HISTORY AND DEFINITION
“Dural tail sign” (DTS), “dural thickening”, “flare sign”, “meningeal sign” are similar terms describing thickening of the dura adjacent to an intracranial neoplasm on contrast-enhanced T1 MR images (Figures 1 and 2). Although this sign has been used in spinal meningioma in the literature, we only use it for intracranial lesions[1]. Nowadays the term DTS is frequently used as in this article. The above-mentioned terms were first described in meningioma by Wilms et al[2] in 1989. In 1990 the triple criteria for DTS were established by Goldsher et al[3] as: (1) Presence of at least two consecutive sections through the tumor at the same site in more than one imaging plane; (2) Greatest thickness adjacent to the tumor and tapering away from it; and (3) Enhancement more intense than that of the tumor itself.
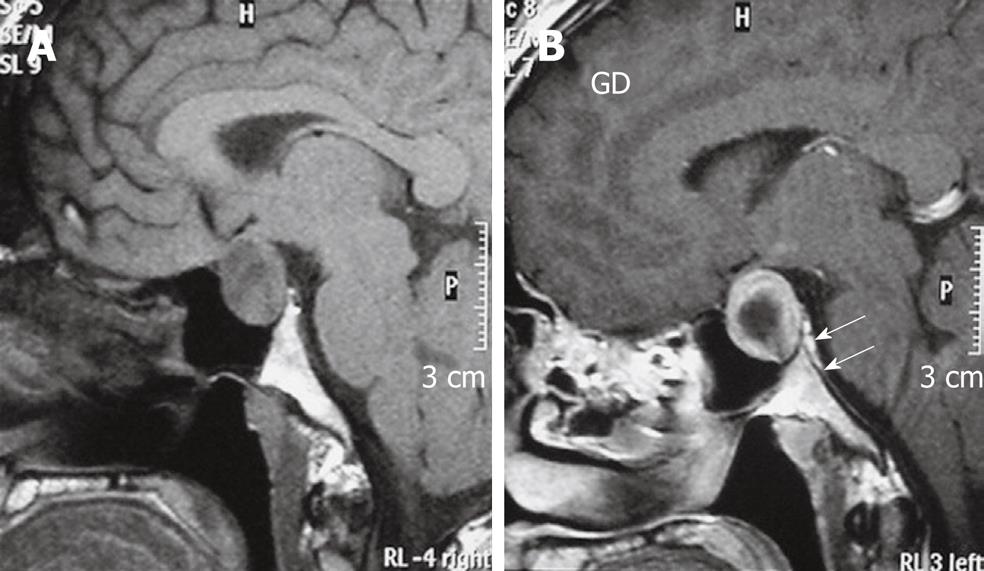
Figure 1 A 65-year-old woman with meningioma and adjacent hyperostosis.
Arrows indicates “dural tail sign”.
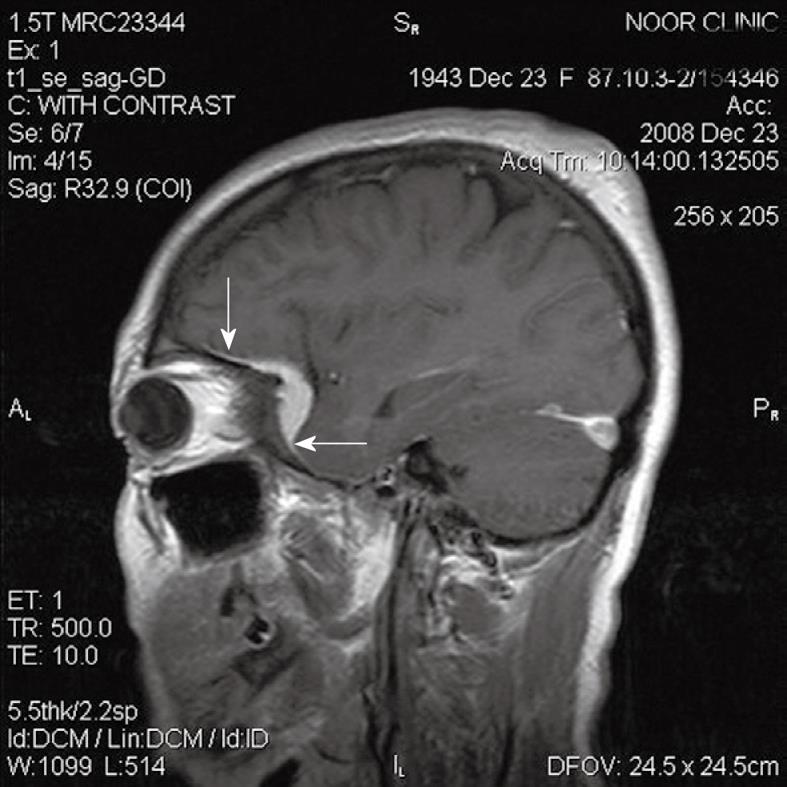
Figure 2 Imaging findings of pituitary macroadenoma.
A: T1W sagittal MRI of brain shows a Pituitary macro-adenoma with extension to the suprasellar cistern; B: After gadolinium injection enhancement is noted in periphery of tumour and dura of dorsum sella (arrows), enhancement is greater than that of the tumour itself. Biopsy proved prolactinoma.
The criteria established by Goldsher et al[3] are still the most useful in describing DTS. Nowadays, as imaging slices tend to be less than 5 mm, there should always be at least three sections showing the dural tail, depending on the slice thickness[4].
No equivalent sign has been described in post-contrast CT scans. Post-contrast CT can show dural thickening in 8% of MR proven DTS[3,5].
Takeguchi et al[6] evaluated the ‘‘dural tail’’ associated with 48 intracranial meningiomas on fluid-attenuated inversion-recovery (FLAIR) and contrast-enhanced T1-weighted images. They noted that the DTS, which was identified on contrast-enhanced magnetic resonance imaging (MRI), was also observed in all the cases of DTS on FLAIR images, and concluded that FLAIR imaging is useful for showing dural abnormalities associated with meningiomas without the need for contrast medium.
DTS PATHOLOGIES AND THEIR DIFFERENTIAL DIAGNOSIS
At the time of DTS description and criteria formation, DTS was thought to be pathognomonic of meningioma, however, many subsequent studies also demonstrated this sign adjacent to various intra- and extra-cranial pathologies as well as spinal lesions.
So far, DTS has been reported in primary and secondary central nervous system (CNS) lymphoma, chloroma, metastasis (extra- and intra-axial), multiple myeloma, glioblastoma multiforme (GBM), aspergillosis, chordoma, schwannoma, pleomorphic xanthoastrocytoma (PMX), hemangiopericytoma, Wegener’s granulomatosis, sarcoidosis, medulloblastoma, eosinophilic granuloma, pituitary adenoma, pituitary apoplexy, and Erdheim-Chester disease[7-24]. DTS can be seen in all locations of the dura adjacent to meningioma in the falx, tentorium and cerebral convexities[3,25]. DTS is less frequently seen in posterior fossa and cystic meningioma[3]. Meningioma in the cerebellopontine (CPA) can show DTS extending to the internal auditory canal and can be mistaken for an acoustic neurinoma[26]. CPA meningiomas sometimes show the DTS but with less enhancement than the primary tumor itself. Previous studies indicated that the DTS has a sensitivity of 58.6% and a specificity of 94.02% for the diagnosis of meningioma[5]. Primary CNS lymphoma is most frequently caused by B cell lymphoma and can be dural-based and can be mistaken for meningioma because of homogenous enhancement and typical DTS. Hodgkin lymphoma is a very rare tumor and can mimic meningioma in T1 enhanced MRI because of a dural-based lesion and DTS[6,8,27,28]. Dural-based metastasis and cortical intraparenchymal metastasis can show DTS. DTS is most often seen in prostate and neuroblastoma metastasis but has been reported in papillary adenocarcinoma, bronchogenic tumors and nasopharynx neoplasms[8,29].
GBM as one of the most frequent intraparenchymal tumors, can extend to the dura and rarely shows dural thickening and DTS. So far no case with tumoral involvement of DTS has been reported in patients suffering from GBM with DTS[8,30].
Adenoid cystic carcinoma, a neoplasm arising from the salivary glands can involve intracranial structures by perineural spread. Dural involvement and DTS have also been reported in this neoplasm[4,10].
Chloroma can mimic meningioma because of dural mass and typical DTS[31]. Hemangiopericytoma can mimic meningioma with positive DTS on imaging but its aggressive behavior, loss of calcification and heterogenous enhancement can differentiate it from meningioma[11].
Intracranial chordoma is seen more often in sphenoidal and occipital regions. DTS has been rarely reported in intracranial chordoma[13].
An acoustic neuroma arises from the vestibular portion of the 8th cranial nerve and can locate in the acoustic canal, CP angle or both. CPA acoustic neuroma can mimic meningioma as an extra-axial mass lesion with positive DTS, but its hypersignal intensity in T2 sequence and narrower dural attachment can differentiate this tumor from meningioma[14,32,33].
Intraparenchymal schwannoma is a relatively uncommon neoplasm and DTS has been reported in one case of intraparenchymal schwannoma[34].
PMX, which is more often seen in cortical regions, typically contains a prominent cystic component and a mural nodule, and sometimes involves the adjacent dura and shows positive DTS but its cystic component, mural nodule and T2 signal should differentiate it from meningioma[15,35].
Wegner’s granulomatosis can involve the dura and intracranial structures and presents with positive DTS adjacent to the PNS sinuses[17,36].
Erdheim-Chester disease, a histiocytic granulomatosis with both intra- and extra-axial structures involvement, has been reported to show positive DTS in post-contrast T1. DTS has also been reported in other granulomatosis diseases (eosinophilic granulomatosis and sarcoidosis)[18,24,37].
Although most authors believe that the DTS adjacent to a sellar mass suggests meningioma, dural thickenings and positive DTS can be seen adjacent to pituitary adenomas in 30% of cases. This sign mostly extends into the planum sphenoidale and carotid sulcus. DTS can also be detected adjacent to pituitary apoplexy[13,19,21,38]. The pituitary gland can be involved in an inflammatory process (hypophysitis). Hypophysitis is rarely reported to present with positive DTS[20].
Gumma, an inflammatory involvement of CNS structures in tertiary syphilis, is seen as an intra-axial peripherally located mass lesion, however, extra-axial mass lesions have been reported and can show DTS mimicking meningioma in these lesions[7,39].
Cavernous hemangioma rarely arises from the dura mater, causing adjacent dural thickening and DTS. Dural cavernous hemangioma mostly involves the sinus cavernous dura[23].
DTS has also been reported adjacent to a posterior cerebral artery aneurysm and in one case of post-operative cerebral aspergillosis[10,22].
Positive DTS has been reported in three cases of medulloblastoma[40,41], two cases of multiple myeloma presenting with extra-axial dural tumors[42,43], one case of primary rhabdomyosarcoma[44], one case of solitary fibrous tumor[45] and one case of papillary middle ear tumor with dural invasion[7].
PATHOPHYSIOLOGY
In contrast to the worldwide accepted diagnostic criteria of DTS, its pathophysiology has not been uniformly established.
It should be noted that DTS is not always due to enhancement of the dura adjacent to tumors. Kuroiwa et al[46] reported a glioma extending into the subarachnoid space, and a meningioma extending to the subdural space and their MRI appearances mimicked the DTS[46].
Wilms et al[2] first reported that thickening of the dura mater represented neoplastic infiltration in or on the surface of the dura in three cases of meningioma with DTS, and described the DTS as indicating tumor invasion. On the other hand, Tokumaru et al[47] found only increased loose connective tissue, hypervascularity and dilated vessels on histologic examination of the enhanced meninges adjacent to four meningiomas. Although two patients showed tumor cell infiltration of the dura mater in this study, tumor cell infiltration was restricted to within 1 mm of the junction of the dura with the meningioma, therefore it was suggested that the dural tail mainly represented reactive changes to the meningioma and not necessarily neoplastic involvement. Subsequently several other histologic studies of the dural tail were described as reactive changes[8,25,26]. Kawahara et al[48] examined the point of attachment of the tumor and the adjacent dura mater in seven patients with DTS and suggested the pathophysiology of the DTS as follow; “initially tumour cells invade vessels and pack them at the point of tumour attachment; then, vessel congestion is induced in the adjacent dura mater, as a result of which it enhances markedly, giving rise to the DTS”. In a histological study on 17 patients with DTS, Rokni-Yazdi et al[49] reported that vascular dilatation was seen in all dural tails which is similar to that of Kawahara et al[48]; however, unlike Kawahara’s study, tumoral invasion of the dura at its point of attachment to the tumor was only reported in 34.1% of cases, so 65.9% of cases did not have invasion and packing of vessels at the point of dural attachment (Figure 3). This was against the Kawahara et al[48] hypothesis regarding the etiology of DTS in all cases; although it may explain the etiology of DTS in those cases with invasion of the dura beneath the tumor. We suggest that invasion of dural vessels by tumor cells and packing at the point of tumor attachment, reactive hypervascularity and tumoral invasion of the dura are three different pathophysiologies of the DTS. Since its description in 1989, only about 80 DTS have been pathologically evaluated and of this relatively small number of pathologic exams, the DTS has been involved with tumoral cells in more than 50% of published examinations and the rest of the pathologic examinations have described vascular congestion and inflammation[2,3,5,25,26,47-51].
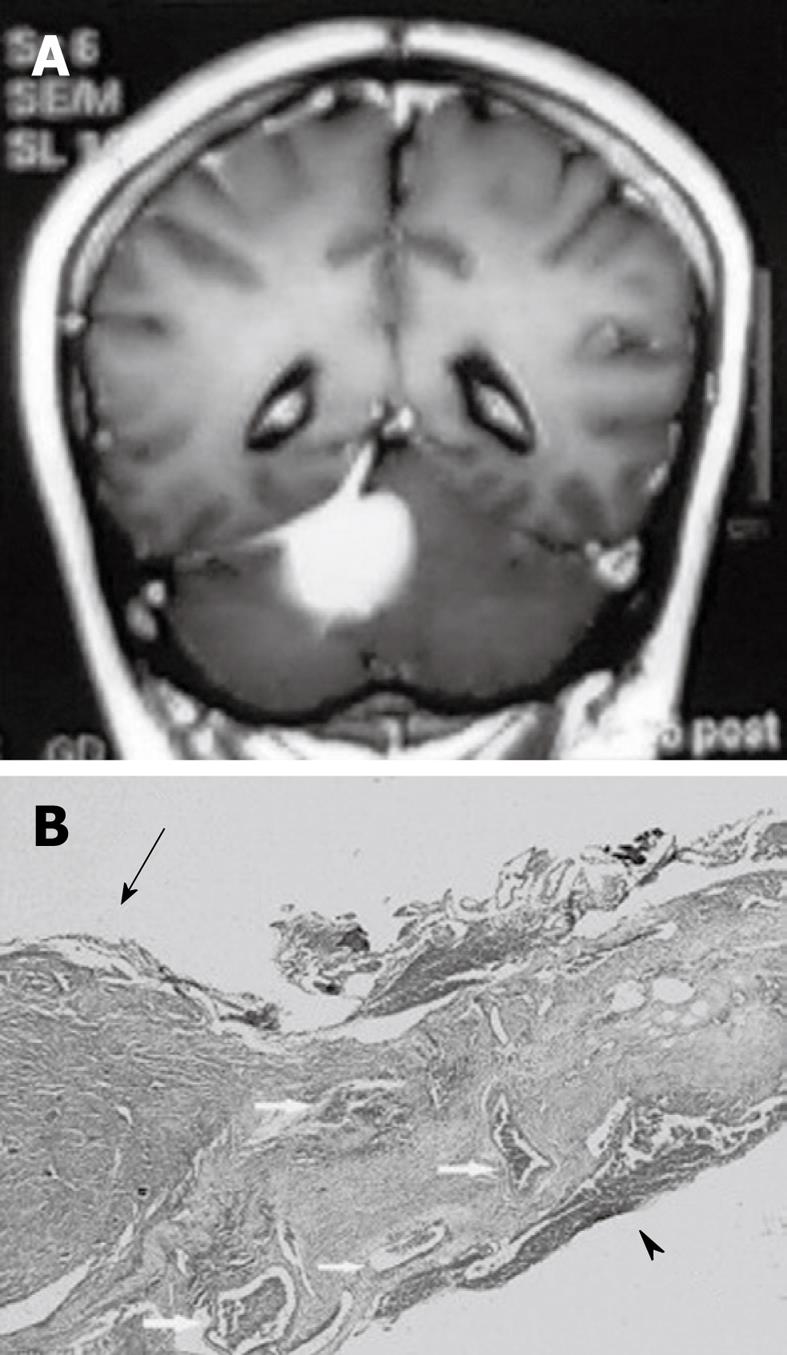
Figure 3 Pathological finding of dural tail sign.
A: A 58-year-old female with a tentorial meningioma and dural tail sign; B: Pathology specimen in this case showed a meningioma (arrow) and the attached dura. The dural tail is noted on the right side of the figure (arrowhead). Both the dura beneath the tumor and dural tail contain dilated blood vessels (arrow). There is no dural invasion. HE 30 ×.
IMAGE PREDICTION OF TUMORAL INVOLVEMENT IN DTS
Takeguchi et al[6] in their study on ‘‘dural tail’’ associated with 48 intracranial meningiomas on FLAIR and contrast-enhanced T1-weighted images, evaluated the histology of five cases with abnormal dura mater.
The dural tail was resected, and infiltration of tumor cells and the changes in reactivity were assessed pathologically and compared to the MRI findings.
The results suggested that in the patient without tumor infiltration, the signal of the dural tail was very high on contrast-enhanced T1-weighted and FLAIR images[6]. Rokni-Yazdi et al[49] in their study on 129 patients with intracranial lesions in which the histology of 17 cases with DTS was evaluated and compared with contrast-enhanced T1 weighted MRI findings, found that pre-operative clinical and imaging criteria did not predict the tumoral involvement of DTS.
Age, sex of patient, pattern of enhancement after contrast injection, size of primary neoplasm and the size of the DTS itself could not predict tumoral invasion into the DTS[49]. It is controversial as to whether the dura mater showing the tail sign should be resected to prevent recurrence. Kawahara et al[48] suggested that removal of the DTS is not a significant factor in recurrence of meningioma. Rokni-Yazdi et al[49] in their study on a limited number of patients with extra-axial tumor and DTS showed that if the dura below the tumor showed tumoral cells involvement, the dural tail was also involved and if the dura below the primary tumor was free of tumoral cells, the dural tail was also spared. Because of this strong correlation, the authors suggested that in the case of extra-axial tumoral resection without dural tail resection, if the dura below the tumor has tumoral cells involvement then the risk of recurrence may increase. However, further studies are needed to confirm this.