INTRODUCTION
The majority of patients suffering from colorectal cancer (CRC) are over 50 years of age, with a relatively equal gender incidence[1]. Recent declines in CRC incidence and mortality are attributable to reduced risk factor exposure, early detection and prevention through polypectomy, and improved treatment[2]. Despite this, CRC remains the third commonest adult cancer with approximately 1 in 19 adults diagnosed with CRC during their lifetime[1].
Imaging plays an important role in screening for CRC. According to the current American Cancer Society guidelines for CRC screening, 5-yearly computed tomography (CT) colonography (CTC) is recommended for asymptomatic patients with average risk[3]. In patients with known CRC, CT plays an important role in both pretreatment staging of disease, as well as assessing for response to treatment. Traditionally, this has been done by anatomical imaging assessment on CT. Advances in technology have further increased the role of CT, by facilitating functional imaging with positron emission tomography (PET) and perfusion studies.
ANATOMICAL IMAGING BY CT
Screening
Although elevated serum carcinoembryonic antigen (CEA) levels are often present in CRC, they are neither sensitive nor specific enough to be used as a screening tool for asymptomatic patients[4]. CTC (otherwise known as virtual colonoscopy) allows a minimally invasive imaging examination of the entire colon and rectum. Compared to optical colonoscopy, the risk for colonic perforation during screening is extremely low, being 0.005%[5] for asymptomatic patients and up to 0.06% for symptomatic patients[6], Use of carbon dioxide with an insufflator that regulates pressure rather than room air for gas insufflation of the colon may further reduce the incidence of perforation[5].
In CTC, high resolution image acquisition of the entire large intestine in a single breath hold is permitted by the use of multi-row detector CT. Integrated 3D and 2D analysis with specialised post-processing software allows for ease of polyp detection, characterization of lesions and location. For optimal assessment, adequate bowel preparation and gaseous distension of the colon are essential. Newer techniques such as faecal tagging reduce the need for vigorous bowel preparation[7] and decreases false positives from the presence of adherent faecal matter. In contrast with optical colonoscopy, extracolonic structures are also evaluated in the same examination. Hellström et al[8] showed that potentially important extracolonic findings, such as lymphadenopathy, aortic aneurysms and solid hepatic and renal masses, were present in 23% of patients.
The American College of Radiology Imaging Network National CT Colonography Trial, which included 2500 patients across 15 institutions in the United States, has shown comparable accuracy between CTC and standard colonoscopy. Pickhardt et al[9] reported a sensitivity of 89% for adenomas greater than 5 mm. For invasive CRC, the pooled CTC sensitivity was higher at 96%. As with other screening techniques, CTC accuracy improves with lesion size. All patients with one or more polyps larger than 10 mm or 3 or more polyps larger than 6 mm should be referred for colonoscopy[10]. However, the management of patients with fewer polyps (fewer than three) in which the largest polyp is 6 to 9 mm or smaller remains controversial at present[11,12].
For patients with suspected CRC, the diagnostic accuracies of contrast-enhanced CTC were even better. Using the tumour, node, and metastasis system, rates of 95%, 85%, and 100% were achieved. The sensitivity of both CTC and optical colonoscopy for cancer detection were both 100%, while the overall sensitivity of CT colonography was even higher than initial colonoscopy for polyp detection (90% vs 78%, P = 0.001, Figure 1)[13].
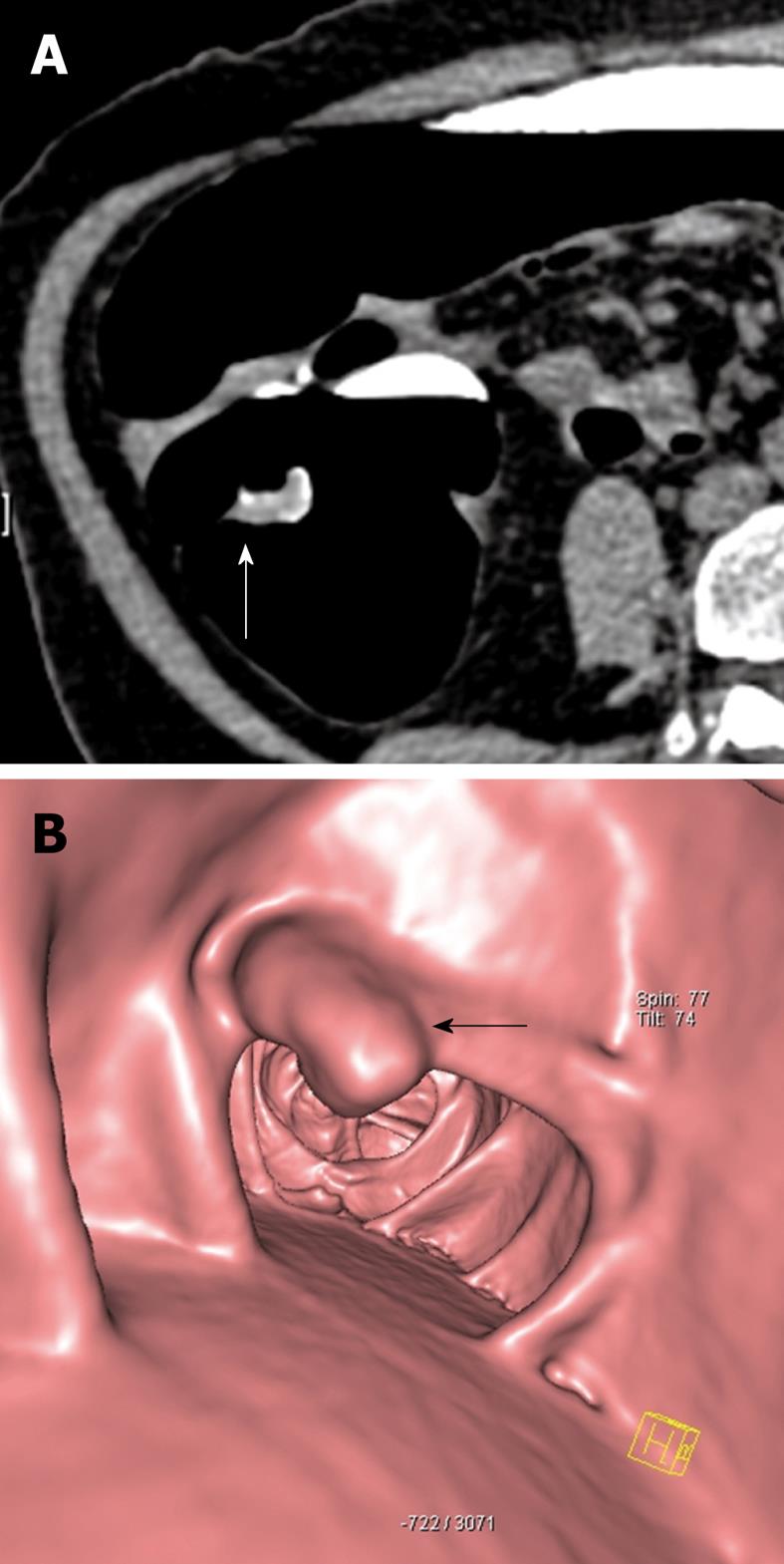
Figure 1 73-year-old female.
Faecal occult blood positive. Sigmoidoscopy was normal. A: Source axial computed tomography (CT) image from CT colonography study in the prone position demonstrates focal thickening (arrow) along a haustral fold in the proximal colon. Note the presence of contrast tagged faecal material coating the lesion; B: 3D reconstructed image of the same lesion showing a polypoid mass (arrow) arising from the haustral fold. Biopsy was positive for adenocarcinoma and the patient underwent curative right hemicolectomy.
The main drawback of CTC is radiation exposure. A single CTC study results in an estimated organ dose to the colon of 7 to 13 mSv, which is an additional 0.044% to the lifetime risk of colon cancer[14]. More efficient low-dose protocols (estimated organ dose ranges of 5 to 8 mSv) have been shown to be feasible with encouraging results[15].
Pre-treatment staging
Preoperative CT is typically performed for the following indications: (1) suspected haematogenous or distal nodal (e.g. paraaortic) metastases; (2) suspected invasion into adjacent organs or abscess formation; (3) unexplained or atypical symptoms; and (4) unusual histologic results. The major goal of CT is to determine if there is direct invasion of adjacent organs, enlargement of local nodes, or evidence of distant metastases[16].
On CT, CRC commonly manifests as focal thickening of the bowel wall and luminal narrowing; hence adequate distension of the bowel is crucial for accurate assessment. CT has a role in the detection of potential complications, such as perforation, fistulation and intussusceptions, which may require early surgical intervention.
The clinical use of CT for local tumour (T) staging of rectal cancer is limited, with a reported accuracy of around 70%[17]. This is attributable to the lack of attenuation differences between tumour and normal visceral soft tissue. In a study by O’Neil looking at patients with rectal cancer, CT consistently overestimated tumour volume and underestimated distance from the anal verge compared to magnetic resonance imaging (MRI)[18]. CT is also poor for the assessment of levator ani invasion in low rectal lesions, although it may assess the more proximal lesions with reasonable accuracy[19] (Figure 2). Similarly, for the more proximal large bowel, CT fares suboptimally, with a sensitivity and specificity rate of 60% and 67%, respectively, for the detection of extramural spread of tumour[20]. This is largely due to failure to detect microscopic disease.
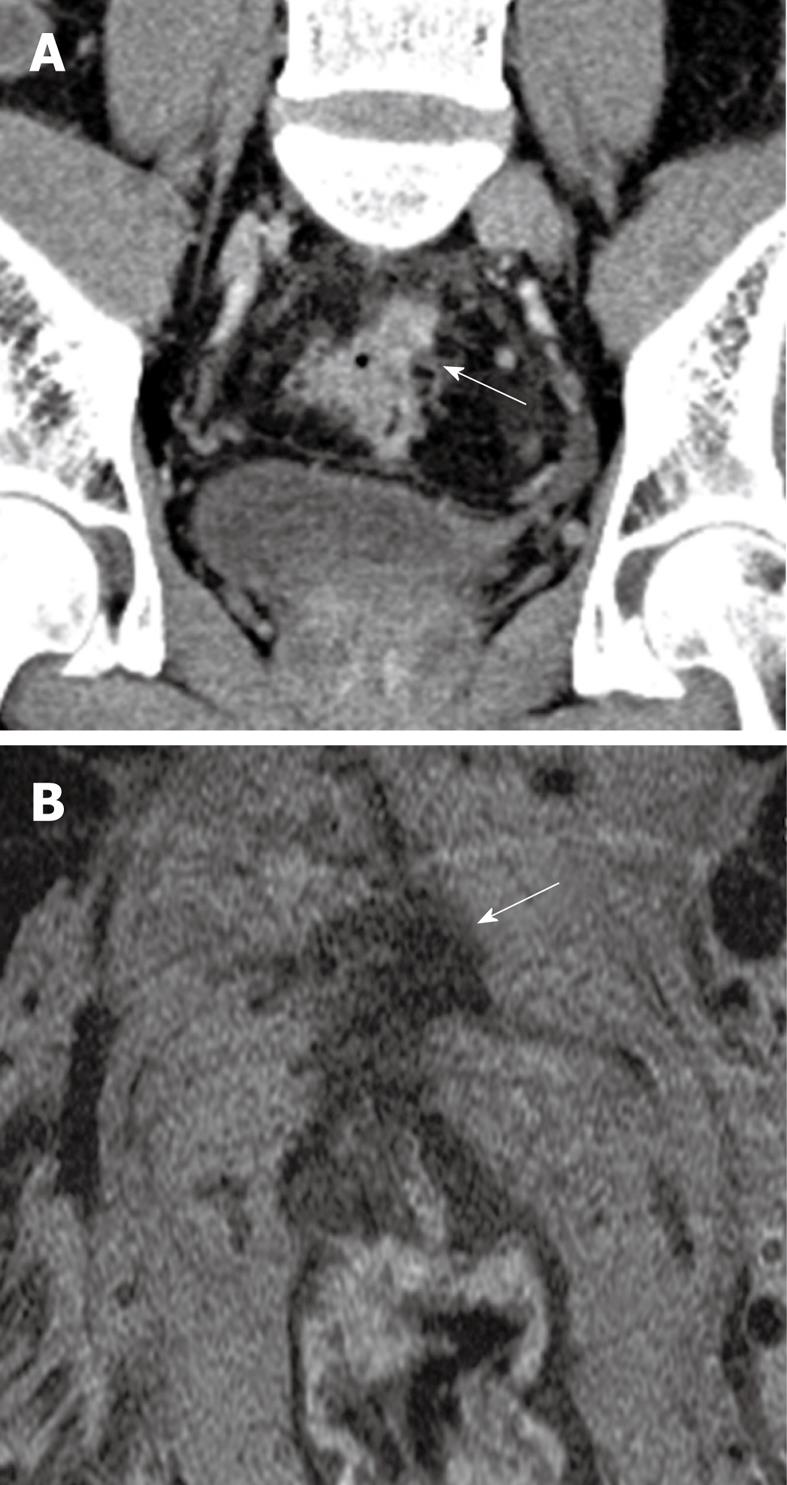
Figure 2 53-year-old male patient.
Presented with lower gastrointestinal bleeding. Endoscopy revealed a fungating mass in the proximal rectum, preventing passage of the scope more proximally. A: Coronal reconstructed contrast-enhanced CT image reveals a spiculated mass at the rectosigmoid junction with a spiculated extraserosal nodular component (arrow); B: Corresponding T2 weighted high resolution magnetic resonance image in the axial plane confirms the findings of extraserosal extension of disease (arrow). The patient was referred for assessment of suitability for neoadjuvant chemoradiation treatment.
CT can be considered to be more efficacious for nodal and metastases (N and M) staging than for T staging. A large meta-analysis by Bipat et al[21] that included 90 studies showed similar accuracies between ultrasound, CT and MRI for the assessment of nodal involvement by rectal cancer. In a study of 137 patients, Valls et al[22] showed good accuracy (85.1%), high positive predictive value (96.1%) and low positive predictive value (3.9%) of CT for the detection of liver metastases. For the detection of CRC metastases, CT imaging in the portal venous phase is the technique of choice. The addition of hepatic arterial phase imaging has been shown not to increase sensitivity, even though it improves the specificity in diagnosing liver metastases in a small number of cases[23].
At present, the optimal imaging strategy for the pretreatment distant staging of CRC remains controversial. For instance, chest CT often detects indeterminate lung lesions, of which only a small proportion develop into definite metastases[24]. Similarly, in rectal cancer, where pelvic MRI has already been performed, CT of the abdomen and pelvis will not provide additional value[25]. Therefore, further studies are required to define optimal preoperative imaging.
Other than the liver, the peritoneum is a major site for metastatic disease (Figure 3). The presence of peritoneal metastasis predicts for a higher local recurrence rate[26]. Furthermore, the Peritoneal Cancer Index, an assessment of the tumour burden attributed to peritoneal disease, has been recognized as an independent prognostic indicator for long-term outcomes. The role of CT in the detection of peritoneal carcinomatosis is limited for small metastases. In the study by de Bree et al[27], CT detection of peritoneal metastases was only moderate (ranging from 9% for subcentimeter lesions to 66% for lesions larger than 5 cm) with significant interobserver differences. A more recent study by Koh et al[28] echoed these findings, with a sensitivity of 11% for lesions smaller than 0.5 cm contrasting with 94% for lesions larger than 5 cm, significantly underestimating the Peritoneal Cancer Index.
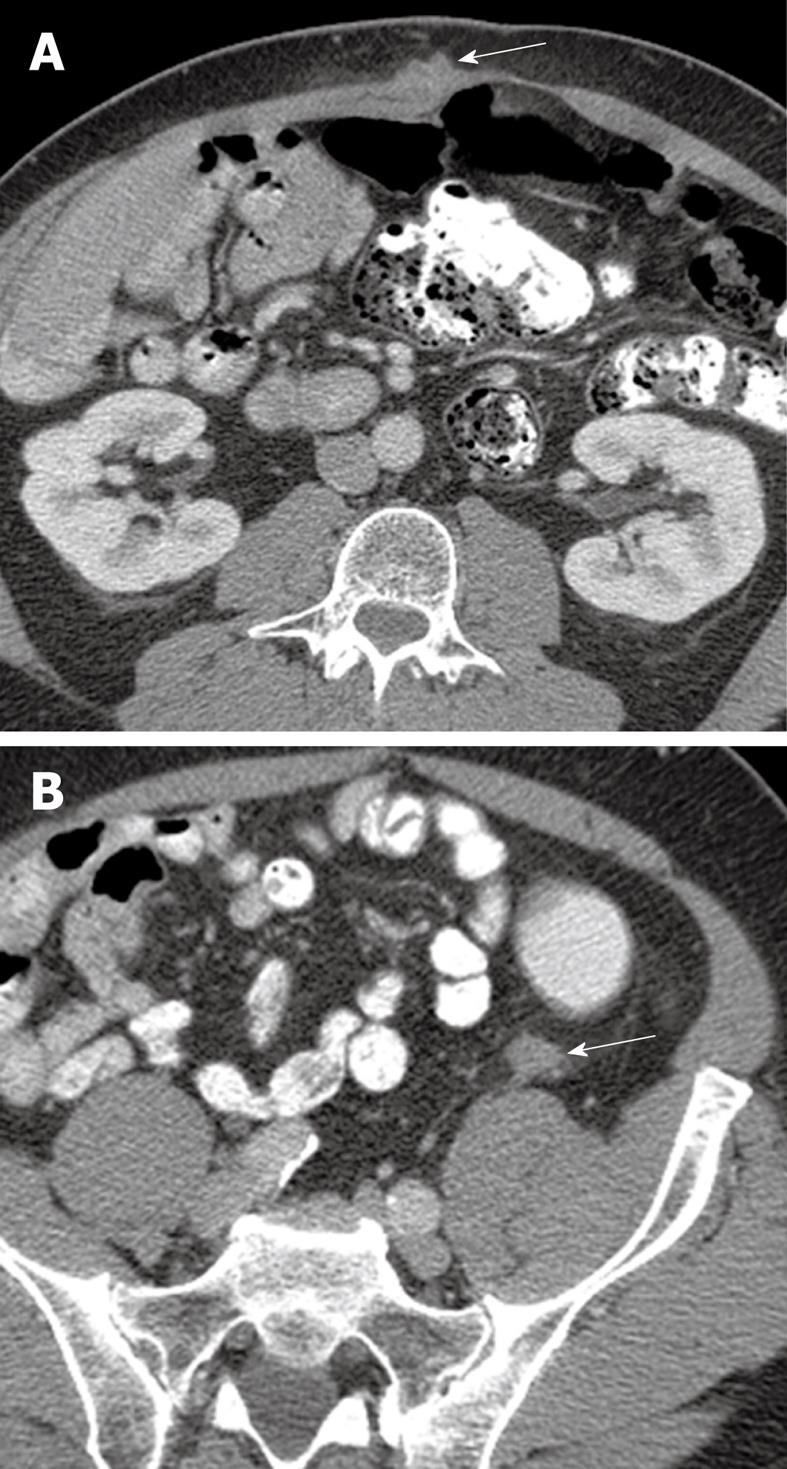
Figure 3 64-year-old male with metastatic adenocarcinoma of the colon.
A: Surveillance axial contrast-enhanced CT image shows a metastatic deposit in the right rectus abdominis muscle (arrow); B: A second metastatic lesion is present in the left paracolic gutter (arrow). The high spatial resolution of CT and the contrast with the adjacent fat allows for easy detection of metastatic disease in these areas.
Post-treatment assessment
For routine surveillance, the American Society of Clinical Oncology currently recommends CEA assays every 3 mo for the first 3 years, CT scan of the chest, abdomen and pelvis annually for the first 3 years and colonoscopy at 3 years in patients with stage 2 and stage 3 CRC[29].
Local disease recurrence is evidenced on CT by the serial progression of a mass, its nodular configuration and invasion of adjacent structures[30]. However, CT cannot reliably differentiate tumour from post-treatment scar formation. For both local and nodal assessment of rectal cancer after neoadjuvant chemoradiation therapy, CT may not be able to reliably predict pathological response, and has a tendency to overstage disease. The study by Huh et al[31] looked at 80 rectal cancer patients following neoadjuvant chemoradiation therapy. It was found that the overall accuracy of CT for restaging the depth of tumour invasion and lymph node metastasis were 46.3% and 70.4%, respectively, while complete pathology-proved remission (11 patients) could not be correctly predicted.
Nevertheless, for the diagnosis of recurrent hepatic metastases, CT has already been shown to be more helpful than laboratory studies (liver function tests, measurement of CEA level)[32]. Specifically, there is a 25% lower mortality in patients undergoing liver imaging compared with nonimaging strategies[29]. This is further supported by the study of 530 patients conducted by Chau et al[33], in which routine post-treatment surveillance with CT and CEA levels in asymptomatic patients were shown to confer a median survival advantage of 13.8 mo over patients who were symptomatic. The reader should note however, given the increased costs, use of routine CT surveillance in these patients is only justified for those who are surgically fit to undergo metastasectomy. Therefore, CT currently still plays an important role in the postoperative surveillance of CRC.
FUNCTIONAL IMAGING BY CT
PET/CT
18Fluoro-deoxyglucose is the most widely used substrate for PET imaging. Fusion PET/CT combines the functional evaluation by PET with the anatomic detail provided by CT (Figures 4 and 5). PET/CT is increasingly shown to be superior to the other imaging modalities in demonstrating recurrent disease activity and has become an integral part of the surveillance strategy for CRC. It has the potential to replace CT as the first-line diagnostic tool for restaging patients for recurrent CRC[34]. In one study, PET/CT revealed unsuspected disease and modified the scope of surgery in around 10% of patients[35]. In another study, FDG PET/CT altered treatment plans in 38% of patients largely through the detection of unsuspected lymphadenopathy[36]. For local disease, PET/CT can improve preoperative target volume delineation by CT for conformal radiation therapy in rectal cancer[37]. Preoperative PET/CT colonography may yield valuable information on the presence of synchronous tumors and for surgical planning[38].
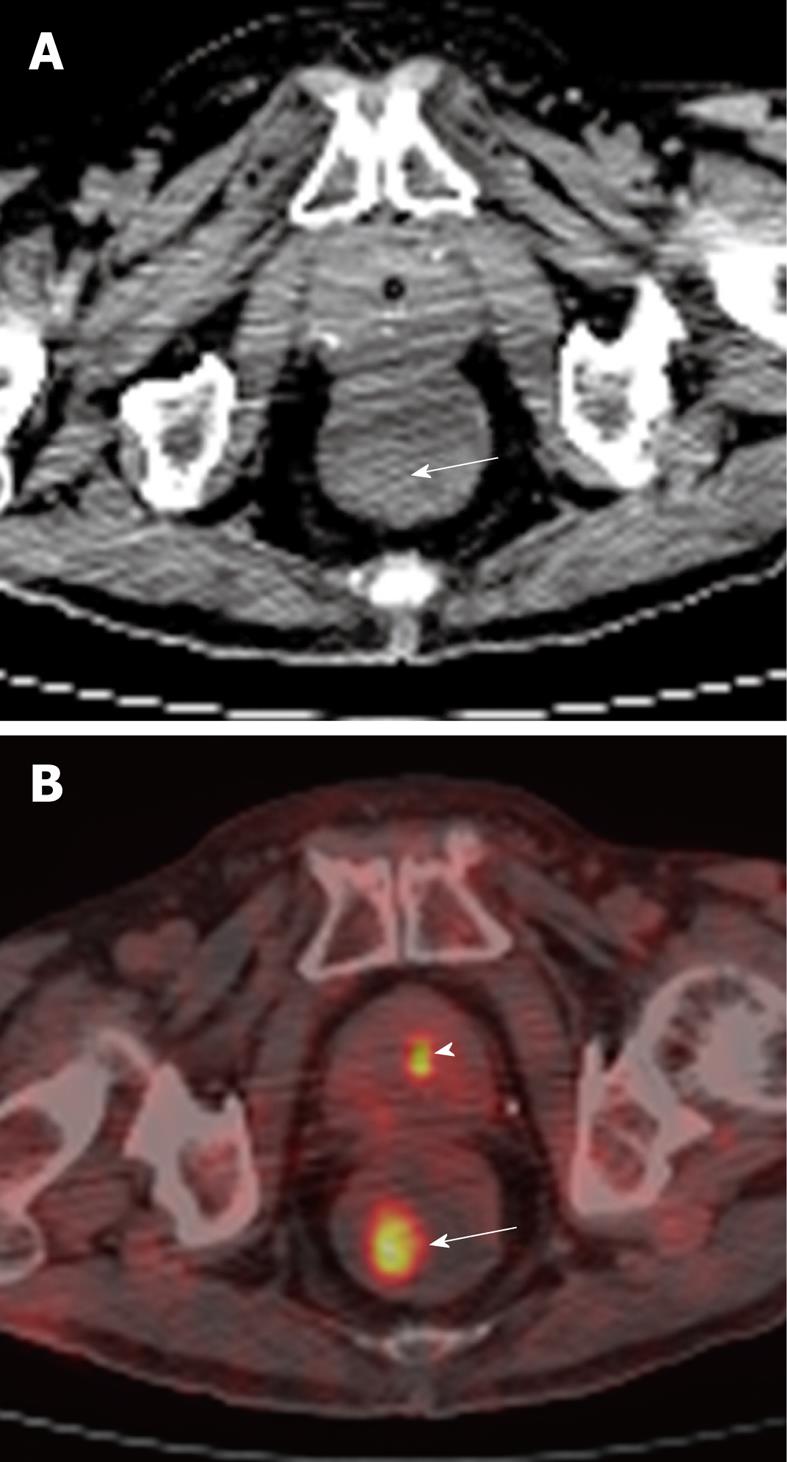
Figure 4 81-year-old male undergoing positron emission tomography (PET)/CT for restaging of diffuse large B cell lymphoma involving the duodenum.
A: Axial non-contrast CT of the rectum showing an incidental subtle soft tissue density polypoid mass (arrow) in the right posterior lateral wall; B: This corresponded to a focal area of hypermetabolic activity (arrow), as demonstrated on fusion PET/CT. Biopsy returned as tubulovillous adenoma. This was excised. Smaller focus of increased tracer activity in keeping with normal physiologic excretion in the urine within the prostatic urethra (arrowhead).
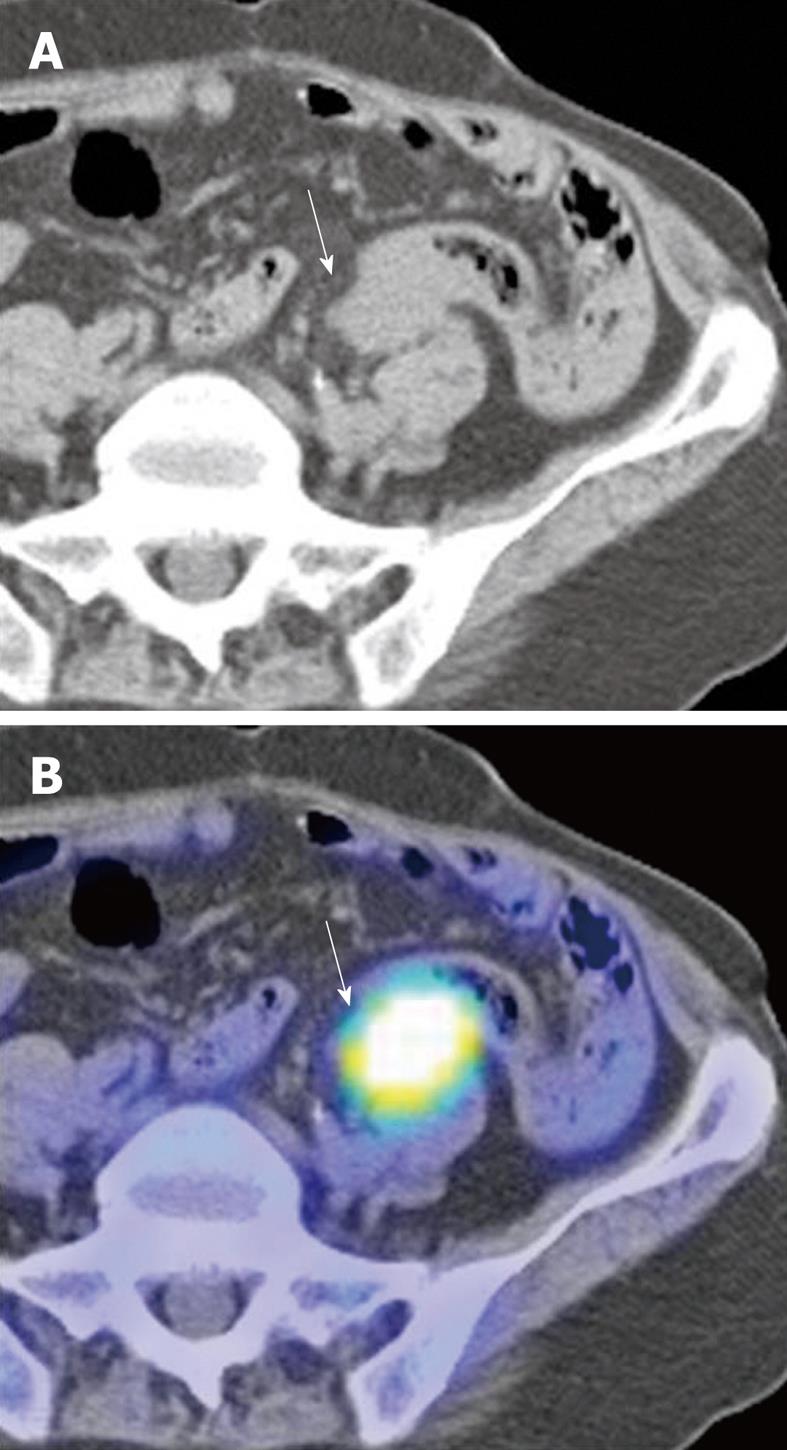
Figure 5 66-year-old Chinese male with adenocarcinoma of the rectum (not shown).
A: Post-operative axial non-contrast CT component of a surveillance PET/CT study showing a soft tissue mass (arrow) abutting the sigmoid colon; B: Corresponding fusion PET/CT image of the lesion (arrow) demonstrates intense hypermetabolic activity consistent with tumour recurrence. (Case courtesy of Dr. Eik Hock Tan, Singapore).
However, by far the greatest value of PET/CT in the management of CRC lies in its ability for whole body lesion detection. In one study, PET/CT showed high accuracy for the detection of liver metastases, with a reported accuracy of up to 99%, sensitivity up to 100% and specificity up to 98%[39]. In the meta-analysis conducted by Kinkel et al[40] that included 110 studies, PET/CT afforded the highest mean weighted sensitivity (92%) and was significantly more sensitive for the detection of hepatic metastases from gastrointestinal cancers than CT. Rappeport et al[41] showed that PET/CT was superior to CT alone for the detection of extrahepatic metastases in CRC patients, with sensitivity and specificity rates of 83% and 96% for PET/CT and 58% and 87% for CT. Contrast-enhanced PET/CT and PET/CT colonography shows promise for improving accuracy in staging of disease[42,43].
PET/CT can distinguish between tumour recurrence and post-surgical scar, as well as pinpoint the site of recurrence in cases with an unexplained rise in serum CEA[44]. It is therefore recommended for evaluation of equivocal findings on serial CT and MRI[45]. To detect recurrent nodal disease, PET/CT is superior to MRI, with a sensitivity of 93%[46]. PET/CT is superior to contrast-enhanced CT in detecting local recurrences at the colorectal anastamosis, intrahepatic recurrences and extrahepatic disease, with sensitivity rates close to or exceeding 90%[47]. Quantitative measurements of standardised uptake value and tumour volume may be used as a marker of tumour burden in cases of tumour recurrence[48]. Note that PET/CT should be performed more than 6 wk following local therapy, as inflammatory changes can result in false positives.
In one study, PET/CT correctly assessed response of liver metastases to Bevacizumab-based therapy in 70% of cases compared to 35% by CT[49]. For evaluation of liver metastases after radiofrequency ablation, PET/CT is comparable to MRI. In the study by Kuehl et al[50], the accuracy and sensitivity for the detection of liver metastases was 91% and 83% for PET/CT and 92% and 75% for MRI, respectively. After treatment of liver metastases with Y-90 microspheres, metabolic response on PET/CT correlates better with CEA levels than anatomic response with CT or MRI[51]. This having been said, it should be noted that complete metabolic response on FDG-PET after neoadjuvant chemotherapy does not necessarily imply complete pathologic response. Therefore, currently, curative resection of liver metastases should not be deferred solely on the basis of FDG-PET findings[52,53].
Perfusion CT
Novel techniques such as perfusion CT[54,55] and combined perfusion CT/PET CT[56] show promise. Perfusion CT is performed at various time intervals after the injection of contrast. A precontrast scan is required for determination of increase in Hounsfield attenuation. Standard imaging protocols are to image at 45 and 130 s after contrast injection. For perfusion CT, iodinated contrast needs to be injected at a high rate, typically at 5 mL/s. Tissue blood flow, blood volume, mean transit time, and vascular permeability-surface area product are calculated based on the enhancement curves.
Aggressive tumors with poor differentiation are thought to be more vascular, and may therefore be distinguished from more well differentiated lesions with the use of perfusion CT. In the study by Sahani et al[57], rectal cancer showed higher tissue blood flow and shorter mean transit times than normal rectum. In another study, similar findings were echoed whereby CT perfusion was able to differentiate cancer from inflammation secondary to diverticulitis[58].
An elevated liver perfusion index has also been found to be associated with the presence of hepatic metastases[59]. Increased arterial perfusion appears to be an indicator of liver metastases, whereas reduced portal perfusion may indicate progressive disease[60]. Perfusion CT may also play a role in predicting progression to metastatic disease. In the study by Goh et al[58], tumour blood flow differed significantly between disease-free and metastatic patients (76.0 mL/min per 100 g tissue vs 45.7 mL/min per 100 g tissue, respectively). Using blood flow < 64 mL/min per 100 g tissue as a cut-off, sensitivity and specificity for the development of metastases were 100% and 73%, respectively.
Perfusion CT has potential for predicting the response of rectal cancer to combined neoadjuvant chemotherapy and radiation therapy. In a study of 19 patients, blood flow, blood volume and permeability-surface area product significantly decreased after combined chemotherapy and radiation therapy (P < 0.009)[61]. To date, however, the technique of perfusion CT remains the subject of research. The main drawback to this technique is the additional exposure to ionising radiation (estimated at 10 mSv). This translates to an added 1 in 2000 risk of lifetime cancer risk. To reduce the risk of ionising radiation, the radiation dose should be carefully optimised on a per patient basis.
There is also a need for standardisation of techniques. For example, the position and size of tumour region of interest analysis and observer variation have been found to substantially influence perfusion values. Region of interest analysis for outlined entire tumour is more reliable for perfusion measurements and more appropriate clinically than use of arbitrarily determined smaller ROIs, although this may mean increased post-processing times[62].