Published online Sep 28, 2010. doi: 10.4329/wjr.v2.i9.368
Revised: August 19, 2010
Accepted: August 26, 2010
Published online: September 28, 2010
AIM: To assess retrospectively the significance of accessory spleen-like mass (ASLM) in oncology patients undergoing positron emission tomography/computed tomography (PET/CT).
METHODS: The results of PET/CT of 913 patients (278 lymphoma; 635 solid tumors) were reviewed. The number, size, location and attenuation of all ASLMs, and spleen attenuation, were recorded. ASLM fluorodeoxyglucose uptake was graded as normal (less than or equal to that in the liver) or representative of malignancy (more than in the liver). Follow-up PET/CT in patients with ASLM was reviewed when available. ASLM size and attenuation for spleen and ASLM were compared by unpaired Student’s t test. The χ2 and Fisher’s exact tests were used to compare ASLM frequency and uptake for lymphomatous and solid tumors, respectively.
RESULTS: ASLM frequency was 14.8%, with 152 ASLMs found in 135 patients. Mean attenuation was lower in ASLM compared with spleen by enhanced and non-enhanced CT (80.7 ± 20.4 HU vs 92.0 ± 14.4 HU, P < 0.0011 and 42.3 ± 9.0 HU vs 51.5 ± 6.3 HU, P < 0.0001, respectively). ASLM incidence was higher in lymphoma patients (56/278, 20.1%) than in those with solid tumors (56/278, 20.1% vs 79/635, 12.4%, P = 0.0036). Pathological uptake was found in four (7.1%) lymphoma patients but not in any patients with a solid tumor (P = 0.028) and it upstaged one patient with lymphoma. Follow-up PET/CT within 3-16 mo was available in 54% of patients with ASLM. Lesion regression was noted in all four pathological ASLMs on follow-up PET/CT after chemotherapy.
CONCLUSION: In patients with lymphoma, ASLM can represent malignancy, and thus further characterization with PET/CT might be warranted. Patients with neoplasia other than ASLM can be confidently diagnosed with accessory spleen.
- Citation: Groshar D, Bernstine H, Goldberg N, Stern D, Sosna J. Accessory spleen-like masses in oncology patients: Are they always benign? World J Radiol 2010; 2(9): 368-373
- URL: https://www.wjgnet.com/1949-8470/full/v2/i9/368.htm
- DOI: https://dx.doi.org/10.4329/wjr.v2.i9.368
Accessory spleen (AS) is a congenital focus of healthy splenic tissue that is separated from the main body of the spleen. It is a frequently normal variant that is found in up to 30% of cases at autopsy examination[1,2]. Most AS is asymptomatic and discovered incidentally during unrelated investigations[3]. The spleen is the largest single lymphatic organ in the body. AS can resemble a lymph node, both on computed tomography (CT) and macroscopically[3,4]. In a recent large study, AS was found in 15.6% of patients from a general population who were undergoing contrast-enhanced abdominal CT. They were recognized to have a distinct appearance on CT[5]. Most appeared as a well-marginated, round, oval or triangular mass, smaller than 2 cm, with attenuation similar to that of splenic tissue, and homogeneous enhancement on contrast-enhanced images[5]. Awareness of the characteristic CT appearance of AS is important for the radiologist to interpret CT studies correctly and avoid mistaking AS for a clinically significant abnormality[3,5].
The presence of AS might pose a problem in cancer patients who are evaluated by CT. AS can increase the suspicion of an enlarged lymph node in lymphoma patients, as well as for a solid tumor in the adrenal gland, pancreas, stomach or intestine, and even in the testes[6-9]. However in the most common location, near the hilum of the spleen or adjacent to the tail of the pancreas, a pathological lymph node or tumor can be mistaken as AS[5,10,11].
Combined positron emission tomography/CT (PET/CT) with 2-[18F] fluoro-2-deoxy-D-glucose (FDG) is increasingly used for staging, restaging, and treatment monitoring in cancer patients. PET allows the detection of increased metabolic activity in tissue that can appear morphologically normal at CT[12], and therefore distinguish a malignant tumor from AS.
We retrospectively assessed the frequency and appearance of accessory spleen-like masses (ASLMs) in a large cohort of oncological patients who were referred for PET/CT. The combined value of FDG uptake and CT for differentiation of AS from tumor resembling AS was assessed.
We retrospectively reviewed PET/CT performed in two separate medical centers during a 3-mo period (February-April 2007). Institutional ethics review board approval was obtained from both medical centers, and informed consent was waived. Databases of all PET/CT examinations performed in both centers were evaluated, and 915 consecutive patients with histopathologically proved malignancy in various stages of medical treatment were identified. Two patients were excluded due to previous splenectomy. The study included 913 patients (519 female, 394 male; mean age 61 years, range 11-89 years). Primary cancers included 278 lymphomas and 635 solid tumors, with colorectal, breast, and lung cancers being most common (Table 1).
| Tumor type | Patients | ASLM |
| Lymphoma | 278 (30.4) | 56 (41.5) |
| Colorectal | 167 (18.3) | 26 (19.3) |
| Breast | 129 (14.1) | 16 (11.8) |
| Lung | 109 (11.9) | 19 (14.1) |
| Gynecological | 68 (7.4) | 10 (7.4) |
| Melanoma | 40 (4.4) | 1 (0.7) |
| Stomach | 33 (3.6) | 0 (0) |
| Genitourinary | 14 (1.5) | 1 (0.7) |
| Others1 | 75 (8.4) | 6 (4.4) |
| Total | 913 (100) | 135 (100) |
All studies were performed using an integrated PET/CT scanner (Discovery ST; GE Medical Systems, Milwaukee WI; or Gemini GXL; Philips Medical Systems, Cleveland, OH, USA). Preparation for PET/CT examination was similar in both centers and included fasting for at least 4 h before FDG administration. Patients were required to drink oral contrast medium (1 L of water with 30 mL Telebrix Gastro and ioxitalamate in a concentration of 300 mgI/mL; Guerbet, Roissy CDG, France). FDG dose varied from 370 to 666 MBq (10-18 mCi) according to patient weight, and was injected at 45-60 min before acquisition of emission images (PET).
Parameters for CT image acquisition were as follows: for Discovery PET/CT, spiral CT at 0.8 s per rotation with 100-300 mAs, 120 kVp, section thickness of 3.75 mm, and 3.75 mm interval. Intravenous contrast material was not administered. PET images were obtained using a weight-based protocol, with 3-4 min acquisition time per bed position. All PET images were reconstructed using an iterative algorithm, with CT-based attenuation correction applied. For Gemini PET/CT, CT parameters were as follows: 0.5 s per rotation with 80-200 mAs, 120 kVp, section thickness of 5 mm, and 2.5 mm interval. Iodine contrast media (Ultravist 300; iopromide 0.623 g/mL; Bayer Schering Pharma AG, Berlin, Germany; in a volume of 1.5 mL/kg) was intravenously administered. Patients with iodine allergy or chronic renal failure, and those who refused contrast, were examined with non-enhanced CT. Immediately after CT, a PET scan was performed. Acquisition time for the emission was 3-4 min per bed position with a one-section overlap. Six to eight bed positions from skull base to mid-thigh resulted in an acquisition time of 18-24 min. CT data were used for attenuation correction. Images were reconstructed using a standard iterative algorithm. Besides the use of intravenous contrast, there was no difference in the PET/CT techniques between the two centers.
All CT, PET and fused PET/CT images were retrospectively examined. An ASLM was defined as a distinct well-marginated, round mass near to the spleen. Axial, coronal, sagittal, and maximum intensity projections were viewed with AW-4.2 Workstation software (GE Medical Systems). The total number of ASLMs per patient, size (in the biggest dimension), location and CT attenuation of the spleen and the ASLM were recorded. Locations of the ASLMs were defined within discrete anatomical areas at the left upper quadrant. The spleen was divided craniocaudally into the upper (above the hilum), middle (hilum level) and lower third (below the hilum). On axial images, the space in front of the spleen was defined as anteromedial or anterolateral, and the space behind the spleen as posteromedial or posterolateral.
Visual analysis of FDG uptake in ASLM was assessed and graded from normal, defined as uptake less than or equal to uptake in the liver, to pathological, defined as intense uptake higher than that of the liver. ASLM with normal uptake was considered as an AS, whereas pathological uptake indicated malignant involvement of the ASLM[13]. Follow-up PET/CT was reviewed for patients with ASLM. ASLM was evaluated for stability or regression in size and uptake.
Values are expressed as mean ± SD (range). Size and attenuation measurements obtained by CT for the spleen and ASLM were compared using the unpaired Student’s t test. The χ2 test was used for comparison of ASLM frequency in patients with lymphomatous vs solid tumors. Fisher’s exact test was used to compare the frequency of pathological ASLM in lymphomatous disease vs solid types of cancer. P < 0.05 was considered statistically significant.
The frequency of ASLM was 14.8%, with 152 ASLMs found in 135 of 913 patients. ASLMs were found in 76 female and 59 male patients with a mean age of 59.8 years (range: 11-89 years) (Table 1). In 18 patients, more than one ASLM was found, with a maximum of four ASLMs in a single patient. All ASLMs were round and well-marginated, with a mean size of 1.2 ± 0.46 cm (range: 0.5-2.9 cm). Only 13 (8.8%) were ≥ 2.0 cm. The most common locations were anteromedial in the lower third (28.9%), anteromedial in the mid-third (19.7%), and anteromedial in the upper third (16.9%).
Mean attenuation for ASLMs was 80.7 ± 20.4 HU (range: 19.0-114.0 HU) in 55 contrast-enhanced CT examinations, and 42.3 ± 9.0 HU (range: 19.3-56.7 HU) in 80 non-enhanced CT studies. Mean attenuation in the spleen was higher both in contrast-enhanced (92.0 ± 14.4 HU, range: 46.0-127.0 HU, P < 0.0011) and non-enhanced CT (51.5 ± 6.3 HU, range: 39-91 HU, P < 0.0001) examinations, with the difference being statistically significant.
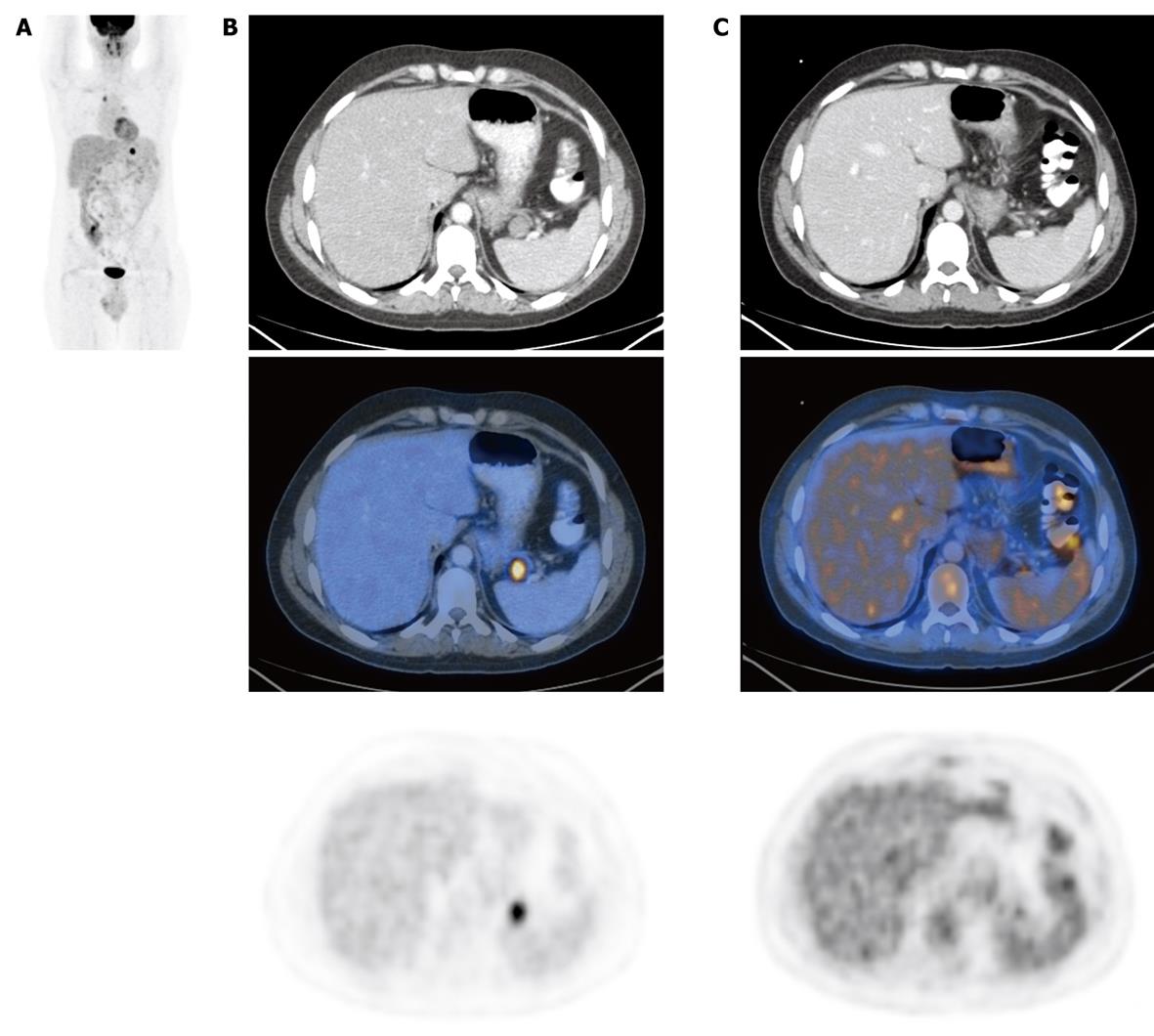
The incidence of ASLM in lymphomatous patients (56 out of 278) was significantly higher than in patients with solid tumors (79 out of 635) (20.1% and 12.4% respectively, P = 0.0036). Intense pathological uptake of FDG in an ASLM was found in four out of 56 (7.1%) lymphomatous patients and in none of the 79 patients with solid tumors (P = 0.028). In one patient, the finding of a positive ASLM changed the management of the disease by upstaging from stage 1 to stage 3 (Figure 1).
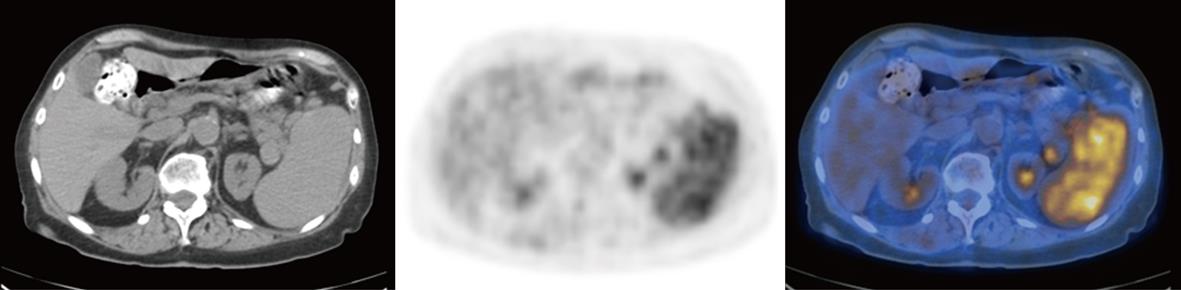
Additional interval PET/CT studies from 3 to 16 mo were obtained in 73 out of 135 (54%) patients with ASLM. In 69 patients (36 with solid tumors and 33 lymphomatous) without FDG uptake, there was no change in shape or size in the follow-up study, which confirmed the ASLM as an AS. All four lymphomatous patients with pathological FDG uptake in ASLM had regression of the disease demonstrated by PET/CT obtained after chemotherapy. In three patients, the ASLM vanished, which strongly suggested a diagnosis of pathological lymph node mimicking an AS. In one additional patient, there was intense pathological uptake of FDG in both the spleen and the ASLM. Lymphomatous involvement of the AS and the spleen was confirmed by stability of the size and location of the ASLM in subsequent PET/CT after chemotherapy, but without FDG uptake (Figure 2).
Three pathologic ASLMs were located anteromedially in the lower third area and one posterolaterally in the mid-third area. The mean size of the pathological ASLMs was larger than the normal ASLMs (1.8 ± 0.19 cm and 1.2 ± 0.46 cm respectively, P < 0.001), with the difference being statistically significant.
We found 152 round masses in the left upper quadrant with CT characteristics suggestive of an AS, in 135 out of 913 (14.8%) oncology patients referred for PET/CT. This is similar to the frequency (15.6%) of AS reported in a large general population referred for contrast-enhanced CT[5]. The significantly higher incidence of ASLM in lymphoma patients (20.1%) vs incidence in patients with solid tumors (12.4%) (P = 0.0036) was an unexpected finding. The higher incidence of ASLM in lymphomatous patients could be explained by lymphatic tissue activation, which might lead to an increase in the size of microscopic ASs previously not seen on conventional imaging. Furthermore, the incidence of pathological ASLM was significantly greater in lymphomatous than in solid tumor patients. ASLMs found in four out of 56 (7.1%) lymphoma patients demonstrated pathologically increased metabolic activity by intense FDG uptake, and changed the management in one patient. No pathological FDG uptake was seen in 79 ASLMs found in patients with solid tumors. By comparing PET/CT findings with previous examinations in the four lymphoma patients, we concluded that an AS was involved with lymphoma in one case, whereas three cases were found to be pathological lymph nodes mimicking a normal AS on CT.
The mean size of ASLMs with normal FDG uptake was significantly smaller than that of the pathological ASLMs. ASLM < 1.5 cm was always benign; however, the mean size of both normal and pathological ASLMs was < 2 cm. The most common anatomical location for ASs was in the anteromedial lower third, as shown by Mortele et al[5]. The most common anatomical location of our small number of pathological ASLMs was also in the anteromedial lower third.
The combined PET/CT study provides important information that is not given by either modality alone. PET detects increased metabolic activity of glucose in tissue that can appear morphologically normal with CT, and therefore might be useful to differentiate benign from malignant lesions[12]. CT provides superior contrast and spatial resolution, with precise anatomical localization and attenuation measurements, but lacks functional information other than nonspecific contrast enhancement and washout. It has been proposed that ASs might be discriminated from pathological lymph nodes or a tumor in the splenic hilum when they enhance on CT to the same degree as the spleen[3,4]. In our study, there was a statistically significant difference in CT attenuation measurements for ASs and the spleen itself, both with and without contrast injection. This low attenuation in ASs has also been reported by Mortele et al[5], and they have suggested that partial volume effects can contribute to this apparently low attenuation because of the small size of these structures. Although the ASLMs have lower density, this is not a reliable sign of malignancy. All four pathological ASLMs detected by PET/CT had attenuation measurements similar to the ASs, and could be at first mistakenly interpreted as an AS based on the characteristic CT findings.
Other imaging techniques like magnetic resonance imaging (MRI) and ultrasound (US) cannot distinguish with high confidence between an AS and a pathological lymph node. The US echogenicity and MRI signal intensity and enhancement of the spleen on images obtained with various pulse sequences are determined by the high blood content, and the neoplastic tissue can resemble normal parenchyma[14-17]. Functional imaging with Tc-99m-labeled sulfur colloid, or Tc-99m-labeled, heat-denatured autologous red blood cells, has been shown to be useful in detecting splenic tissue and confirming the presence of an accessory or ectopic spleen[1,7,18].
The findings of our study could have clinical implications. Although PET/CT with FDG offers higher accuracy and sensitivity than PET or CT alone for lymphoma detection, the role of PET/CT for routine evaluation of lymphoma patients is still being defined[19,20]. Also, PET/CT is not available everywhere and CT is still usually performed in these patients. In lymphoma patients with ASLM on CT, further functional or metabolic imaging might be suggested to distinguish an AS from a pathological lymph node. In patients with other types of neoplasia, ASLM could be diagnosed as an AS.
Our study had some limitations. Low-grade lymphomas and some solid tumors might have low FDG activity, and thus FDG PET/CT could underestimate the real incidence of pathological ASLM. Functional imaging with radiocolloid or heat-denatured autologous red blood cells was not performed to confirm the presence of splenic tissue in all ASs. However, ASs were confirmed by sequential PET/CT imaging in more than half of the patients, and the lack of FDG uptake in the ASs was highly suggestive of a normal structure rather than a tumor. We combined data from two medical centers. Although the techniques varied with regard to intravenous use of iodine, all other portions of the PET/CT examinations were similar.
In conclusion, ASLM frequency in oncology patients is similar to the frequency published in the general population. It was higher in lymphomatous patients, of which about 7% showed malignant involvement. In lymphoma patients, awareness of an ASLM is important for the radiologist to interpret the CT findings correctly and raise the possible need to perform further functional or metabolic studies to avoid mistaking AS for a pathological mass. In patients with other types of neoplasia, ASLM can be confidently diagnosed as an AS.
Accessory spleen (AS) is a congenital focus of healthy splenic tissue that is separated from the main body of the spleen. It is a frequently normal variant found in up to 30% of cases at autopsy examination. Most ASs are asymptomatic, and discovered incidentally during unrelated investigations. The presence of an AS might pose a problem in cancer patients evaluated by computed tomography (CT). Combined positron emission tomography/CT (PET/CT) with 2-[18F] fluoro-2-deoxy-D-glucose (FDG) is increasingly used for staging, restaging, and treatment monitoring in cancer patients. PET allows the detection of increased metabolic activity in tissue that can appear morphologically normal at CT, and therefore, distinguish a malignant tumor from AS.
An assessment of the frequency and appearance of accessory spleen-like masses (ASLMs) in a large cohort of oncology patients referred for PET/CT. The combined value of FDG uptake and CT examinations for differentiation of AS from tumors that resemble AS was assessed.
In lymphoma patients, awareness of ASLMs is important for the radiologist to interpret the CT findings correctly, and raise the possible need to perform further functional or metabolic studies to avoid mistaking AS for a pathological mass. In patients with other types of neoplasia, ASLMs can be confidently diagnosed as AS.
An ASLM in an oncology patient can be confidently diagnosed as a splenule in non-lymphoma patients. In lymphoma patients PET/CT might be warranted for further characterization.
An ASLM is defined as a distinct well-marginated, round mass near the spleen.
This paper is very interesting and should be published because PET/CT of spleen-like masses could be an important subject in clinical routine work. Some improvements should be done.
Peer reviewer: Silvia Obenauer, MD, Associate Professor of Radiology, Department of Radiology, Heinrich.Heine University Duesseldorf, Moorenstr. 5, 40225 Düsseldorf, Germany
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
| 1. | Dodds WJ, Taylor AJ, Erickson SJ, Stewart ET, Lawson TL. Radiologic imaging of splenic anomalies. AJR Am J Roentgenol. 1990;155:805-810. |
| 2. | Freeman JL, Jafri SZ, Roberts JL, Mezwa DG, Shirkhoda A. CT of congenital and acquired abnormalities of the spleen. Radiographics. 1993;13:597-610. |
| 3. | Gayer G, Zissin R, Apter S, Atar E, Portnoy O, Itzchak Y. CT findings in congenital anomalies of the spleen. Br J Radiol. 2001;74:767-772. |
| 4. | Moore KL, Dally AF. Abdomen. Clinically oriented anatomy. 4th ed. Philadelphia: Lippincott Williams & Wilkins 1999; 175-350. |
| 5. | Mortelé KJ, Mortelé B, Silverman SG. CT features of the accessory spleen. AJR Am J Roentgenol. 2004;183:1653-1657. |
| 6. | Hayward I, Mindelzun RE, Jeffrey RB. Intrapancreatic accessory spleen mimicking pancreatic mass on CT. J Comput Assist Tomogr. 1992;16:984-985. |
| 7. | Ota T, Kusaka S, Mizuno M. A splenic pseudotumor: an accessory spleen. Ann Nucl Med. 2003;17:159-160. |
| 8. | Stiris MG. Accessory spleen versus left adrenal tumor: computed tomographic and abdominal angiographic evaluation. J Comput Assist Tomogr. 1980;4:543-544. |
| 9. | Tsuchiya N, Sato K, Shimoda N, Satoh S, Habuchi T, Ogawa O, Kato T. An accessory spleen mimicking a nonfunctional adrenal tumor: a potential pitfall in the diagnosis of a left adrenal tumor. Urol Int. 2000;65:226-228. |
| 10. | Harris GN, Kase DJ, Bradnock H, Mckinley MJ. Accessory spleen causing a mass in the tail of the pancreas: MR imaging findings. AJR Am J Roentgenol. 1994;163:1120-1121. |
| 11. | Robertson F, Leander P, Ekberg O. Radiology of the spleen. Eur Radiol. 2001;11:80-95. |
| 13. | Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, Wiseman GA, Kostakoglu L, Scheidhauer K, Buck A. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571-578. |
| 14. | Burgener FA, Tan RK, Meyers SP, Zaunbauer W. Differential diagnosis in magnetic resonance imaging. New York: Thieme 2002; . |
| 15. | Elsayes KM, Narra VR, Mukundan G, Lewis JS Jr, Menias CO, Heiken JP. MR imaging of the spleen: spectrum of abnormalities. Radiographics. 2005;25:967-982. |
| 16. | Gayer G, Hertz M, Strauss S, Zissin R. Congenital anomalies of the spleen. Semin Ultrasound CT MR. 2006;27:358-369. |
| 17. | Hahn PF, Stark DD, Glastad K. Biliary system, pancreas, spleen, and alimentary tract. Magnetic resonance imaging. 2nd ed. St. Louis: Mosby 1992; 1769-1853. |
| 18. | Valdés Olmos RA, Horenblas S, Kartachova M, Hoefnagel CA, Sivro F, Baars PC. 99mTc-labelled heat-denatured erythrocyte SPET-CT matching to differentiate accessory spleen from tumour recurrence. Eur J Nucl Med Mol Imaging. 2004;31:150. |
| 19. | Jhanwar YS, Straus DJ. The role of PET in lymphoma. J Nucl Med. 2006;47:1326-1334. |
| 20. | Kirby AM, Mikhaeel NG. The role of FDG PET in the management of lymphoma: what is the evidence base? Nucl Med Commun. 2007;28:335-354. |