INTRODUCTION
The small bowel (SB) has been a challenging organ for clinical and radiological evaluation. Crohn’s disease (CD) is the most common SB disease. Detection of disease and its extent are the two clinically important questions. Moreover, there is increasing interest in determining the degree of inflammatory activity of the disease. For symptomatic patients, it is also important to determine whether the symptoms are functional or due to active inflammatory activity vs fibrotic stenosis. In recent years, magnetic resonance imaging (MRI) has emerged as a promising technique in patients with CD. In this article, we are going to review the role of MRI in the diagnosis of CD.
CROHN’S DISEASE
CD is a chronic, transmural inflammatory disorder of the entire gastrointestinal tract. It is the most common SB disease in United States and Europe (3.1 to 14.6/100 000 in North America and 0.7 to 9.8/100 000 in Europe)[1]. The etiology of CD is unknown. It has been proposed that the condition is immune-mediated, with an abnormal mucosal response to unknown luminal antigens[2]. It commonly involves the SB, in particular the terminal ileum. The initial lesion starts as a focal inflammatory infiltrate in mucosa and submucosa leading to hyperemia and edema. As the disease progresses, superficial ulcers develop. In severe cases, transmural inflammation and even serosal involvement is present. In long-standing cases, chronic obstruction due to scarring, luminal narrowing, and stricture formation may arise. Extramural manifestations are fistulae, abscesses, adhesions, creeping fat, malabsorption and enlargement of lymph nodes.
DIAGNOSTIC MODALITIES
More than 70% of CD patients develop disease in the SB. Since endoscopic techniques are often limited to more proximal segments of the SB, either enteroclysis or SB follow-through (SBFT) has been the gold standard for the diagnosis of CD[3,4]. SB enteroclysis has been shown to be more reliable when compared to SBFT in demonstrating early mucosal changes[5,6]. It has been claimed that SBFT is inaccurate in detecting active CD of the SB[7-9]. Both methods give limited and indirect information about the bowel wall and extraluminal extension of disease and their diagnostic accuracy is dependent on examiner experience. Additionally, these techniques require an extensive bowel preparation, and their indication is limited in young patients due to the amount of ionizing radiation exposure. Newer techniques, such as video capsule endoscopy (VCE), push endoscopy and double-balloon endoscopy (DBE), have been developed to compensate for the above-mentioned disadvantages. VCE demonstrates the mucosal surface of the SB wall. However, tissue sampling and therapeutic interventions are not possible and it is contraindicated in patients with obstruction. In a recent study comparing VCE and magnetic resonance enterography (MRE), it has been claimed that VCE can depict and characterize subtle mucosal lesions missed at MRE, whereas MRE gives additional information about mural, perienteric and extraenteric involvement[10]. DBE provides visualization of the entire SB and endoscopic therapy and, moreover, it allows obtaining tissue sample for analysis[11]. Ileocolonoscopy has been a most valuable tool for diagnosis and follow-up of CD in the colon and terminal ileum, but inspection of the terminal ileum fails in up to 27.8% of examinations[12].
CROSS-SECTIONAL IMAGING MODALITIES
Transmural and extramural extent of disease cannot be visualized with SB barium examinations (enteroclysis or SBFT), VCE, DBE or ileocolonoscopy. Recent advances in computer technology have furthered the usefulness of cross-sectional imaging, leading to improved spatial and temporal resolution to obtain high-resolution imaging.
Ultrasonography, computed tomography (CT) and MRI are the three techniques often used in abdominal examination. While CT is the modality of choice in the USA, in Europe MRI and US are preferred[11]. US is mostly performed without using enteric contrast medium. However, there are some studies reporting a higher sensitivity following the enteric contrast medium administration[13,14]. Although US can be used to assess both small and large bowel, diseases of the duodenum and jejunum are often missed. Moreover, the rectum and distal sigmoid cannot be visualized accurately. As the spatial resolution is insufficient to detect superficial pathology, it is less suitable for early diagnosis. Doppler US is useful only to assess whether the disease is in active phase or remission, however, it cannot give information about the severity of active disease[15]. In a recent study, evaluating the role of US and MRI to assess extension and inflammatory activity of CD, Martínez et al[16] reported that both US and MRI are sensitive to localize the affected bowel segments and to detect transmural complications. They found a significant correlation between color Doppler flow and bowel wall enhancement on MRI.
CT is not as sensitive as barium studies in detecting mucosal lesions but it is valuable in demonstrating intramural and extraluminal findings. CT enterography (CTE) is a fast, noninvasive technique that uses thin sections and large volumes of enteric contrast material to better delineate the wall and lumen of the SB[17,18]. The use of neutral enteric contrast agents, such as water, combined with intravenous (IV) contrast material allows excellent visualization of hypervascular lesions and hyperenhancing segments[7,8,19]. However, due to repetitive use for follow-up in young CD patients, its role is limited by the amount of ionizing radiation. CTE is less suitable for detecting mild disease, as superficial lesions are not accurately visualized[9]. Extramural complications are well demonstrated on CTE[20] but exposure to ionizing radiation with repeated tests in a relatively young patient population is a matter of concern. MRI has been introduced as an alternative method to detect CD, and it can be performed as either MRE or enteroclysis[21-23]. MRE has been shown to be useful to detect active ileitis, to assess disease activity and to identify extraenteric complications[21,23,24]. In a recent series, which systematically reviewed the evidence on the accuracy of MRI for grading CD activity, MRI was found to correctly grade 91% of patients with frank disease, 62% of patients with mild disease and 62% of patients in remission. Thus, it was concluded that MRI correctly graded disease activity in a large proportion of patients with frank disease[25].
ADVANTAGES OF MRE
MRE is currently used as an alternative modality to CTE due to its potential advantages. Lack of ionizing radiation is an important feature of MRE. MRE has improved soft tissue contrast, which is important for detecting subtle pathologic areas. It is particularly helpful for detection, staging and follow-up of perianal fistulae. MRE also enables static and dynamic studies that provide real-time and functional imaging. By using multiphase imaging techniques, bowel peristalsis and distensibility can be evaluated. MRE helps to determine the cause of bowel narrowings, i.e. whether they are due to contractions or to fixed strictures. Due to the safety profile of gadolinium contrast agents, the technique may be preferred in patients who are allergic to iodine contrast medium.
FACTORS ASSOCIATED WITH EXPOSURE TO DIAGNOSTIC RADIATION
Mean cumulative exposure dose (CED) to significant levels of ionizing radiation is of particular concern in patients with CD, as the disease presents in adolescence and has a life-long duration. The United States National Research Council estimates that for every 1000 patients undergoing a 10 mSv CT examination of the abdomen, one patient will develop a radiation-induced cancer in their lifetime[26]. In a population-based cohort, CTE was shown to deliver approximately 1.5-2 times the effective dose of conventional abdominopelvic CT scanning. It was found that CD patients received a CED of 36.9 mSv over a follow-up of 10.9 years[27]. Desmond et al[28] measured a mean CED value of 36.1 mSv and the value exceeded 75 mSv in 15.5% of patients followed between 1992 and 2007. As there is an increased time-life risk of developing intestinal malignancies, especially SB lymphoma and liver and biliary tract tumors[29], imaging modalities which impart no radiation dose have a definite advantage in imaging CD patients.
WHAT CLINICIANS EXPECT FROM RADIOLOGISTS REGARDING CD
Diagnosis of CD still remains a clinical challenge. A combination of clinical information is required, with radiologic imaging playing a key role. The radiologic information is sought for two purposes. One is to noninvasively and accurately diagnose CD, so that gastroenterologists may avoid treating patients who do not have true CD with intensive medical therapies, which have a potential for morbidity. The other role of radiologic examinations is to evaluate the extent, activity and severity of disease, and to exclude penetrating disease. The extent of the disease influences the medical and surgical approach. The presence of perienteric inflammation, fistulae and partial SB obstruction may also alter the management decision. A standardized reporting system and radiologic activity index may be achieved by a detailed radiologic imaging, and this may help assessing the disease activity[30].
PATIENT PREPARATION PROTOCOLS
As a collapsed bowel loop may obscure lesions or mimic pathologic wall thickening, bowel distension is the single most important factor for any method of choice. For this purpose, a large amount of orally administered enteric contrast material is used in MRI examination, to achieve SB luminal distension. Oral contrast agents not only distend the lumen but also decrease the susceptibility to develop artifacts by displacing intraluminal air. Positive contrast agents increase intraluminal signal [hyperintense on both T1-weighted (T1W) and T2- weighted (T2W) images]. They consist of paramagnetic substances such as gadolinium chelates, ferrous and manganic ions and manganese ions[31-33]. They reduce T1 relaxation time, while T2 relaxation time is usually not affected. Due to water content of the solutions, they will also be seen as hyperintense on T2W images. Wall thickening is well delineated on T1W images but, due to the increased intensity of the bowel lumen, they may hinder evaluation of inflammatory enhancement or intraluminal lesions. Negative contrast agents reduce intraluminal signal (hypointense on both T1W and T2W images). They include superparamagnetic particles such as perfluorooctyl bromide, iron oxides and oral magnetic particles[31,34-36]. Barium sulfate can also be used as a negative contrast agent, when administered at high concentrations. These agents induce local dishomogeneity in the magnetic field affecting both T1 and T2 relaxation time. They are helpful in the detection of bowel wall thickening on T1W images and, due to their decreased signal intensity, bowel wall enhancement will be more remarkable on T1W images. These agents are preferred for the visualization of inflamed bowel wall and surrounding fat on T2W images. In fact, since the bowel lumen will be seen as hypointense, the hyperintense inflammation will be more conspicuous. Biphasic contrast agents demonstrate different signal intensities on different sequences[37,38]. Water, hyperosmolar (mannitol-based solutions) and iso-osmolar watery solutions and barium sulfate are seen as low intensity signal on T1W and high intensity signal on T2W images[31]. Following IV contrast administration, the low signal intensity on T1W images provide a better resolution between bowel lumen and hyperenhancing wall inflammation or masses. Water is readily available, better accepted by the patients and cheap, but it is rapidly absorbed from distal bowel. Therefore, adequate distension may not be obtained. Potential limitations of these agents are gas formation and osmotic effects. For this reason, nonosmotic agents such as methylcellulose, polyethylene glycol and locust bean gum have also been used[37,39,40]. Manganese and gadolinium chelates are also biphasic agents seen as low intensity signal on T2W and high intensity signal on T1W images when they are administered at high concentrations[31,33]. In a recent study, alternative oral contrast agents, such as rose hip syrup, black current extract, iron-deferoxamine and cocoa, were investigated as oral contrast agents for MRI. Cocoa, with its differing relaxation and signal enhancement, provided good contrast between lumen and water, and between fat and gadolinium enhancement, and it was found to be a promising oral contrast agent[41]. Currently, there is no universal consensus on an optimal oral contrast agent or ingestion protocol and none of them can be considered ideal. After the appropriate administration of oral contrast, the patient should be placed on the MRI table. The preferred scanning position is prone, in order to separate bowel loops and to enable maximal bowel coverage in coronal planes[42,43]. However, for comfort reasons, MRI is usually performed in the supine position. An antispasmodic agent is given immediately before the examination, either by intramuscular or IV route. Gadolinium is infused IV as soon as noncontrast enhanced sequences are completed. It has been shown that IV contrast contributes to the delineation of active inflammation in CD[44]. There have also been various series evaluating the role of rectal contrast administration in assessing the active inflammation of the colon. It has been claimed that rectal contrast can improve reader agreement[45,46].
PULSE SEQUENCES
Although there is no consensus on a universal protocol, an appropriate SB examination should consist of fast and ultrafast T1W and T2W sequences in both axial and coronal planes. Contrast enhanced T1W sequences are obtained by using gradient echo technique with fat saturation. The most commonly used sequence in SB imaging is fast low-angle shot using both 2D and 3D acquisitions. These are routinely used to identify increased enhancement in inflamed bowel wall[47,48]. 3D imaging provides better spatial resolution and SNR than 2D imaging and the volumetric data can be reconstructed in any planes. But it is more susceptible to motion which may cause blurring in the abdominal wall. Fast T1W gradient echo sequences without fat supression or T1W fast spin echo sequences may also be applied before IV contrast administration. T2W sequences are generated by rapid acquisition and relaxation enhancement with ultrafast acquisition time. They are known as half-acquisition single-shot fast spin echo (SSFSE) or SSFSE sequences, depending on the manufacturers. They are heavily T2W sequences, complementary to gadolinium-enhanced gradient echo sequences, and produce high contrast between the lumen and the bowel wall. As these sequences are highly resistant to magnetic susceptibility or chemical shift artifacts, the wall thickness may be evaluated accurately. Moreover, the sinus tracts and fistulas are well visualized. These sequences are sensitive to intraluminal motion and there may be intraluminal low intensity signal artifacts. Visualization of the mesenteric structures is impaired on these sequences due to k-space filtering effects. In recent years, high resolution, ultra-fast sequences based on steady-state free precession have emerged as the predominant technique for imaging of SB. These sequences are called true fast imaging with steady state precession, balanced fast field echo or fast imaging employing steady-state acquisition (FIESTA) sequences, depending on the manufacturer. They are relatively insensitive to motion artifacts, provide uniform intraluminal signal and lead to a high contrast between the bowel wall, lumen and mesentery. Mesenteric vessels and lymph nodes are better visualized on these sequences than on the single-shot sequences. The disadvantage of the sequence is a black-boundary artifact at the interface of the bowel wall and mesenteric fat that may hinder small lesions[49]. Although it is claimed that this artifact is also a potential limitation for bowel wall thickness assessment, Fidler[50] reported that it did not represent a significant limitation in their routine practice. There is still not a consensus on the scan delay: it has been reported that, in normal volunteers, peak wall enhancement for MRI was at 60-70 s (portal venous phase)[51].
MRE VS MR ENTEROCLYSIS
MR enteroclysis is an emerging technique for SB imaging that combines the advantages of conventional enteroclysis with those of cross-sectional imaging. It enables visualization of luminal, mural and extramural abnormalities. It is performed with intubation of the duodenum or proximal SB by subsequently administering enteric contrast agents. It provides superior distension and improves depiction of mucosal abnormalities. However, as placement and positioning of an intestinal tube are still necessary for the examination, it is not well tolerated by the patient. MRE is performed by ingestion of a large volume of enteric contrast. It obviates the need for a nasoenteric intubation, so the technique is well tolerated by the patients. As yet, there is no consensus about the modality of choice in the radiologic community. In a recent prospective study performed with 40 CD patients, the two techniques were compared and luminal distension and visualization of superficial mucosal, mural and mesenteric abnormalities were evaluated. No statistically significant differences were found in assessing the diagnostic efficacy as to the visualization of mural stenoses and fistulae. The number of detected mesenteric findings was very high with both techniques[42]. The study concluded that MRE might have a role in patients who refuse or have failed intubation and also during follow-up.
MRE VS OTHER DIAGNOSTIC MODALITIES
Various studies have been performed to compare MRE with other techniques as a diagnostic modality in CD patients. Lee et al[52] compared the usefulness of CTE, MRE and SBFT in 30 patients to detect active terminal ileitis and extraenteric complications. Differences in areas under the ROC curves for three modalities were not significant. Sensitivity values for detection of extraenteric complications were significantly higher for CTE and MRE. In another recent series, MRE and CTE were compared in follow-up of CD patients, in which polyethylene glycol was used as oral contrast medium. MRE showed a good sensitivity in detection of CD activity and it was suggested as an accurate method in monitoring the activity of CD compared to CT[39]. Siddiki et al[53] compared, in a prospective study, MRE and CTE in 33 CD patients and found similar sensitivities for MRE and CTE (90.5% vs 95.2%, respectively). Horsthuis et al[54] performed a meta-analysis on the accuracy of US, MR, scintigraphy, CT and positron emission tomography (PET) in the diagnosis of inflammatory bowel disease. They found no significant differences in the diagnostic accuracy among the imaging techniques. Tillack et al[55] compared the diagnostic performance of MRE and wireless VCE in detecting and classifying SB CD proximal to the terminal ileum. As for the presence or absence of pathology, results of MRE and VCE were in total agreement for the evaluated segments (85%). In judging lesion severity, both yielded identical results. The researchers concluded that both modalities were complementary and MRE should be used in more severe cases of CD and in patients who might have involvement beyond the mucosa of the SB. In a pilot study, performed to compare double-balloon enteroscopy and MR enteroclysis in diagnosing suspected CD, the presence of pathology and its localization, degree and extent of involvement were evaluated. Both techniques had the potential to become diagnostic standards that complement each other in patients with suspected complex SB CD[56].
SAMPLE PROTOCOL
At our institution, MRI examinations are performed with a 1.5-T GE Signa MR scanner (GE Healthcare, Milwaukee, WI). Patients fast for 6 h before the MRI examination. A total of 1350 mL of Volumen (E-Z-EM Inc.) is administered orally over the course of 45 min prior to scanning. Immediately prior to starting the examination, when the patient is being placed in the scanner, 1 mg of intramuscular glucagon (Glucagen; Bedford Laboratories, Bedford, Ohio) is administered. After acquiring a standard three-plane scout image, the following sequences are obtained through the abdomen and pelvis using a 4-channel phased array body coil: (1) Axial and coronal FIESTA with and without fat suppression (TR/TE 3.4/1.4, matrix 224 × 224, flip angle 45, slice thickness/gap: 7 mm/0 mm); (2) Axial and coronal T2W SSFSE with and without fat suppression (TR/TE infinite/90, matrix 256 × 256, slice thickness/gap: 6 mm/0 mm); (3) Pre- and post-contrast T1W LAVA with additional dynamic post-contrast images (TR/TE 3.5-3.9/1.6-1.9, matrix 192 × 256, flip angle 10, interpolated slice thickness 2.2 mm); and (4) Axial and/or coronal diffusion-weighted images (b values, 0 and 600 s/mm2; TR, 8000 ms; TE, 75 ms; matrix, 128 × 128-224; slice thickness, 7 mm; gap, 0 mm). A number of signals are acquired. For each sequence, the upper abdomen and pelvis are scanned separately. Gadodiamide (Omniscan; Nycomed-Amersham, Princeton, NJ) is administered IV at a dose of 0.1 mmol/kg, followed by a 20-mL saline flush at the rate of 2.0 mL/s. For the dynamic-contrast enhanced MRI (DCE-MRI) examinations, T1W, three-dimensional, gradient-echo, and free-breathing coronal DCE-MR images covering the entire abdomen are acquired (repetition time, 3.5-3.9 ms; echo time, 1.6-1.9 ms; matrix size, 160 × 256; flip angle, 10°; interpolated slice thickness, 3 mm) with temporal resolution of 5 to 12 s for approximately 4 to 7 min. The dynamic scans are started immediately with the injection of contrast without delay. Post-contrast high resolution T1W images are obtained after completion of the DCE-MRI sequence acquisition. Acquisition time for each sequence ranges from 5 to 8 min. Field of view ranges between 32 and 40 cm and ASSET factor of 2 is used in all sequences. Total scan time is between 35-50 min.
MR FINDINGS IN CD
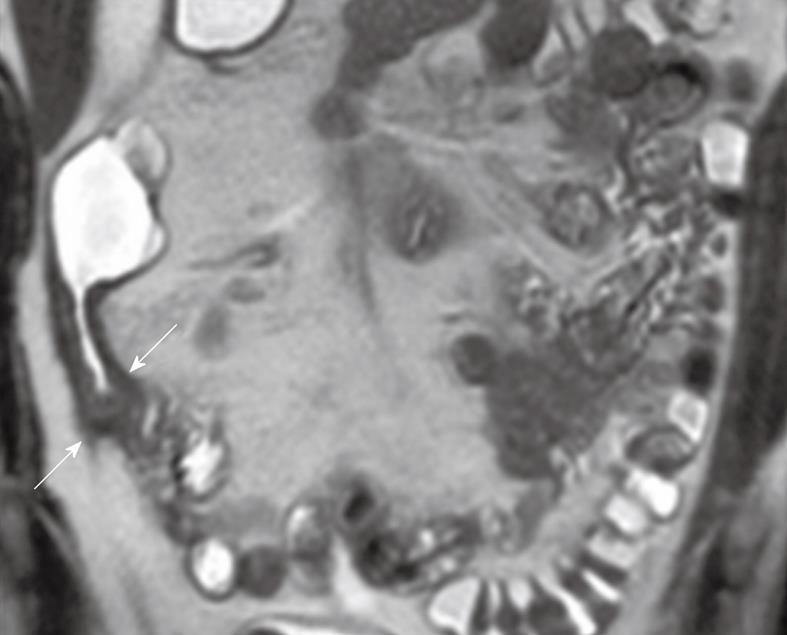
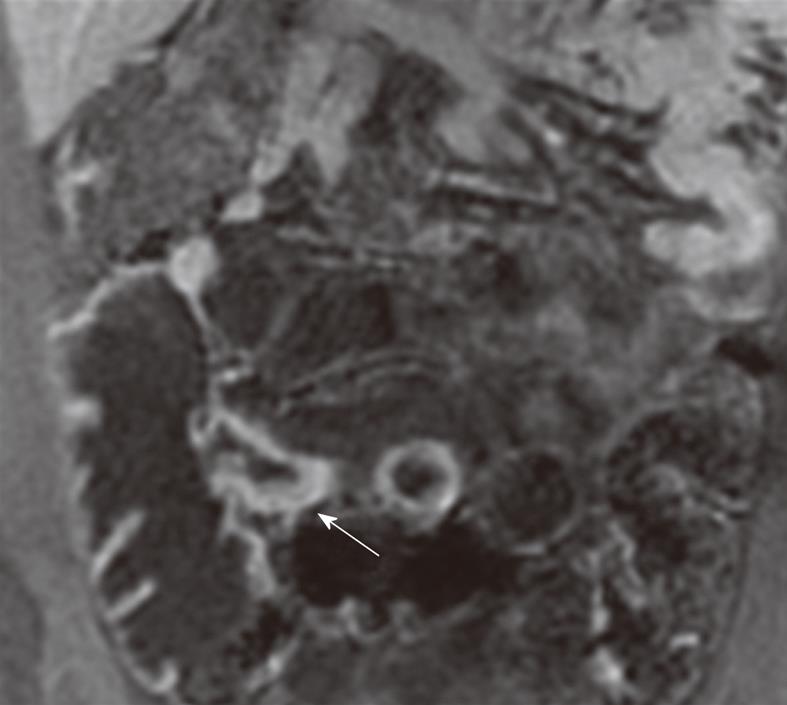
There have been various MR imaging findings proposed as imaging biomarkers of CD activity[48,57,58]. Bowel wall thickening is a significant but yet not entirely specific feature of CD. Mural thickness increases with acute inflammation, given the associated histologic findings of edema and inflammatory infiltrate. A wall thickness of greater than 3 mm in an appropriately distended segment should be considered abnormal (Figures 1 and 2). Increased enhancement of bowel wall is also an important finding of active inflammation, which is associated with mucosal hyperemia (Figure 2). The degree of bowel wall thickening and enhancement has also a high degree of correlation with the CD activity index and the histologic grading. Many investigators[59,60] have suggested that a greater degree of mural enhancement is seen when inflammatory activity increases but Punwani et al[61], in their recent series performed with 18 CD patients, failed to find this correlation in their study group. The enhancement pattern of the inflamed bowel has also been evaluated[24]. A layered pattern (mural stratification) of bowel enhancement has been reported to have good correlation with active inflammation[62,63]. Mural stratification is the abnormal separation of the contrast-enhancing outer gut margin (serosa/muscularis propria) from the contrast-enhancing inner gut margin (mucosa/muscularis mucosa). The layered appearance consists of an inner enhancing ring produced by the hyperemic mucosa and an outer ring by enhancing muscle and serosa with an intermediate low-density ring is produced by submucosal edema. A similar target sign may be seen due to a low intensity signal ring formed by hypertrophied fat and fibrosis of the submucosa. This sign is seen in chronic stage, and thus it is important to distinguish between spasms and strictures of active inflammation from fat-halo sign, as seen in the chronic stage. There is also a strong association between mural signal intensity on T2W images and inflammatory activity[59,61] (Figure 1). Low signal wall thickening on T2W images with lack of increased enhancement is indicative of chronic or inactive CD. Comb sign is another finding of CD which is produced by distended, enhancing, mesenteric vessels supplying the inflamed bowel segment. Fibro-fatty proliferation (fat-wrapping) of the mesentery around the inflamed bowel is a secondary finding, which leads to separation of bowel loops. Fat stranding adjacent to thickened bowel wall may also be present in CD patients. Introduction of ultrafast pulse sequences in MR examination protocols has significantly improved the identification of mesenteric lymph nodes in patients with CD. Some investigators state that enhancement of lymph nodes is indicative of CD activity[59]. Gourtsoyianni et al[64] studied mesenteric lymph nodes in patients with different subtypes of CD and they concluded that enhancement ratio of lymph nodes identified on MR may vary across different subtypes of CD. Such differences may be valuable in clinical practice. CD may lead to some complications such as fistulae, phlegmon, abscesses and bowel obstruction. CD may be subgrouped into 3 categories: fistula-forming/perforating, fibrostenotic and perianal. In fistula-forming/perforating disease, the large sinus tracks or fistulae may be visualized by enteric contrast agent and they are seen as linear hyperintense tracks. Fibrostenotic CD is seen as a fixed narrowing of the affected segment without any bowel wall thickening or inflammation on MRI. Chronic strictures are seen as hypointense on both T1 and T2W images and may show minimal enhancement. In perineal CD, MRI helps in diagnosis and demonstrating the anatomy of perineal fistulae.
Figure 1 Coronal T2-weighted single-shot fast spin echo (SSFSE) image shows wall thickening and increased mural T2-signal intensity in the terminal ileum wall (arrows).
Figure 2 Coronal contrast-enhanced 3D GRE T1-weighted image shows wall thickening and increased enhancement of TI wall (arrow).
ADVANCED MR TECHNIQUES IN CD
New MR applications have been applied to obtain additional information about the structural organization of tissues on bowel imaging. Diffusion-weighted imaging (DWI) and DCE-MRI techniques are still being studied for their potential to provide more quantitative and accurate assessment of fibrosis and active inflammation in the bowel wall.
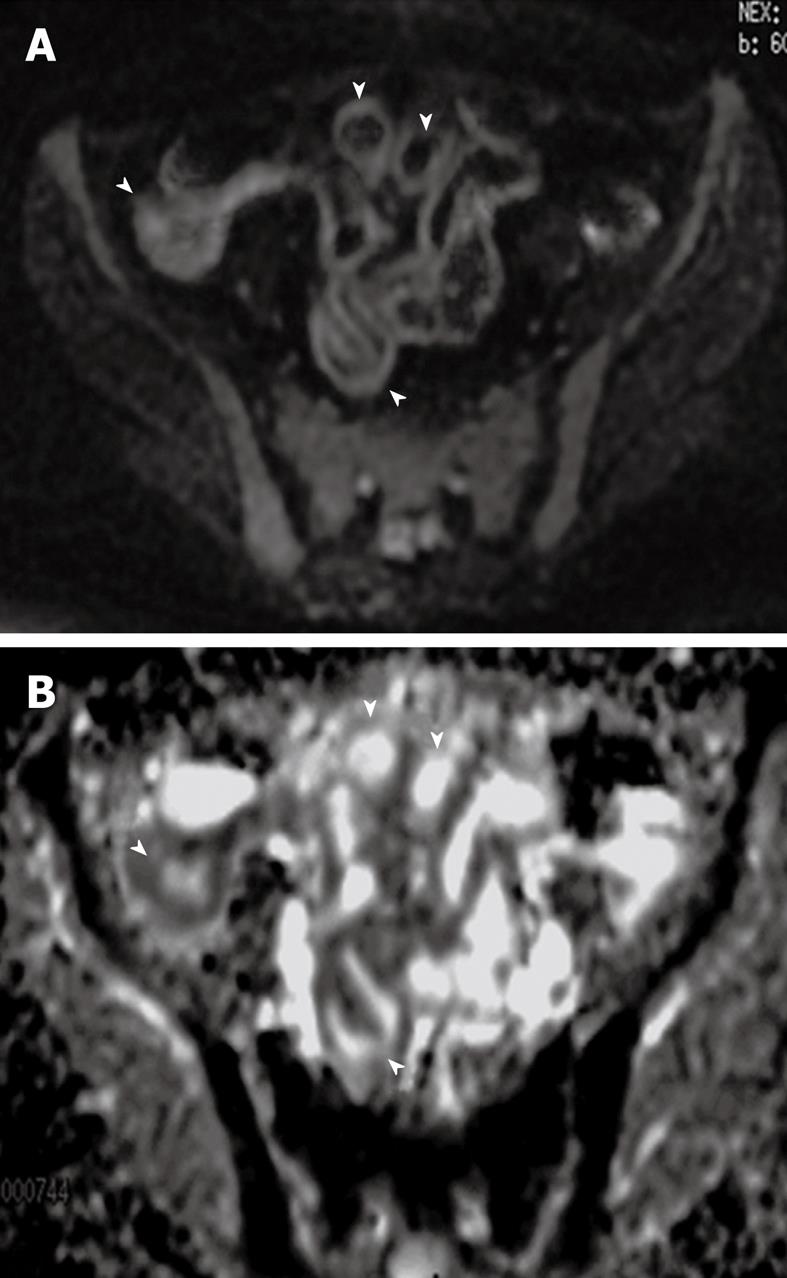
DWI reflects the changes in the mobility of water molecules and yields qualitative and quantitative information reflecting tissue cellularity and cell membrane integrity. It complements morphological information obtained by conventional MRI. There are some studies on the role of DWI in detection of bowel inflammation in CD. The apparent diffusion coefficient (ADC) may facilitate quantitative analysis of disease activity. Visual assessment of DWI may provide higher accuracy, and the calculation of the ADC may facilitate the quantitative analysis of disease activity (Figure 3). Considering its relatively light patient burden, DWI may contribute to the follow-up of CD patients. Oto et al[65] reviewed DWI images of 11 CD patients and measured ADC values in a pilot study. They concluded that inflamed bowel segments showed higher signal and decreased ADC values compared to normal segments on DWI sequence. Kiryu et al[66] found an accuracy of 93.3% in the SB, based on visual evaluation and lower ADC values in the disease-active than that in disease-inactive area in CD patients.
Figure 3 DWI and apparent diffusion coefficient (ADC) map of inflamed bowel wall.
A: Thickened inflamed bowel wall with high signal on DWI image (arrowheads); B: On ADC map, inflamed bowel wall presents a dark signal (arrowheads).
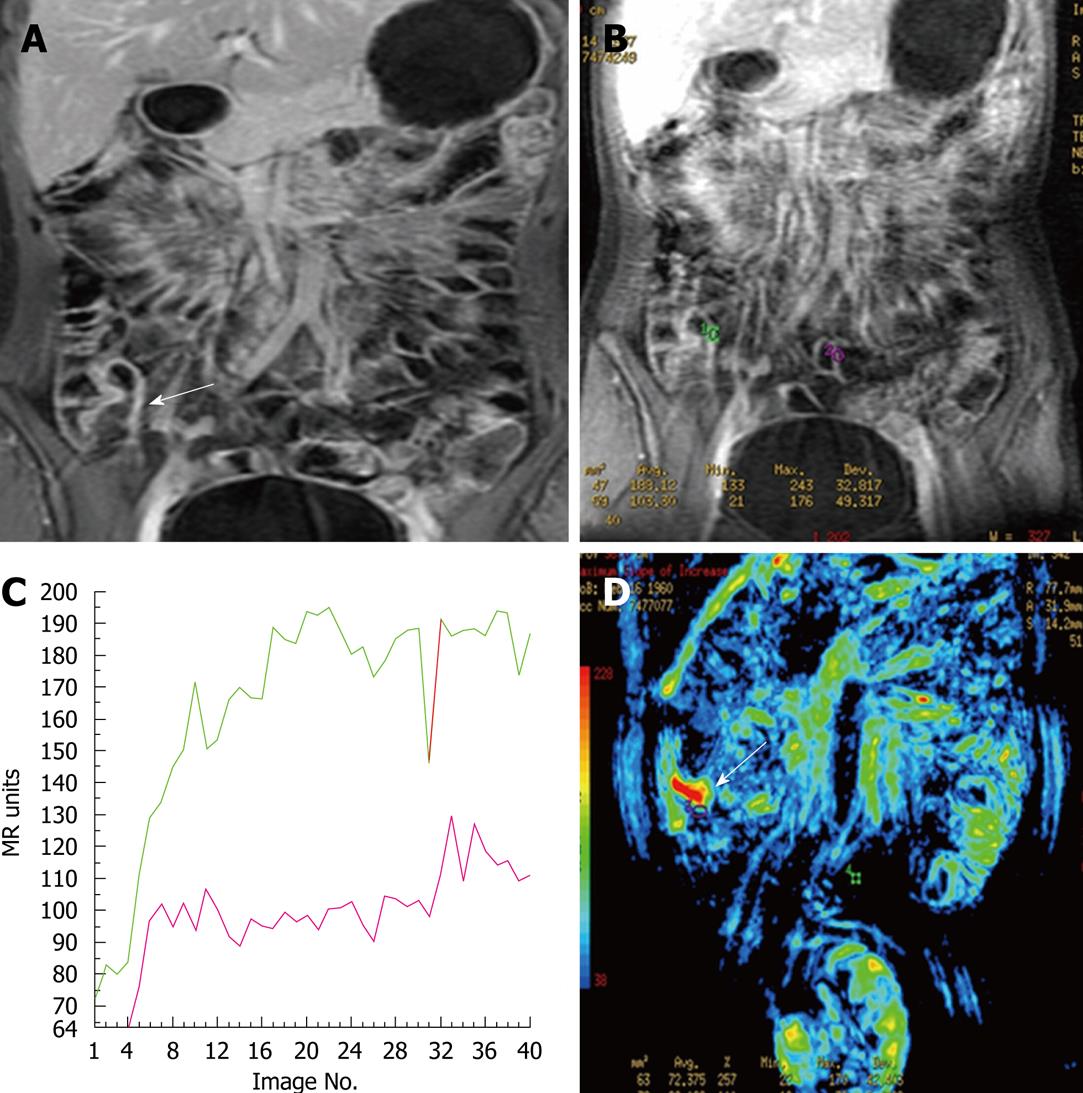
DCE-MRI is a method useful to investigate microvascular structure and function by tracking the pharmacokinetics of injected low-molecular weight contrast agents. It is sensitive to alterations in vascular permeability, extracellular, extravascular and vascular volumes and blood flow[67]. The microvasculature neoangiogenesis has been introduced as a recent component of inflammatory bowel disease pathogenesis[68] and local vascularization is known to increase with the activity of the disease[23]. In clinical DCE-MRI, T1W image signals are repeatedly measured after the IV injection of a contrast agent, typically a low-molecular weight gadolinium chelate. When the tissue is highly permeable, the contrast agent will rapidly leak from the vasculature into the extravascular space and result in fast enhancement in the DCE-MRI images. Therefore; the evaluation of the increased enhancement in the pathologic bowel wall can be useful in determining the site and the degree of activity of CD (Figure 4). There are some initial qualitative and semiquantitative studies on the ability of DC-MRI in assessing CD activity[60,63,23,69-71]. Horsthuis et al[72] assessed the efficacy of DCE-MRI in perianal CD in 33 patients and found a significant correlation between time intensity curves and perianal activity disease index.
Figure 4 Dynamic-contrast enhanced magnetic resonance imaging (DCE-MRI) findings in inflamed terminal ileum and in a normal segment.
A: Coronal contrast-enhanced 3D GRE T1-weighted image shows wall thickening and increased enhancement of TI wall (arrow); B: Dynamic contrast-enhanced image showing ROI placed on inflamed terminal ileum (green ROI) wall and normal ileal segment (purple ROI); C: The time-intensity curves plotted as a function of time for terminal ileum and normal ileal segment. Perfusion parameters of TI (green curve) is higher than the normal ileal segments (purple curve); D: Color map generated from dynamic contrast enhanced study shows increased perfusion in terminal ileum (arrow).
The assessment of stenoses in CD is an important clinical problem and observation of intestinal motility has been of prime importance for an accurate diagnosis for both the gastroenterologist and the radiologist. Yet, there is no established technique that can reliably distinguish inflammatory from scarred stenoses. The physician needs to decide whether anti-inflammatory or surgical therapy should be carried out. Cinematographic techniques have recently been evaluated for this purpose[73,74]. A continuous liquid infusion through a nasojejunal probe during data acquisition provides optimal distension of the intestinal loops and makes it possible to differentiate between functional and scarred stenoses, even on static images.
CONCLUSION
Cross-sectional imaging techniques have a crucial role in SB imaging. MRI has many advantages over CT, including lack of ionizing radiation, improved soft tissue contrast, ability to provide real-time and functional imaging and the safety profile of utilized contrast agents. Limitations of MRI are inferior temporal and spatial resolution, difficulty in access to the scanners and cost. With increasing awareness of radiation exposure caused by CT examinations, and improvements in MRI techniques, MRE will emerge as a diagnostic modality of choice in CD patients.
Peer reviewers: Joon Koo Han, MD, PhD, Professor, Department of Radiology, Seoul National University College of Medicine, 103, Daehangno, Jongno-gu, Seoul 110-744, South Korea; Arne S Borthne, MD, PhD, Associate Professor, Diagnostic Imaging Center, Akershus University Hospital, Sykehusveien 27, Nordbyhagen, NO-1478 Lørenskog, Norway
S- Editor Cheng JX L- Editor Negro F E- Editor Zheng XM