MT, Meyer JR. Serial CT and MR imaging of carmustine wafers. AJNR Am J Neuroradiol. 2000;21:119-123. [PubMed] |
| 13. | Hammoud DA, Belden CJ, Ho AC, Dal Pan GJ, Herskovits EH, Hilt DC, Brem H, Pomper MG. The surgical bed after BCNU polymer wafer placement for recurrent glioma: serial assessment on CT and MR imaging. AJR Am J Roentgenol. 2003;180:1469-1475. [PubMed] |
| 14. | Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22:633-638. [PubMed] |
| 15. | Kong DS, Kim ST, Kim EH, Lim DH, Kim WS, Suh YL, Lee JI, Park K, Kim JH, Nam DH. Diagnostic dilemma of pseudoprogression in the treatment of newly diagnosed glioblastomas: the role of assessing relative cerebral blood flow volume and oxygen-6-methylguanine-DNA methyltransferase promoter methylation status. AJNR Am J Neuroradiol. 2011;32:382-387. [PubMed] |
| 16. | Giese A, Kucinski T, Knopp U, Goldbrunner R, Hamel W, Mehdorn HM, Tonn JC, Hilt D, Westphal M. Pattern of recurrence following local chemotherapy with biodegradable carmustine (BCNU) implants in patients with glioblastoma. J Neurooncol. 2004;66:351-360. [PubMed] |
| 17. | Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963-1972. [PubMed] |